ABSTRACT
The PhoQ/PhoP two-component signal transduction system is conserved in various Gram-negative bacteria and is often involved in the expression of virulence in pathogens. The small inner membrane protein SafA activates PhoQ in Escherichia coli independently from other known signals that control PhoQ activity. We have previously shown that SafA directly interacts with the sensor domain of the periplasmic region of PhoQ (PhoQ-SD) for activation, and that a D179R mutation in PhoQ-SD attenuates PhoQ activation by SafA. In this study, structural comparison of wild-type PhoQ-SD and D179R revealed a difference in the cavity (SD (sensory domain) pocket) found in the central core of this domain. This was the only structural difference between the two proteins. Site-directed mutagenesis of the residues surrounding the SD pocket has supported the SD pocket as a site involved in PhoQ activity. Furthermore, the SD pocket has also been shown to be involved in SafA-mediated PhoQ control.
Graphical Abstract
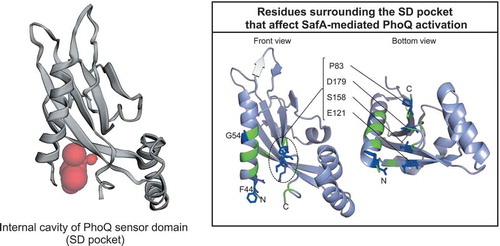
Residues surrounding the SD pocket that affect SafA-mediated PhoQ activation
Bacteria adapt and survive against various environmental stresses, such as changes in temperature, pH, and osmolarity. These stresses are sensed and responded by systems such as the two-component signal transduction system (TCS). The simplest TCSs are composed of a signal-responding histidine kinase sensor (HK) and its cognate response regulator (RR). Upon receiving a signal, the ATP-dependent auto-phosphorylation of the HK is regulated (either activated or inactivated), from the phosphorylated histidine, to an aspartate of its cognate RR. RRs usually dimerize upon phosphorylation, and most function as transcriptional regulators, controlling the expression of genes necessary for adaptation to environmental changes [Citation1]. TCSs often control the expression of virulence in pathogens. Because they are only found in bacteria, fungi, and plants, but not in mammals, TCSs are extensively studied as targets for drug development [Citation2].
The PhoQ/PhoP TCS, conserved in various Gram-negative bacteria, is involved in the expression of virulence in many pathogens. When PhoQ HK is activated by signals such as restricted divalent cations [Citation3,Citation4], cationic antimicrobial peptides [Citation5], and acidic pH [Citation6,Citation7], it autophosphorylates its conserved histidine residue, followed by phosphotransfer to its cognate RR, PhoP, and regulates a wide range of genes responsible for magnesium homeostasis, cell wall composition, stress resistance, and virulence [Citation8,Citation9]. PhoQ, located in the inner membrane, senses the signals mostly at its sensory domain situated in the periplasm. However, in case of sensing acidic pH, the signal is sensed not only at its sensory domain in the periplasm but also in the cytoplasmic domain [Citation10], as is reported for osmo-sensing EnvZ [Citation11]. Another study has shown that the change in cytoplasmic pH regulates the phosphatase activity of HKs [Citation12].
We have previously identified an additional factor that activates PhoQ in Escherichia coli. This factor is a small membrane protein, SafA, whose expression is induced by another TCS, EvgS/EvgA [Citation13]. EvgS/EvgA is activated by acidic pH, regulates the expression of acid resistance genes, and renders cells in their exponential growing phase resistant to the acidic environment [Citation14,Citation15]. safA is found in E. coli and Shigella but not in other bacteria utilizing the PhoQ/PhoP system. SafA is a single-spanning inner membrane protein of 65 amino acid residues and activates the PhoQ/PhoP system by direct interaction with the periplasmic region of PhoQ [Citation13,Citation16]. Although high concentration of divalent cations represses the PhoQ/PhoP system by promoting the phosphatase activity of PhoQ against phosphorylated PhoP [Citation17], SafA does not affect the phosphatase activity but instead promotes the autophosphorylation activity of PhoQ and accumulates phosphorylated PhoP [Citation18]. Activation of the PhoQ/PhoP system increases intracellular RpoS level in exponentially growing cells and enhances EvgS/EvgA-triggered acid resistance. Thus, SafA serves as a connector of EvgS/EvgA and PhoQ/PhoP for enhanced acid resistance [Citation19]. Other connectors between TCSs, such as MzrA, connecting CpxA/CpxR and EnvZ/OmpR in E. coli, and PmrD, connecting PhoQ/PhoP and PmrB/PmrA in Salmonella, have been reported [Citation20–Citation22]. These connectors of TCSs are assumed to contribute to the formation of signal transduction networks.
