ABSTRACT
Six-week-old male KK-Ay mice received drinking water with S-adenosylmethionine (SAM), α-glycerophosphocholine (GPC), or SAM+GPC for 10 weeks. The serum glucose of SAM+GPC at 15 weeks old, total cholesterol of GPC at 12 weeks old, and triglyceride of GPC at 15 weeks old and of SAM at 16 weeks old were reduced. SAM+GPC reduced serum leptin and food intake.
Abbreviations: SAM: S-adenosylmethionine; GPC: α-glycerophosphocholine
The administration of a methionine-choline-deficient (MCD) diet is a common method of inducing fatty liver. The feeding of db/db mice, a model for diabetic dyslipidemia, on an MCD diet caused S-adenosylmethionine (SAM) depletion, and led to the development of non-alcoholic steatohepatitis (NASH) [Citation1]. MCD diet-induced NASH can be reversed in rats by switching to a diet with sufficient methionine and choline [Citation2]. SAM treatment was reported to improve insulin sensitivity and prevent body weight gain in OLETF rats, an animal model of type 2 diabetes mellitus [Citation3]. SAM plays an important role in normal cell function related to methionine metabolism, and the synthesis of glutathione, polyamine, and choline. Choline, recognized as an essential nutrient, is needed for neurotransmitter synthesis, cell-membrane signaling, and lipid transport. α-Glycerophosphocholine (GPC) is known to be a useful choline compound and is present in various foods [Citation4]. SAM and GPC are widely distributed in sake cake (sake-kasu), a byproduct of Japanese sake fermentation. In this study, we investigated the effect of the combination of SAM and GPC on glucose and lipid metabolism in KK-Ay mice, obese-diabetic mice showing hyperglycemia, hyperlipidemia, and hyperinsulinemia.
S-(5′-adenosyl)-L-methionine p-toluenesulfonate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Neoliquid GPC85 (85% α- glycerophosphocholine-water solution) was purchased from NOF Corporation (Tokyo, Japan).
Five-week-old male KK-Ay mice (CLEA Japan, Inc., Tokyo, Japan) were maintained under controlled conditions (22 ± 2°C, 12-h light/dark cycle, lights on 0:00–12:00). The animals were housed (n = 4–5 per cage) in plastic cages (225 × 338 × 140 mm) with free access to food and water. After a 1-week acclimation period, the mice received drinking deionized water with 0.06 mg/ml SAM, 0.06 mg/ml GPC, or 0.06 mg/ml SAM+0.06 mg/ml GPC (experimental groups; n = 8, 8, and 9, respectively) or just deionized water (control group; n = 8) for 10 weeks. The mice had free access to a modified AIN-76 diet (Oriental Yeast, Tokyo, Japan) comprising 20% casein, 0.3% DL-methionine, 15% cornstarch, 50% sucrose, 5% cellulose, 5% corn oil, 1% vitamins, 3.5% minerals, and 0.2% choline bitartrate. This study was approved by the Animal Care Committee of the National Research Institute of Brewing, Japan (ethical approval no. 26–1). All animals received humane care as outlined in the Guide for the Care and Use of Laboratory Animals (National Research Institute of Brewing, Animal Care Committee).
The activities of serum alanine aminotransferase (ALT, EC 1.1.1.27) and aspartate aminotransferase (AST, EC 2.6.1.1) as well as the levels of serum glucose, triglyceride, total cholesterol, and HDL cholesterol were measured calorimetrically by the DRICHEM commercial assay system (Fuji Film, Tokyo, Japan). Thiobarbituric acid-reactive substances (TBARS), insulin, adiponectin, IGF-1, and leptin were quantified with a TBARS Assay Kit (Cayman, MI, USA), Mercodia Mouse Insulin ELISA Kit (Mercodia, Uppsala, Sweden), Quantikine ELISA Mouse Adiponectin/Acrp30 Kit (R&D Systems Inc., MN, USA), Quantikine ELISA Mouse/Rat IGF-1 Kit (R&D Systems Inc.), and Mouse/Rat Leptin Enzyme Immunoassay Kit (SPI Bio, Montigny le Bretonneux, France), respectively, in accordance with the manufacturer’s instructions.
