ABSTRACT
The effects of dietary protein contents and regular exercise on the oxidation of supplemented leucine were examined. In the short-term study, male BALB/cCrSlc mice were fed diets containing 0, 10, 20, 35, and 60% protein: energy ratios for 1 week. In the long-term study, exercised and sedentary mice were fed diets containing 20, 35, and 60% protein ratios for 9 weeks. After the feeding periods, the mice were a bolus administered oral supplements of l-[1-13C] leucine. Expired gas was analyzed, and oxidized leucine was expressed as a relative 13CO2/12CO2 ratio. In the short-term study, the peak 13CO2/12CO2 ratio significantly increased with diet protein concentrations. Moreover, the long-term study also showed that the peak 13CO2/12CO2 ratio was significantly increased by high protein diets in both exercised and sedentary mice. Our results indicate that supplemental leucine oxidation is associated with consumption of a high-protein diet, irrespective of exercise status.
Abbreviations: AUC: area under the curve; EX: exercise; RQ: respiratory quotient; SED: sedentary; VO2/W: oxygen uptake per body weight
Graphical Abstract
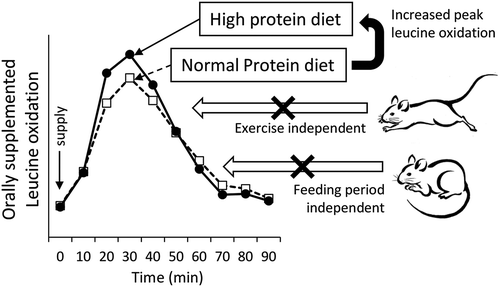
The peak leucine oxidation increases with diet protein concentrations in short- and long-term study, irrespective of exercise status.
Leucine is a branched-chain amino acid, which is initially metabolized by muscle enzymes [Citation1,Citation2]. Leucine plays a regulatory role in muscle protein synthesis, which has been shown to activate the mammalian target of rapamycin (mTOR) pathway [Citation3–Citation5]. Although not enough evidence has been accumulated to conclude that leucine supplementation is effective in muscles of athletes, previous studies suggest that taking leucine partly promotes muscle recovery in injured athletes [Citation6] and attenuates muscle membrane disruption in cyclists [Citation7]. Moreover, two meta-analyses documented that leucine supplementation has beneficial effects on muscle mass in older individuals [Citation8,Citation9]. Therefore, leucine supplementation is a potential approach to attenuate skeletal muscle degradation.
Previous studies support a recommendation of leucine supplementation, especially after exercise [Citation10], whereas how nutritional factors are associated with supplemental leucine metabolism has not been well-examined, either with or without exercise. Excess protein intake is reported to increase nitrogen excretion, suggesting that excess amino acids are metabolized as energy sources [Citation11,Citation12]. Although associations between dietary protein content and supplemental leucine oxidation have not been well examined, it was also reported that intravenous, whole body leucine oxidation was elevated by high-protein diets in neutered cats [Citation13] and healthy adult men [Citation14]. Therefore, it is likely that nutritional factors, especially protein intake, may influence supplemental leucine metabolism.
In addition to dietary conditions affecting leucine metabolism, the rate of leucine oxidation is increased during endurance exercise [Citation15,Citation16], and endurance athletes show high levels of leucine oxidation at rest [Citation17]. Because these results suggest that endurance exercise induces whole leucine wastage, it is likely that habitual endurance exercise increases the need for leucine supplementation. Indeed, previous investigations have recommended high protein intake in endurance athletes [Citation18,Citation19]. Their results suggest the importance of leucine supplementation in habitual endurance exercise, even during rest; however, combined effects of dietary protein contents and endurance exercise on oral leucine oxidation have not been investigated previously.
We hypothesized that supplemental leucine oxidation is associated with dietary protein contents and endurance exercise. In the present study, we examined the effects of different protein contents and regular exercise on the oxidation of orally supplemented leucine in adult mice. The results provide evidence whether leucine supplementation is required, depending on dietary protein contents and habitual endurance exercise.