SafA and MzrA control the activity of HKs by direct interaction and belong to the group of auxiliary proteins of HKs. Two other small auxiliary proteins that modulate PhoQ activity have also been reported. One is MgrB, a small membrane protein of 47 residues, whose expression is activated by phosphorylated PhoP and represses PhoQ activity by direct interaction with the periplasmic region of PhoQ. Thus, MgrB is a part of the negative feedback loop of the PhoQ/PhoP system. MgrB is broadly conserved among several genera of Enterobacteriaceae [Citation7]. The other protein, UgtL, is a membrane protein of 132 residues with two predicted transmembrane domains. UgtL is conserved only in Salmonella, similar to SafA, which is only found in E. coli and Shigella. Expression of ugtL is promoted by the PhoQ/PhoP system, and UgtL enhances acid-induced activation of PhoQ by direct interaction. The first predicted transmembrane domain and the periplasmic domain (24 residues) of UgtL are necessary for activation [Citation23].
Likewise, SafA directly interacts with the periplasmic sensory domain of PhoQ (PhoQ-SD) for PhoQ activation. The C-terminal region of SafA binds to PhoQ-SD, as observed by both in vivo and in vitro studies [Citation16]. The Asp179 residue of PhoQ, which stabilizes the PhoQ-SD dimer, is also related to the PhoQ–SafA interaction. PhoQ D179R mutant shows low binding to SafA, and the activation of this mutant by SafA is attenuated [Citation16]. However, the binding sites of SafA, MgrB, and UgtL have not been clarified.
In the present study, we compared the crystal structures of PhoQ-SD D179R mutant with wild-type PhoQ-SD and found a significant difference in the internal cavity positioned proximal to the dimer interface. Analysis of this cavity revealed its importance in controlling PhoQ activity.
Materials and methods
Bacterial strains and plasmids
The E. coli strains used in this study are listed in . Strain MC4100 ΔphoQ PmgtA-lacZ harbors a lacZ gene under control of the PhoP-regulated mgtA promoter and was used for reporter assays. Plasmids used in this study are listed in . pBAD-phoQ-ha-safA was constructed by inserting a HA-tag into pBAD-phoQ-safA by using primers ha-safA F and ha-safA R (Table S1). The ha-safA fragment from pBAD-phoQ-ha-safA was amplified by primers HindIII-HA-SafA and safA-BamHI (Table S1), digested with BamHI (Takara Bio, Shiga, Japan) and HindIII (Takara Bio), and cloned into pHSG576 to construct pHSG576 ha-safA.
Table 1. Bacterial strains used in this study.
Table 2. Plasmids used in this study.
Protein purification
To purify proteins for crystallization, E. coli KRX strains (Promega KK, Tokyo, Japan) transformed with pETphoQ(43–190)-TEV and pETphoQ(43–190) D179R-TEV were proliferated in Terrific Broth (1.2% tryptone, 2.4% yeast extract, 0.72% glycerol, 17 mM KH2PO4, and 72 mM K2HPO4) supplemented with kanamycin at 37°C with aeration to a cell density of OD600 = 0.4–0.5, followed by addition of rhamnose at a final concentration of 0.1%. After 18 h of induction at 25°C with aeration, cells were harvested and washed with lysis buffer (50 mM Tris-HCl, pH 8.0, and 100 mM NaCl). The washed cells were resuspended in lysis buffer, lysed by sonication, and centrifuged at 2300 × g for 10 min at 4°C. The supernatant was affinity purified using Ni(II)-NTA agarose beads (Qiagen, Venlo, Netherlands). TEV protease (laboratory stock) was added to the eluted protein samples for cleaving the His tag, dialyzed against lysis buffer, and passed through Ni(II)-NTA agarose beads to eliminate the cleaved His tag. Protein samples for purification were further purified by gel filtration using a Superdex75 column (GE Healthcare, Buckinghamshire, England).
Crystallization
Wild-type PhoQ-SD was crystalized at 4°C via vapor diffusion in sitting drops formed by mixing the protein (10 mg·mL−1) with the reservoir solution containing 0.1 M N-cyclohexyl-2-aminoethanesulfonic acid (CHES; pH 9.2), 32% polyethylene glycol (PEG) 3350, and 0.2 M ammonium tartrate. PhoQ-SD D179R was crystalized at 4°C via vapor diffusion in sitting drops formed by mixing the protein (10 mg·mL−1) with the reservoir solution containing 0.1 M CHES (pH 9.2), 26% PEG 3350, and 0.2 M ammonium tartrate.
