ABSTRACT
Exocrine gland-secreting peptide 22 (ESP22) is a 10-kDa protein secreted in tears of juvenile mice. ESP22 inhibits sexual behaviors in adults, leading to a reduction in reproduction rate. We herein identified the 24 amino acid sequence within ESP22 that was essential for exhibiting sexual rejection activity. This synthesizable peptide can be useful for controlling mouse overpopulation.
In mice (Mus musculus), sexual behavior is controlled by chemosensory cues including proteinaceous pheromones. A 7-kDa protein in tear fluids of male mice, known as exocrine gland-secreting peptide 1 (ESP1), enhances sexual behavior in female mice [Citation1,Citation2]. ESP22, a 10-kDa peptide that belongs to the ESP family, is secreted from tear fluids of juvenile mice and inhibits sexual behaviors in both male and female adult mice [Citation3–Citation5]. ESP1 and ESP22 are detected by specific G protein-coupled receptors expressed in the vomeronasal organ (VNO). This information is sent to the accessory olfactory bulb (AOB) and subsequently to higher brain areas [Citation6,Citation7]. ESP22 suppresses sexual behavior in female mice even in the presence of ESP1, leading to a reduction in reproduction rate. In this study, our aim was to identify a short active fragment in ESP22 that could be synthesized and used at a field level to solve the problem of mouse overpopulation.
Using a secondary structure prediction tool (the PSIPRED Protein Analysis Workbench) [Citation8], it was predicted that ESP22 had four helices and two β-sheet structures. Based on this prediction, we chemically generated three peptides of ESP22: an N-terminal fragment (3–35 a.a.), a middle fragment (30–74 a.a.), and a C-terminal fragment (66–89 a.a.) ()). C57BL/6 virgin female mice were exposed to each peptide fragment (500 µg). The number of VNO neurons in which phosphorylated ribosomal protein pS6 (a neural activation marker) was induced [Citation9] was quantified. An increased number of pS6-positive neurons was observed in the VNO sections of mice exposed to the N-terminal peptide but not in those of mice exposed to the middle or C-terminal peptides (). The activity was equivalent to that of 100 µg of full length recombinant ESP22 (). We also observed an increased number of c-Fos-positive neurons in the posterior part of the AOB of female mice exposed to the N-terminal peptide, suggesting that the information was sent to the brain ().
Figure 1. The N-terminal ESP22 peptide activates vomeronasal sensory neurons (VSNs) and accessary olfactory bulb (AOB) neurons.
(a) Predicted secondary structures of ESP22 and three synthetic peptides utilized in this study. Grey cylinder and arrow show helix and beta-sheet structure, respectively, predicted by the PSIPRED Protein Analysis Workbench [Citation8] Three synthetic ESP22 peptides are highlighted in bold: an N-terminal fragment (3–35 a.a.), a middle fragment (30–74 a.a.), and a C-terminal fragment (66–89 a.a.). (b) Representative immunohistochemical images of pS6-expressing VSNs in the vomeronasal organ of C57BL/6 virgin female mice stimulated with control buffer, N-terminal, middle, or C-terminal ESP22 peptides (500 µg each), or full length recombinant ESP22 (rESP22) (100 µg). Arrowheads denote the expression of pS6. Scale bar, 50 µm. (c) Number of pS6-positive cells per section. A total of 18 sections from each animal were quantified. Control, n = 6 mice; N-terminal peptide, n = 5 mice; middle peptide, n = 3 mice; C-terminal peptide, n = 3 mice; recombinant ESP22 (100 µg), n = 5 mice. Error bars, S.E.M. **p < 0.01 by Steel-Dwass test. (d) Representative ISH images of c-Fos-expressing AOB neurons in C57BL/6 virgin female mice stimulated with control buffer, or N-terminal peptide (500 µg). c-Fos cRNA probes (red) were used in conjunction with nuclear DAPI staining (blue). Abbreviations: GL, glomerular layer; MCL, mitral/tufted cell layer. Scale bar, 100 µm. (e) Number of total c-Fos-positive cells in AOB sections. A total of 16 sections from each animal were quantified. n = 5 mice. Error bars, S.E.M. *p < 0.05 by Mann-Whitney U test. Each dot represents data of an individual mouse in panel (c) and (e). Immunohistochemistry and ISH were performed as described previously [Citation5,Citation10].