Total RNA was extracted from the liver using RNeasy Mini Kit (Qiagen, Hilden, Germany), in accordance with the manufacturer’s instructions. QuantiTect Reverse Transcription Kit (Qiagen) was used for reverse transcription of isolated total RNA (1 μg). Real-time quantitative PCR was performed using QuantiTect SYBR Green® PCR (Qiagen) system with specific QuantiTect Primer Assay (Qiagen) primers. Relative expression levels were determined using the ΔΔCt method. The expression of the target genes was normalized to that of Gapdh as an endogenous control gene.
The data were analyzed by one-way ANOVA followed by the Tukey–Kramer HSD test. The level of significance was set at p < 0.05.
Although the significant reduction of food and fluid intake was observed in the GPC and SAM+GPC groups, liver weight, final body weight and gains in body weight were not significantly different among the four groups (). The modified AIN-76 diet does not contain SAM and GPC, so animals received these substances only from the prepared drinking water. The doses of SAM and GPC in this study were estimated at 15 weeks old. SAM and SAM+GPC groups received 23.7 and 17.2 mg/kg body weight/day of SAM, respectively. GPC and SAM+GPC groups received 14.3 and 16.4 mg/kg body weight/day of GPC, respectively.
Table 1. Effects of SAM and GPC on parameters in KK-Ay mice.
The serum level of glucose was significantly lower in the SAM+GPC group than in the control group at 15 weeks old (−40%, p < 0.05) (). The serum level of total cholesterol was significantly lower in the GPC group than in the control group at 12 weeks old (−24%, p < 0.05) (). The serum level of triglyceride was significantly lower in the GPC group than in the control group at 15 weeks old (−38%, p < 0.05), and in the SAM group than in the control group at 16 weeks old (−39%, p < 0.05) (). Compared with the control group, reductions of these levels were often observed in experimental groups on other conditions mentioned above, but theses did not reach significance.
Figure 1. Effects of SAM and GPC on glucose and lipid metabolism.
The graphs show serum glucose (a), total cholesterol (b), and triglyceride (c) at 12, 15, and 16 weeks old. Values are mean ± SE (n = 8–9). Means in a bar without a common superscript letter differ significantly at p < 0.05 (Tukey–Kramer HSD test).
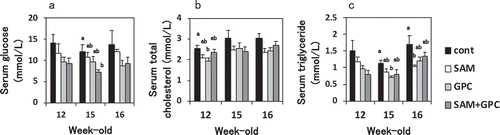
The serum level of leptin was significantly lower in the SAM+GPC group than in the control group (−55%, p < 0.05) (). The SAM or GPC group also showed a lower leptin level than the control group, but this did not reach significance. Reductions of the serum levels of insulin were observed in all experimental groups (p = 0.07) (). The level of ALT, a marker of liver injury, appeared to be lower in all experimental groups than in the control group (p = 0.09) (). None of the four groups exhibited significant differences in TBARS in the liver and serum AST, HDL cholesterol, adiponectin, and IGF-1 levels ().
The levels of leptin and insulin in circulation are increased and display leptin and insulin resistance in obese rodents such as KK-Ay mice [Citation5,Citation6]. In the SAM+GPC group, the leptin level was significantly decreased, suggesting the improved leptin resistance. The SAM or GPC group also tended to show the decreased leptin level, it is possible that each substance possesses such activity and combination of SAM+GPC enhances the activity. Decrease in the food intake, and partial decreases in the serum glucose, triglyceride and total cholesterol seems to be mediated by the improvement of leptin resistance in the experimental groups.
In the SAM+GPC, SAM, GPC groups, there was no significant difference, but the insulin level tended to be decreased, suggesting the improved insulin resistance. Improvement of insulin sensitivity and resistance by SAM and choline was reported [Citation3,Citation7,Citation8], and similar effect of SAM and GPC as a choline source was observed in this study. Partial decreases in the serum glucose seems to be mediated by the improvement of insulin resistance in the experimental groups. Adiponectin is also involved in the insulin resistance, but the effect of SAM and GPC on adiponectin was not found in this study.