Structure determination
X-ray diffraction data were collected using a wavelength of 1.0 Å at beamline BL41XU at SPring-8, Japan, and processed using HKL2000 [Citation25] and the CCP4 program suite [Citation26]. The initial phase of the wild-type PhoQ-SD crystal was obtained using the molecular replacement (MR) method. The structure of PhoQ-SD (PDB code: 3BQ8) was used as the search model for MR using the program Phaser [Citation27]. The structure was refined with Phenix [Citation28], and model building was carried out with Coot [Citation29]. The initial phase of D179R mutant data was obtained by MR using the model of wild-type. X-ray data collection and refinement statistics are shown in .
Table 3. X-ray data collection and refinement statistics of PhoQ-SD.
Site-directed mutagenesis
Site-directed mutagenesis in PhoQ-SD was performed by PCR using Prime STAR MAX DNA polymerase (Takara Bio), pBAD-phoQ as the template, and primers listed in Table S1. Likewise, the D179R mutation was induced in pETphoQ(43–190)-TEV utilizing the primers used for D179R mutation. After PCR, the plasmid used as the template was digested with DpnI (New England Biolabs, Ipswich, MA, USA) and transformed in JM109. Plasmids were purified from the transformants, and mutation was confirmed by sequencing.
β-galactosidase assay
LacZ activity was determined according to the protocol described previously [Citation30]. Cells were proliferated at 37°C in a 96-well plate shaker [DWMaxBP-304P shaker (TAITEC, Saitama, Japan)] in LB medium (10 g·L−1 Bacto Tryptone, 5 g·L−1 yeast extract, and 5 g·L−1 NaCl; pH 7.2) supplemented with 50 µg·mL−1 ampicillin, 25 µg·mL−1 chloramphenicol, 1 mM IPTG, 1 mM cAMP, 0.002% L-arabinose, and with or without 10 mM MgCl2. Culture aliquots (80 µL) were added to individual wells of a clear 96-well plate containing 20 µL of Reporter Lysis 5 × Buffer (Promega). Cells were lysed by freezing at −80°C and subsequent thawing at 37°C for approximately 20 min. One hundred microliters of 1.32 mg·mL−1 2-nitrophenyl β-D-galactopyranoside (ONPG; Sigma-Aldrich, St. Louis, MO, USA) in Z-buffer {0.26% β-mercaptoethanol in phosphate buffer (pH 7)} was then added to each well and mixed thoroughly. Absorbance at 420 and 550 nm for each well was read 20 times within 40 min at 25°C using Vient® nano (BioTek, Winooski, VT, USA). β-galactosidase activity was calculated by the following equation:
Arbitrary units [AU] = (A420 − 1.75 × A550)/[(incubation time (min) at 25°C) × (A600 of the bacterial culture at the time of collection)].
Western blot analysis
The cell culture used for the reporter assay was adjusted to OD600 = 0.3, and 200 µL of this culture was mixed with equal volume of 10% trichloroacetic acid (TCA), vortexed, and maintained on ice for 20 min. Protein samples were collected by centrifugation (20,400 × g, 15 min, 4°C), and the pellet was washed with 500 µL of acetone and centrifuged (20,400 × g, 15 min, 4°C). After allowing the pellet to stand at room temperature for 10 min, the pellet was suspended with 50 µL of SDS-PAGE sample buffer, vortexed for 30 min at room temperature for solubilization, and subsequently incubated at 37°C for 5 min. Protein samples were separated by SDS-PAGE (12.5% Laemmli SDS-PAGE gel for PhoQ protein and 15% Bis-Tris gel for SafA protein), transferred to a polyvinylidene difluoride membrane (Immobilon P membrane for PhoQ and Immobilon PSQ membrane for SafA; Merck Millipore, Burlington, MA, USA), and probed with antiserum against PhoQ [Citation16] and anti-HA-tag antibodies [HA probe (F-7) AC; Santa Cruz Biotechnology, Dallas, TX, USA]. We detected signals with the ECLTM Western Blotting Detection Reagent or ECLTM Prime Western Blotting Detection Reagent (GE Healthcare) using the LAS4000 mini lumino-image analyzer (GE Healthcare). The nonspecific bands detected by anti-PhoQ (** in and ) were used as loading controls.
Accession numbers
The coordinate and structure factors for E. coli PhoQ-SD have been deposited in the RCSB Protein Data Bank (PDB), accession code 6A8U (wild-type) and 6A8V (D179R mutant).