Materials and methods
Animals
Sixty-nine male wild-type BALB/cCrSlc mice (age, 8 weeks) were obtained from Shimizu Laboratory Animal Supply Co., Ltd (Kyoto, Japan). The mice were housed under controlled conditions of constant temperature (21°C–25°C), relative humidity (45%–65%), and light/dark cycle (12/12 h). The mice were exposed to light from 00:00 to 12:00 h, and exposed to dark from 12:00 to 00:00 h. The mice were supplied water ad libitum. This study was approved by the ethics committee of Ryukoku University.
Short-term study
After acclimatization for 1 week, the mice were divided into different protein diet groups. All pellet diets (D11080501, 10% protein: energy ratio; D12450B, 20% protein: energy ratio; D05060901, 35% protein: energy ratio; and D04080301, 60% protein: energy ratio), except the 0% protein pellet diet, were purchased from Research Diets Inc. (NJ, USA). The protein sources in the diets were casein and L-cystine (98.5 and 1.5%, respectively). Nutrient ingredient and composition of experimental diets were shown in , and the number of mice in each group was 7–15 (). Mice receiving nutrient-matched diet containing the 0% protein: energy ratio were fed AIN-93G-based diet (Oriental Yeast Co., Ltd., Chiba, Japan) containing corn starch instead of milk casein and cystine. The 0% protein diet was 10% for fat and 90% carbohydrates energy ratio, composed of 553 g; corn starch, 350 g; sucrose, 25 g; soybean oil, 20 g; lard, 50 g; cellulose, 35 g; AIN-93G mineral mix, 10 g; AIN-93 vitamin mix, 2 g; choline bitartrate. The mice were fed each diet ad libitum for 1 week prior to the metabolic study.
Table 1. Dietary composition.
Table 2. Dietary composition, body weight and expired gas analysis in sedentary mice fed the diets for 1 week.
Long-term study
Because the 140 g casein/kg diet (12% protein) was recommended in previous research [Citation20], it is considered that mice fed 0 and 10% protein diets show dietary protein insufficiency, resulting in severe weight loss caused by protein catabolism throughout the long-term study. Therefore, mice fed 20, 35, and 60% protein diets were used for the long-term study which followed the short-term study. First, mice fed each diet were divided into exercise (EX) or sedentary (SED) groups (n = 7–11; ). In the first week of the study, mice in the EX group were exercised on a treadmill (40% incline, 10 m/min, and 30 min/day; Mousebelt-2000; ARCO System, Chiba, Japan) two times. From the second week, the mice were exercised on a treadmill with high intensity for 7 weeks, and the exercise program comprised five exercise sessions per week. The mice exercised on the treadmill with a non-skid belt, set at a 40% incline; the treadmill speed was maintained at 5 m/min for the first 5 min and was increased to 10 m/min for the next 5 min. After 10 min, the speed was increased by 1 m/min every 1 min until the mice were exhausted [Citation21].
Table 3. Body weight, organ weight, expired gas analysis in sedentary and trained mice fed the diets for 9 week.
Metabolic study
In both short- and long-term study, the mice were placed in metabolic cages at 9:00 h after fasting for 2 h. Their expired gas was analyzed using a CO2 and O2 mass-spectrometric analyzer (ARCO-2000; ARCO System). The gas analysis system has been previously described in detail [Citation22–Citation24]. In the long-term study, the metabolic measurements were performed at least 2 days after the previous exercise session to exclude the acute effects of exercise. After performing baseline measurements for 3 h, the mice were orally administered a single dose (0.8 mg/g body weight) of l-[1-13C] leucine (99 atom percent excess; Cambridge Isotope Laboratories, MA, USA) with water solution. Oxidation of the orally administered l-[1-13C] leucine was assessed based on the relative abundance of 13CO2 (13CO2/12CO2 ratio). The administered leucine (0.8 mg/g body weight) was able to discriminate from the baseline 13CO2/12CO2 ratio. CO2 and O2 masses were analyzed for 3 h under fasting condition after l-[1-13C] leucine administration. After refeeding for 1 h, the CO2 and O2 mass were analyzed for 24 h with consumption of the indicated diets. Thus, the metabolic study was successively performed over 28 h. Respiratory quotient (RQ) was calculated from the expired volumes of CO2 and O2 (VCO2 and VO2, respectively) by using the formula VCO2/VO2, and oxygen uptake was expressed per body weight (VO2/W).