![Figure 1. The N-terminal ESP22 peptide activates vomeronasal sensory neurons (VSNs) and accessary olfactory bulb (AOB) neurons.(a) Predicted secondary structures of ESP22 and three synthetic peptides utilized in this study. Grey cylinder and arrow show helix and beta-sheet structure, respectively, predicted by the PSIPRED Protein Analysis Workbench [Citation8] Three synthetic ESP22 peptides are highlighted in bold: an N-terminal fragment (3–35 a.a.), a middle fragment (30–74 a.a.), and a C-terminal fragment (66–89 a.a.). (b) Representative immunohistochemical images of pS6-expressing VSNs in the vomeronasal organ of C57BL/6 virgin female mice stimulated with control buffer, N-terminal, middle, or C-terminal ESP22 peptides (500 µg each), or full length recombinant ESP22 (rESP22) (100 µg). Arrowheads denote the expression of pS6. Scale bar, 50 µm. (c) Number of pS6-positive cells per section. A total of 18 sections from each animal were quantified. Control, n = 6 mice; N-terminal peptide, n = 5 mice; middle peptide, n = 3 mice; C-terminal peptide, n = 3 mice; recombinant ESP22 (100 µg), n = 5 mice. Error bars, S.E.M. **p < 0.01 by Steel-Dwass test. (d) Representative ISH images of c-Fos-expressing AOB neurons in C57BL/6 virgin female mice stimulated with control buffer, or N-terminal peptide (500 µg). c-Fos cRNA probes (red) were used in conjunction with nuclear DAPI staining (blue). Abbreviations: GL, glomerular layer; MCL, mitral/tufted cell layer. Scale bar, 100 µm. (e) Number of total c-Fos-positive cells in AOB sections. A total of 16 sections from each animal were quantified. n = 5 mice. Error bars, S.E.M. *p < 0.05 by Mann-Whitney U test. Each dot represents data of an individual mouse in panel (c) and (e). Immunohistochemistry and ISH were performed as described previously [Citation5,Citation10].](/cms/asset/46852ac3-fdd3-4f86-9360-ebf75001eca3/tbbb_a_1554427_f0001_c.jpg)
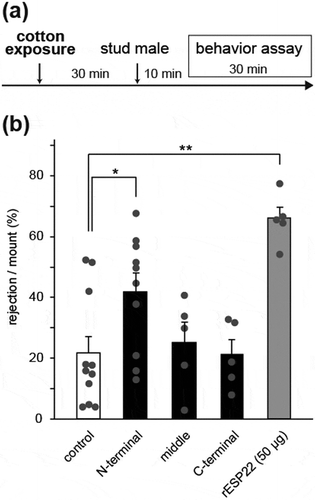
To confirm the behavioral output of the N-terminal peptide, we performed a sexual behavior assay in C57BL/6 virgin female mice at a hormone-primed pseudo-estrus state [Citation5]. We started the sexual behavior assay 30 minutes after cotton exposure to stabilize females by following a previously-reported pheromone-mediated behavioral paradigm [Citation2,Citation5,Citation10], ()). Female mice pre-exposed to cotton swabs coated with 500 µg of the N-terminal peptide showed sexual rejective behavior toward male mice more frequently than females exposed to a control buffer ()). On the other hand, there was no increase of rejection behavior in female mice pre-exposed to the middle or C-terminal peptides ()). Together with the neural activity assays in the VNO and AOB (), these results suggest that the N-terminal region of ESP22 including the 1st and 2nd helices is sufficient to elicit sexual rejective behavior in females.
Figure 2. The N-terminal ESP22 peptide enhances sexual rejection in virgin female mice.
(a) Timeline for sexual behavior assays using adult female mice pre-exposed to a synthetic ESP22 peptide or control buffer. (b) Quantification of the sexual behaviors of control buffer-, ESP22 peptide (500 µg)-, and recombinant ESP22 (rESP22)-exposed female mice. Each dot represents data of an individual female mouse. Control, n = 11; N-terminal peptide, n = 10; middle peptide, n = 5; C-terminal peptide, n = 5; rESP22 (50 µg), n = 5. Error bars, S.E.M. **p < 0.01 and *p < 0.05 by non-repeated measures ANOVA with Dunnet correction.
Next, the N-terminal peptide was further trimmed to include only the 2nd helix region (12–35 a.a., shown in red) of ESP22 ()). We found that females pre-exposed to the 2nd helix fragment (500 μg) showed ~90% rejection ratio over male mounting, which was more robust than the activity of full length recombinant ESP22 (). Dose-response experiments demonstrated that 50 μg of the 2nd helix peptide was sufficient to elicit rejection behavior (). The reason for the lower activity of full length recombinant ESP22 than the 2nd helix peptide could be due to the N-terminal His-tag on the recombinant ESP22. Together, these results suggest that the 2nd helix is the core active site in ESP22.