Results and discussion
Difference between the structures of PhoQ-SD and its D179R mutant
The connector SafA is an inner membrane protein that is induced by the activation of the acid-responding EvgS/EvgA TCS. SafA activates PhoQ HK of the PhoQ/PhoP TCS by direct interaction; the C-terminal region of SafA binds to the periplasmic region of PhoQ (PhoQ-SD). To determine the binding site of SafA, we first aimed to co-crystalize PhoQ-SD with SafA(41–65) peptide, which corresponds to the periplasmic C-terminal region of SafA. This peptide can bind to PhoQ-SD, in vitro, which has been confirmed by means of surface plasmon resonance and NMR spectroscopy, and can also activate PhoQ HK, in vivo, when added to the cell culture of a PhoQ/PhoP reporter strain [Citation16]. Despite multiple trials, we were unable to obtain co-crystals, possibly because of the low solubility of the SafA(41–65) peptide.
Next, we tried another approach. In our previous studies, the D179R mutation in PhoQ-SD has been the only mutation in PhoQ that reduced activation by SafA. This mutation also reduced the binding affinity of SafA to PhoQ-SD, as confirmed in vitro by surface plasmon resonance between SafA(41–65) peptide and PhoQ-SD D179R [Citation16]. Thus, PhoQ-SD D179R has changed to a conformation that is not suitable for SafA binding. This suggested that by determining the conformation of PhoQ-SD D179R and comparing it with the wild-type structure, we would be able to determine the conformational change in PhoQ-SD D179R that inhibited the binding of SafA, and hence understand the region in the wild-type PhoQ that is necessary for SafA binding. Therefore, we crystallized wild-type PhoQ-SD corresponding to PhoQ(43–190) and its D179R mutant and determined their structures ().
Figure 1. Crystal structures of wild-type and D179R PhoQ-SD.(a) Structures of wild-type (PDB code: 6A8U) and D179R (PDB code: 6A8V) PhoQ-SD are shown as ribbon diagrams. Asp179 (wild, red; D179R, magenta) and Lys186 (wild, blue; D179R, cyan) residues are shown as stick representation. (b) Surface structures of wild-type and D179R PhoQ-SD are shown. The SD pocket in the wild-type structure is circled with a broken line. (c) SD pocket estimated by CASTp program [Citation35]. The location and volume of the pockets are shown with colored balls (wild, red, 42 Å3; D179R blue, 45 Å3). (d) Superposition of wild-type and D179R PhoQ-SD structures shown as ribbon diagrams.
![Figure 1. Crystal structures of wild-type and D179R PhoQ-SD.(a) Structures of wild-type (PDB code: 6A8U) and D179R (PDB code: 6A8V) PhoQ-SD are shown as ribbon diagrams. Asp179 (wild, red; D179R, magenta) and Lys186 (wild, blue; D179R, cyan) residues are shown as stick representation. (b) Surface structures of wild-type and D179R PhoQ-SD are shown. The SD pocket in the wild-type structure is circled with a broken line. (c) SD pocket estimated by CASTp program [Citation35]. The location and volume of the pockets are shown with colored balls (wild, red, 42 Å3; D179R blue, 45 Å3). (d) Superposition of wild-type and D179R PhoQ-SD structures shown as ribbon diagrams.](/cms/asset/427994b4-88d8-4201-9406-dec4c00b68e2/tbbb_a_1562879_f0001_c.jpg)
The main-chain structure of wild-type PhoQ-SD in our crystallographic analysis showed very similar conformation to that of the reported crystal structure (PDB code: 3BQ8) [Citation31] with root mean-squared deviation (r.m.s.d.) of 0.7 Å. The molecular arrangement in the reported PhoQ-SD structure has been proposed as a functional dimer, whereas the molecular packing of the wild-type and the D179R mutant of PhoQ-SD are distinct from that observed in the reported PhoQ-SD structure. In our case, the protein seemed to be packed as monomers. The PISA [Citation32] program for the analysis of protein interface and assembly also indicated that two chains of PhoQ-SD in the asymmetric unit are packed as monomers. In our experiments, the wild-type and the D179R mutant of PhoQ-SD were crystallized in very similar conditions. The only difference between the two was in the concentration of PEG3350 (32% for the wild-type and 26% for D179R mutant), which may not give much effect to their structures. The differences in cell dimensions of crystal (by 3 ~ 9 Å) between the wild-type and the D179R mutant were derived from the small difference of contacting sites with neighboring molecules. The differences in the molecular orientation in the lattice were less than 8 degrees. PhoQ-SDs are expected to be functionally dimerized in situ on the membrane; however, it has been shown that E. coli PhoQ-SD is monomeric in solution with a lower limit of 600 µM as the measured Kd of dimerization by analytical ultracentrifugation [Citation33].