Statistical analysis
All statistical analyses were performed using SPSS version 23.0 (SPSS Inc., IL, USA). Two-way repeated ANOVA (time × group) was used to examine differences in 13CO2/12CO2 ratio, VO2/W, and RQ during metabolic measurements. Another two-way ANOVA (exercise × diet) was used to assess the main effects of variables on exercise and diet conditions in long-term study. A post hoc Tukey-Kramer’s test was used to identify significant differences among mean values when a significant main effect or interaction was identified. One-way ANOVA was used to assess differences in other variables. All measurements are presented as mean ± standard error. P < 0.05 was considered statistically significant.
Results
Effects of 1 week feeding different protein diets on supplemental leucine oxidation
Body weight between the groups was not different at the beginning of the study. After feeding for 1 week, body weights of mice fed the 0% protein diet were significantly lower than those of mice fed the other diets (P < 0.05; ). However, no significant differences were observed in the body weights of mice fed the other protein diets for 1 week.
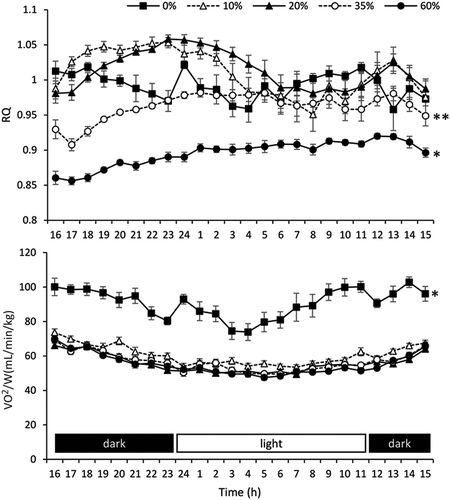
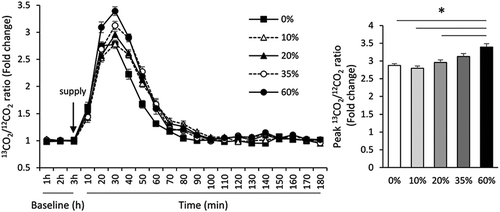
We observed that the 3-h baseline measurement values of the 13CO2/12CO2 ratio and RQ were not different among mice fed the different diets. Baseline oxygen uptake in mice fed the 0% protein diet was significantly higher than that of mice fed the other diets (P < 0.05; ). The 13CO2/12CO2 ratio peaked at 30 min after supplementation of l-[1-13C] leucine, and returned to baseline at 90 min after the oral administration of l-[1-13C] leucine in mice in all the groups (). Results of the two-way repeated ANOVA showed a significant higher 13CO2/12CO2 ratio for mice fed 60% protein diets than for mice fed 0% protein diets (P < 0.05). In addition, the peak 13CO2/12CO2 ratio was significantly higher for mice fed the 60% protein diet than for mice fed the 0, 10, and 20% protein diets (P < 0.05). The AUC of the 13CO2/12CO2 ratio was significantly higher for mice fed 35% or 60% protein diets than for mice fed the 0% protein diet (P < 0.05; ).
Figure 1. Effects of the consumption of different protein diets for 1 week on oral leucine oxidation in mice; * indicates a significant difference (P < 0.05) compared with 0, 10, 20% protein diet groups.
Expired gas was monitored for 24 h without fasting (). Results of the two-way repeated ANOVA with Tukey-Kramer’s test showed that the 24-h RQ was significantly lower in mice fed the 60% protein diet than in mice fed the other diets (P < 0.05). Moreover, the 24-h RQ was significantly lower in mice fed the 35% protein diet than in mice fed the 10 and 20% protein diets (P < 0.05). Mice fed the 0% protein diet showed significantly higher 24-h VO2/W than mice fed the other diets (P < 0.05).