Figure 3. The 2nd helix ESP22 peptide enhances sexual rejection in virgin female mice. (a) Schematic illustration of predicted secondary structures of ESP22. Cylinders show the helix structure predicted by the PSIPRED Protein Analysis Workbench [Citation8]. The position of the 2nd helix and the sequence (12–35 a.a.) are highlighted in red. (b) Raster plot representing mounting episodes made by the male mouse, with the magenta bars representing attempts associated with rejection responses by the female mice. Mounting attempts without rejection are shown by the grey bars. Control buffer-exposed female mice, n = 6; the 2nd helix peptide-exposed female mice 0.5 µg, n = 3; 5 µg, n = 4; 50 µg, n = 3; 500 µg, n = 3. (c) Quantification of the sexual behaviors of control buffer- and 2nd helix peptide-exposed female mice. Each dot represents data of an individual female mouse. Control, n = 6; 0.5 µg, n = 3; 5 µg, n = 4; 50 µg, n = 3; 500 µg, n = 3. Error bars, S.E.M. **p < 0.01 by non-repeated measures ANOVA with Dunnet correction.
![Figure 3. The 2nd helix ESP22 peptide enhances sexual rejection in virgin female mice. (a) Schematic illustration of predicted secondary structures of ESP22. Cylinders show the helix structure predicted by the PSIPRED Protein Analysis Workbench [Citation8]. The position of the 2nd helix and the sequence (12–35 a.a.) are highlighted in red. (b) Raster plot representing mounting episodes made by the male mouse, with the magenta bars representing attempts associated with rejection responses by the female mice. Mounting attempts without rejection are shown by the grey bars. Control buffer-exposed female mice, n = 6; the 2nd helix peptide-exposed female mice 0.5 µg, n = 3; 5 µg, n = 4; 50 µg, n = 3; 500 µg, n = 3. (c) Quantification of the sexual behaviors of control buffer- and 2nd helix peptide-exposed female mice. Each dot represents data of an individual female mouse. Control, n = 6; 0.5 µg, n = 3; 5 µg, n = 4; 50 µg, n = 3; 500 µg, n = 3. Error bars, S.E.M. **p < 0.01 by non-repeated measures ANOVA with Dunnet correction.](/cms/asset/b1898bc9-08e9-4220-8e8b-febc108af74e/tbbb_a_1554427_f0003_c.jpg)
In this study, we identified the core region in ESP22 that was crucial for sexual rejection activity. Unlike the full length ESP22, the 24 amino acid peptide can be chemically synthesized. The peptide can be dissolved in water or agar jelly and applied to mice. Mice exposed to the peptide show reduced sexual behavior, resulting in a reduction in reproduction rate. Therefore, this peptide can be utilized to control the mouse population. The overpopulation of mice has occurred in urban areas, causing various problems as they can rapidly increase their population size due to their shorter life cycle. Mating depends on sexual behavior, which can be inhibited by the ESP22 peptide. ESP22 is a pheromone and is not a chemical that is often hazardous to the natural environment. Thus, the peptide is species-specific and safe to use. Although further field studies are necessary to confirm the utility of the peptide, the current results provide a potential solution to solve mouse overpopulation.
Author contributions
T.O. and K.T. designed the study. T.O. performed the behavior assays and the immunohistological assays for the VNO sections. T.I. performed the immunohistological analysis for the AOB sections. R.K. examined secondary structure of ESP22. T.O. and K.T. wrote the paper with contributions from other authors.
Acknowledgments
We thank members of the Touhara lab for their helps. T.O. was supported by research fellowship for young scientist from JSPS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kimoto H, Haga S, Sato K, et al. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901.
- Haga S, Hattori T, Sato T, et al. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122.
- Kimoto, H., Sato K, Nodari F, et al. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol. 2007;17:1879–1884.
- Ferrero DM, Moeller LM, Osakada T, et al. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502:368–371.
- Osakada T, Ishii KK, Mori H, et al. Sexual rejection via a vomeronasal receptor-triggered limbic circuit. Nat Commun. 2018;9:4463.
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562.
- Holy TE. The accessory olfactory system: innately specialized or microcosm of mammalian circuitry? Annu Rev Neurosci. 2018;41:501–525.
- Buchan DWA, Minneci F, Nugent TCO, et al. Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acids Res. 2013;41:349–357.
- Abe T, Touhara K. Structure and function of a peptide pheromone family that stimulate the vomeronasal sensory system in mice. Biochem Soc Trans. 2014;42:873–877.
- Ishii KK, Osakada T, Mori H, et al. A labeled-line neural circuit for pheromone-mediated sexual behaviors in mice. Neuron. 2017;95:123–127.