Gene expression in the liver was analyzed by real-time PCR (). Compared with the SAM group, the GPC group showed a 1.4-fold increase in expression of Mat1a (p < 0.05), the gene encoding methionine adenosyltransferase related to SAM synthesis and hepatocyte function. GPC might contribute to hepatocyte protection. On the other hand, there was no effect on the expression of the Gnmt gene encoding glycine-N-methyltransferase related to SAM degradation. The ingestion of SAM and/or GPC did not affect the expression of Ppara and Pparg related to lipid metabolism, Srebf1, Irs2, and Foxo3a related to glucose metabolism, Sod2 and Cat related to oxidative stress, and Tgfb1 related to liver fibrosis. Compared with the control group, the expression of Irs2, which mediates insulin resistance, seemed to be increased in the SAM, GPC, and SAM+GPC groups (p = 0.47), but did not reach significance due to considerable individual variation.
Figure 2. Effects of SAM and GPC on gene expression related to glucose, lipid, and SAM metabolism, oxidative stress, inflammation and liver fibrosis in the livers of KK-Ay mice.
Values are mean ± SE (n = 8–9). Means in a bar without a common superscript letter differ significantly at p < 0.05 (Tukey–Kramer HSD test).
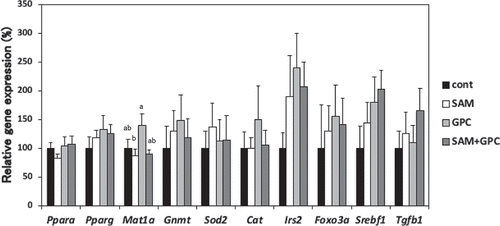
The oral administration of SAM (15 and 30 mg/kg per day) reduced oxidative stress in OLETF rats [Citation9]. GPC administration (16.56 mg/kg) reduced oxidative stress in a rat liver ischemia-reperfusion injury model [Citation10]. Although the anti-oxidative activity of SAM or GPC was reported [Citation9,Citation10], such activity was not found in the gene expression related to oxidative stress and TBARS level in the liver in this study. The doses of SAM and GPC in these previous studies [Citation9,Citation10] were almost the same as in the current work. It is possible that liver oxidative stress was relatively low because KK-Ay mice were not fed an MCD or high-fat diet. It is thus necessary to confirm the doses and administration period of SAM and GPC that are optimal for the promotion of anti-oxidative activity in such experimental system.
Author contributions
H.I., K.M., and T.F. conceived the experiments and wrote the manuscript. H.I., K.M., M.O., and S.S. performed the experiments and analyzed the data. All authors read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wortham M, He L, Gyamfi M, et al. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig Dis Sci. 2008;53:2761–2774.
- Mu YP, Ogawa T, Kawada N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine-choline-deficient diet. Lab Invest. 2010;90:245–256.
- Jin CJ, Park HK, Cho YM, et al. S-Adenosyl-L-methionine increases skeletal muscle mitochondrial DNA density and whole body insulin sensitivity in OLETF rats. J Nutr. 2007;137:339–344.
- Biswas S, Giri S. Importance of choline as essential nutrient and its role in prevention of various toxicities. Prague Med Rep. 2015;116:5–15.
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770.
- Ohashi A, Matsushita Y, Kimura K, et al. Conjugated linoleic acid deteriorates insulin resistance in obese/diabetic mice in association with decreased production of adiponectin and leptin. J Nutr Sci Vitaminol. 2004;50:416–421.
- Moon MK, Kim M, Chung SS, et al. S-Adenosyl-L-methionine ameliorates TNFα-induced insulin resistance in 3T3-L1 adipocytes. Exp Mol Med. 2010;42:345–352.
- Zeisel SH. Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin Chem Lab Med. 2013;51:467–475.
- Lim S, Moon MK, Shin H, et al. Effect of S-adenosylmethionine on neointimal formation after balloon injury in obese diabetic rats. Cardiovasc Res. 2011;90:383–393.
- Strifler G, Tuboly E, Görbe A, et al. Targeting mitochondrial dysfunction with L-alpha glycerylphosphorylcholine. PLoS One. 2016;11:e0166682.