Characteristics of exercised and sedentary mice fed different protein diets for 9 weeks
As shown in , average exercise time was significantly longer for EX mice fed the 20% protein diet than for EX mice fed the 60% protein diet (P < 0.05). After 9 weeks, the body weights of EX mice were significantly higher than those of SED mice (P < 0.05). The absolute weights of the soleus, plantaris, and gastrocnemius muscles were significantly higher in EX mice than in SED mice (P < 0.05). However, no significant differences in these skeletal muscles were observed in relative weight/100 g body weight between EX and SED mice. EX mice had lower absolute and relative epidydimal fat weights and higher absolute and relative kidney weights than SED mice (P < 0.05).
Effect of the 9-week intake of different protein diets on supplemental leucine oxidation in exercised and sedentary mice
Baseline measurements showed no significant differences in 13CO2/12CO2 ratios, and RQ between EX and SED mice (). Baseline VO2/W was significantly higher in SED mice that than in EX mice (P < 0.05), but there was no significant difference in VO2/W during 3 h after supplementation with l-[1-13C] leucine. RQ during the trial after leucine supplementation was slightly but significantly higher in the EX mice (P < 0.05).
Consistent with the results of the short-term study, the 13CO2/12CO2 ratio peaked at 30 min after oral l-[1-13C] leucine administration in both SED and EX mice (). Results of two-way ANOVA for the peak 13CO2/12CO2 ratio showed a significant main effect of dietary protein concentration (P < 0.05), but not of exercise (P = 0.525). The post hoc test revealed that the peak 13CO2/12CO2 ratio was significantly higher in mice fed the 60% protein diet than in mice fed the 20% protein diet (P < 0.05).
Figure 3. Effects of the consumption of different protein diets and exercise for 9 weeks on oral leucine oxidation in mice; * indicate a significant different (P < 0.05) between the 20 and 60% protein diet groups. EX, exercise group; SED, sedentary group.
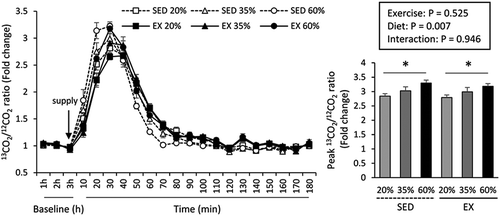
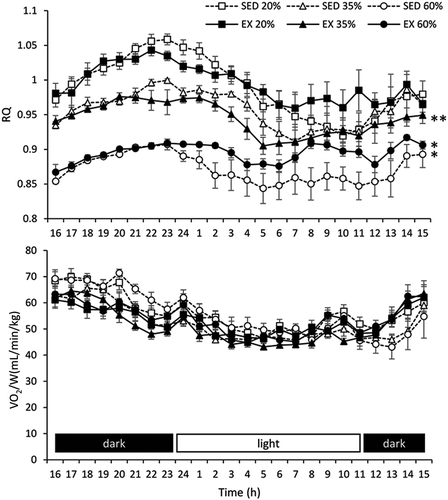
We next monitored 24-h expired gas, and lower 24-h RQ was obtained for both SED and EX mice fed the 60% protein diet compared with that for mice fed the other diets (P < 0.05; ). EX mice fed the 35% protein diet had lower 24-h RQ than both SED and EX mice fed the 20% protein diet (P < 0.05; ). The 24-h VO2/W did not differ among the groups, and no significant differences in 24-h RQ and VO2/W were observed between SED and EX mice fed the same protein diet.
Figure 4. The 24-h RQ and VO2/W in the long-term study; * indicates a significant difference (P < 0.05) compared with mice fed the other protein diets, and ** indicates a significant difference (P < 0.05) compared with SED and EX mice fed the 20% protein diet. EX, exercise group; RQ, respiratory quotient; SED, sedentary group; VO2/W, oxygen uptake per body weight.
Discussion
Our study examined the effects of different protein diets and endurance exercise on the metabolism of supplemental leucine in mice. Analysis of expired gas using a CO2 and O2 mass-spectrometric analyzer showed that peak leucine oxidation (peak 13CO2/12CO2 ratio) was associated with both short- and long-term dietary protein consumption. On the other hand, there was no significant difference in the peak leucine oxidation between EX and SED mice. These results suggest that supplemental leucine is rapidly metabolized as energy sources under enough protein intakes, and the peak oxidation was associated with dietary protein contents but not with endurance exercise.
Results of the short-term (1-week) study showed almost similar 13CO2/12CO2 ratio peaks in mice fed the 0 and 10% protein diets; however, the peak 13CO2/12CO2 ratio increased with an increase in other dietary protein concentration (). Because the American Institute of Nutrition recommends that the diet of adult rodents should contain 12% protein concentration [Citation20], the similar peak oxidation of leucine may be associated with insufficient protein intake in mice fed the 0 and 10% protein diets. Unlike the peak 13CO2/12CO2 ratio, the AUC of the 13CO2/12CO2 ratio was significantly lower in mice fed the 0% protein diet (). Therefore, the AUC, rather than the maximal oxidation capacity might be more correlated with a dietary protein deficit. In addition, because both the 13CO2/12CO2 peak and AUC increased with diet protein concentrations ( and ), the short-term study indicates that high protein diet induces leucine oxidation after a bolus of leucine intake, which may be related to supply above protein requirements.
In the short-term study, results of the 24-h metabolic measurements showed lower 24-h RQ for mice fed a low-carbohydrate and high-protein diet (), which was consistent with that observed previously [Citation25]. The results suggested that decreased glucose oxidation in these mice. Because no significant difference was observed in 24-h VO2/W among mice fed 10–60% protein diets (), the lower 24-h RQ was not be caused by increases in fat oxidation and energy expenditure. It is likely that the 24-h RQ is associated with protein oxidation as energy sources. In addition, the mice fed the 0% protein diet showed significantly higher 24-h VO2/W than mice fed the other diets (). This result was in concordance with the results of the baseline and trial VO2/W in mice fed the 0% protein diet (). A study involving a rodent model of activity-based anorexia that received a limited amount of diet showed a significant increase in spontaneous exercise volume, which in turn decreased body weight [Citation26]. Therefore, the increase in VO2 consumption in mice fed the 0% protein diet might be because of poor nutritional status and/or weight reduction associated with higher physical activity in search of diet.
In the long-term study, no significant effects were observed in the baseline 13CO2/12CO2 ratio among all the groups, and thus the habitual dietary and exercise conditions did not influence the 13CO2 levels. In addition, the long-term study showed that the peak 13CO2/12CO2 ratio after leucine supplementation increased with an increase in dietary protein concentration in both EX and SED mice (), as seen in the short-term study. There was no significant difference in the peak 13CO2/12CO2 ratio between EX and SED mice. The EX mice showed higher weight gain, muscle mass, and fat loss than SED mice, suggesting that high-intensity treadmill exercise affects muscle anabolism in EX mice (). Although it has been reported that skeletal muscles metabolize branched chain amino acids, including leucine [Citation2], the our results suggested that dietary protein contents had more influence on the peak leucine oxidation than changes in body composition.
In contrast, the long-term study showed that no significant association was observed between the AUC of the 13CO2/12CO2 ratio and dietary protein intake (). The results suggested that long-term intake of excess protein altered total leucine oxidation. Although the underlying mechanisms remain unclear, it was shown that a high protein diet induced gluconeogenesis in rodents and human [Citation27–Citation29]. Therefore, the lack of relationship between excess protein intake and supplemental leucine in the long-term study may be regulated by gluconeogenesis, resulting in the similar 13CO2/12CO2 AUCs among the groups. Although the relationship was not clear, slightly but significantly higher RQ after leucine supplementation was found in EX mice compared with SED mice (, average difference of 0.01, P < 0.05), suggesting that slightly more glucose might be metabolized during the 3 h after leucine supplementation in EX mice.
Twenty-four hours of metabolic chamber analysis revealed that the 24-h RQ and VO2/W values obtained in the long-term study were similar to those obtained in the short-term study (). The 24-h RQ was significantly lower in EX and SED mice fed high protein diets, and there was no significant difference in 24-h VO2/W among the groups. In addition, the 24-h RQ and VO2/W values were not significantly different between the EX and SED mice, suggesting that these values at rest were not affected by exercise status. It was reported that neither the 24-h RQ nor energy expenditure differed between male endurance athletes and untrained males [Citation30]. These results suggested that, unlike after leucine supplementation, dietary protein contents have more influence than endurance exercise on 24-h RQ.
Our study has several limitations. First, because we sought to examine effects of endurance exercise and high protein diets on body composition and leucine supplementation, the EX mice performed high-intensity exercise, which induced muscle mass gain. Thus, we could not evaluate the relationship between low-intensity exercise and leucine supplementation. Second, this study used the experimental diets with varying ratio of protein and carbohydrate, whereas it reports that high-protein, low-carbohydrate diet increases plasma levels of glucoregulatory hormone such as cortisol [Citation31]. Cortisol induces amino acid oxidation including leucine [Citation32], and thus further research is needed to determine whether the effects of low-carbohydrate diet on gluconeogenic hormone alter the relationship between dietary protein intake and leucine oxidation. It was suggested that equal protein diets with varying ratios of fat and carbohydrate did not influence glucoregulatory hormones including cortisol, epinephrine, and norepinephrine [Citation33]. Therefore, equal-protein, different carbohydrate diets may provide additional insight into relationship between dietary consumption and leucine oxidation. Third, the mice in the long-term study consumed sufficient dietary calories and protein. The results may be different from effects of leucine supplementation in humans who habitually consume lower energy and micronutrients intake, such as elderly individuals [Citation34] and during weight loss, as well as those who require protein restriction, such as patients with kidney diseases [Citation35]. Additional human studies are needed to examine the relationship between different protein intake and leucine metabolism.
In conclusion, peak leucine oxidation after leucine supplementation is associated with both short- and long-term protein intakes, and supplemental leucine oxidation does not correlate with exercise status and body composition. The results suggest that the peak leucine oxidation reflect differences in dietary protein contents irrespective of feeding duration and fitness level.
Author Contribution
HT, NA, and KI carried out animal experiments and measurements in the study. HT and KI participated in the design of the study. HT performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by a grant from the Kao Research Council for the Study of Healthcare Sciences (to HT). All authors declare that they do not have any conflicts of interest related to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shimomura Y, Fujii H, Suzuki M, et al. Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: regulation of the activity and gene expression by nutrition and physical exercise [Review]. J Nutr. 1995 Jun;125(6 Suppl.):1762S–1765S. PubMed PMID: 7782942; eng.
- Suryawan A, Hawes JW, Harris RA, et al. A molecular model of human branched-chain amino acid metabolism [Research support, U.S. Gov’t, Non-P.H.S. Research support, U.S. Gov’t, P.H.S.]. Am J Clin Nutr. 1998 Jul;68(1):72–81. PubMed PMID: 9665099; eng.
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling [Research support, Non-U.S. Gov’t research support, U.S. Gov’t, P.H.S. Review]. Proc Nutr Soc. 2004 May;63(2):351–356. PubMed PMID: 15294054; eng.
- Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway [Research support, U.S. Gov’t, P.H.S.]. J Nutr. 2000 Oct;130(10):2413–2419. PubMed PMID: 11015466; eng.
- Wilkinson DJ, Hossain T, Hill DS, et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol. 2013 Jun 1;591(11):2911–2923. PubMed PMID: 23551944; PubMed Central PMCID: PMC3690694. eng.
- Laboute E, France J, Trouve P, et al. Rehabilitation and leucine supplementation as possible contributors to an athlete’s muscle strength in the reathletization phase following anterior cruciate ligament surgery [Randomized controlled trial]. Ann Phys Rehabil Med. 2013 Mar;56(2):102–112. PubMed PMID: 23260415; eng.
- Thomson JS, Ali A, Rowlands DS. Leucine-protein supplemented recovery feeding enhances subsequent cycling performance in well-trained men [Randomized controlled trial research support, Non-U.S. Gov’t]. Appl Physiol Nutr Metab = Physiologie Appliquee, Nutrition Et Metabolisme. 2011 Apr;36(2):242–253. PubMed PMID: 21609286; eng.
- Xu ZR, Tan ZJ, Zhang Q, et al. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis [Meta-analysis research support, Non-U.S. Gov’t review]. Br J Nutr. 2015 Jan 14;113(1):25–34. PubMed PMID: 25234223; eng.
- Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis [Meta-analysis review]. J Nutr Health Aging. 2015 Apr;19(4):437–446. PubMed PMID: 25809808; eng.
- Layman DK. Role of leucine in protein metabolism during exercise and recovery [Review]. Can J Appl Physiol = Revue Canadienne De Physiologie Appliquee. 2002 Dec;27(6):646–663. PubMed PMID: 12501002; eng.
- Tarnopolsky MA, Atkinson SA, MacDougall JD, et al. Evaluation of protein requirements for trained strength athletes [Clinical trial randomized controlled trial research support, Non-U.S. Gov’t]. J Appl Physiol. 1992 Nov;73(5):1986–1995. (1985). PubMed PMID: 1474076; eng.
- Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults [Meta-analysis research support, Non-U.S. Gov’t research support, U.S. Gov’t, P.H.S.]. Am J Clin Nutr. 2003 Jan;77(1):109–127. PubMed PMID: 12499330; eng.
- Wester TJ, Weidgraaf K, Hekman M, et al. Amino acid oxidation increases with dietary protein content in adult neutered male cats as measured using [1-13C]leucine and [15N2]urea [Research support, Non-U.S. Gov’t]. J Nutr. 2015 Nov;145(11):2471–2478. PubMed PMID: 26355002; eng.
- Forslund AH, Hambraeus L, Olsson RM, et al. The 24-h whole body leucine and urea kinetics at normal and high protein intakes with exercise in healthy adults [Research support, Non-U.S. Gov’t research support, U.S. Gov’t, P.H.S.]. Am J Physiol. 1998 Aug;275(2 Pt 1):E310–E320. PubMed PMID: 9688634; eng.
- Lamont LS, McCullough AJ, Kalhan SC. Beta-adrenergic blockade heightens the exercise-induced increase in leucine oxidation [Research support, Non-U.S. Gov’t research support, U.S. Gov’t, P.H.S.]. Am J Physiol. 1995 May;268(5 Pt 1):E910–E916. PubMed PMID: 7762645; eng.
- Phillips SM, Atkinson SA, Tarnopolsky MA, et al. Gender differences in leucine kinetics and nitrogen balance in endurance athletes [Research support, Non-U.S. Gov’t]. J Appl Physiol. 1993 Nov;75(5):2134–2141. (1985). PubMed PMID: 8307870; eng.
- Lamont LS, McCullough AJ, Kalhan SC. Comparison of leucine kinetics in endurance-trained and sedentary humans [Clinical trial comparative study research support, Non-U.S. Gov’t research support, U.S. Gov’t, P.H.S.]. J Appl Physiol. 1999 Jan;86(1): 320–325. (1985).
- Tarnopolsky MA, MacDougall JD, Atkinson SA. Influence of protein intake and training status on nitrogen balance and lean body mass [Comparative study research support, Non-U.S. Gov’t]. J Appl Physiol. 1988 Jan;64(1): 187–193. (1985).
- Lemon PW. Effects of exercise on dietary protein requirements [Research support, Non-U.S. Gov’t review]. Int J Sport Nutr. 1998 Dec;8(4):426–447. PubMed PMID: 9841962; eng.
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet [Congresses]. J Nutr. 1993 Nov;123(11):1939–1951. PubMed PMID: 8229312; eng.
- Ishihara K, Taniguchi H. Fat max as an index of aerobic exercise performance in mice during uphill running. PLoS One. 2018;13(2):e0193470. PubMed PMID: 29474428; PubMed Central PMCID: PMCPMC5825145.
- Ishihara K, Oyaizu S, Onuki K, et al. Chronic (-)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J Nutr. 2000 Dec;130(12):2990–2995. PubMed PMID: 11110858.
- Ishihara K, Oyaizu S, Mizunoya W, et al. Use of 13C-labeled glucose for measuring exogenous glucose oxidation in mice. Biosci Biotechnol Biochem. 2002 Feb;66(2):426–429. PubMed PMID: 11999421.
- Matsumura S, Saitou K, Miyaki T, et al. Mercaptoacetate inhibition of fatty acid beta-oxidation attenuates the oral acceptance of fat in BALB/c mice [Research support, Non-U.S. Gov’t]. Am J Physiol Regul Integr Comp Physiol. 2008 Jul;295(1):R82–91. PubMed PMID: 18480247; eng.
- Klaus S. Increasing the protein: carbohydrateratio in a high-fat diet delays the development of adiposity and improves glucose homeostasis in mice [Research support, Non-U.S. Gov’t]. J Nutr. 2005 Aug;135(8):1854–1858. PubMed PMID: 16046708; eng.
- Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2 [Research support, Non-U.S. Gov’t]. Eur Neuropsychopharmacol. 2010 Sep;20(9):622–631. PubMed PMID: 20471226; eng.
- Azzout B, Chanez M, Bois-Joyeux B, et al. Gluconeogenesis from dihydroxyacetone in rat hepatocytes during the shift from a low protein, high carbohydrate to a high protein, carbohydrate-free diet [Research support, Non-U.S. Gov’t]. J Nutr. 1984 Nov;114(11):2167–2178. PubMed PMID: 6491768; eng.
- Kaloyianni M, Freedland RA. Contribution of several amino acids and lactate to gluconeogenesis in hepatocytes isolated from rats fed various diets. J Nutr. 1990 Jan;120(1):116–122. PubMed PMID: 2303909; eng.
- Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet [Randomized controlled trial research support, Non-U.S. Gov’t]. Am J Clin Nutr. 2009 Sep;90(3):519–526. PubMed PMID: 19640952; eng.
- Schulz LO, Nyomba BL, Alger S, et al. Effect of endurance training on sedentary energy expenditure measured in a respiratory chamber [Comparative study]. Am J Physiol. 1991 Feb;260(2 Pt 1):E257–E261. PubMed PMID: 1996628; eng.
- Slag MF, Ahmad M, Gannon MC, et al. Meal stimulation of cortisol secretion: a protein induced effect. Metabolism. 1981 Nov;30(11):1104–1108. PubMed PMID: 6270500.
- Brillon DJ, Zheng B, Campbell RG, et al. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol. 1995 Mar;268(3 Pt 1):E501–E513. PubMed PMID: 7900796.
- Bisschop PH, Pereira Arias AM, Ackermans MT, et al. The effects of carbohydrate variation in isocaloric diets on glycogenolysis and gluconeogenesis in healthy men. J Clin Endocrinol Metab. 2000 May;85(5):1963–1967. PubMed PMID: 10843182.
- Lorenzo-Lopez L, Maseda A, de Labra C, et al. Nutritional determinants of frailty in older adults: A systematic review [Meta-analysis review research support, Non-U.S. Gov’t]. BMC Geriatr. 2017 May 15;17(1):108. PubMed PMID: 28506216; PubMed Central PMCID: PMC5433026. eng. DOI:10.1186/s12877-017-0496-2
- Pedrini MT, Levey AS, Lau J, et al. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis [Meta-analysis]. Ann Intern Med. 1996 Apr 1;124(7):627–632. PubMed PMID: 8607590; eng.