In our wild-type structure, Asp179 and Lys186 formed a salt bridge to stabilize the conformation of the C-terminal region of this domain ()). This salt bridge has also been observed in the crystal structure of S. enterica PhoQ-SD [Citation34]. Because of the salt bridge, a cavity is created between Asp179 and helix H1 (,). We named this cavity as the SD pocket. When the surface accessible surface of the SD pocket was analysed by CASTp program [Citation35], the volume of this cavity was estimated to be 42 Å3 ()). In the case of the D179R mutant, the lack of a salt bridge induced a large difference in the conformation of these residues (D179R and K186) and the molecular shape (,). The side-chain of Arg179 shifted closer to helix H1, thereby occupying the SD pocket ()). Although a shallow depression was still observed on the surface of the D179R mutant by CASTp ()), the deeper cavity between D179 and helix H1 that was observed in the wild-type had disappeared. The region of the disordered helix H2 () ~ helix H3 (residues 126–152) also showed some difference with the average deviation of 1.1 Å when the two structures were superposed with overall r.m.s.d. of 0.7 Å ()). However, these two helices are apart from the cavity, and a similar level of deviation was detected in the same region between the two chains of wild-type PhoQ-SD, suggesting that the deviation of these two helices was not caused by the mutation. Importantly, the SD pocket is located near the N- and C-termini of PhoQ-SD, providing a vacant space facing the cell membrane. The location of this pocket would be reasonable for SafA binding because it can enable a small membrane protein, with approximately only 25 residues in the periplasm region, to approach this pocket. Based on these reasons, we assumed that SafA interacts with the residues in the SD pocket.
We sought to determine whether the SD pocket was limited to our monomer structure (). A close examination of the dimer PhoQ-SD structure (PDB code: 3BQ8) revealed a cavity between helix H1 and Asp179 (). In the dimer structure, Asp179 of mol. B forms a salt bridge with Arg50′ in helix H1 of mol. A [Citation31], and also with Lys186 of mol. B ()). These salt bridges allow the formation of a pocket between helix H1 and Asp179 in mol. B, as observed in our monomer structure ( and ). This comparison indicates that both monomeric and dimeric forms of PhoQ-SD have SD pockets.
Figure 2. SD pocket also exists in the PhoQ-SD dimer structure.(a) PhoQ-SD dimer structure (PDB code: 3BQ8) is shown as a ribbon diagram. Each subunit is labeled molecule A (mol. A) and molecule B (mol. B). In mol. A, R50ʹ is shown in orange. In mol. B, Asp179 is shown in red, Lys186 in blue. (b) SD pocket estimated by CASTp program [Citation35]. The location and volume of the pocket are shown with red balls (276 Å3). H1: helix H1.
![Figure 2. SD pocket also exists in the PhoQ-SD dimer structure.(a) PhoQ-SD dimer structure (PDB code: 3BQ8) is shown as a ribbon diagram. Each subunit is labeled molecule A (mol. A) and molecule B (mol. B). In mol. A, R50ʹ is shown in orange. In mol. B, Asp179 is shown in red, Lys186 in blue. (b) SD pocket estimated by CASTp program [Citation35]. The location and volume of the pocket are shown with red balls (276 Å3). H1: helix H1.](/cms/asset/5b638a62-17d1-4cf7-802f-74c741634681/tbbb_a_1562879_f0002_c.jpg)
Effect of PhoQ D179 mutations against PhoQ activity and SafA-mediated activation
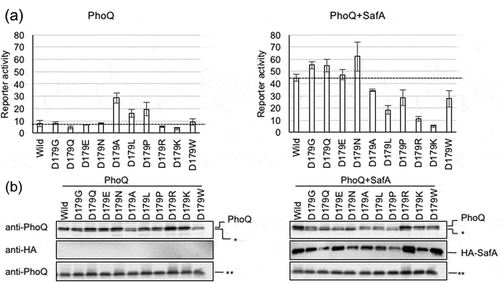
We examined how Asp179 contributed to SafA-mediated activation of PhoQ. PhoQ activity was measured by expressing PhoQ from pBAD-phoQ and SafA from pHSG576 ha-safA plasmids. These plasmids were co-transformed in strain MC4100 ΔphoQ PmgtA-lacZ, which is a reporter strain for the PhoQ/PhoP system. The codon coding for Asp179 was mutated to express the different variants in pBAD-phoQ, thus substituting the Asp by different amino acids. Their PhoQ activities and SafA-mediated PhoQ activation effects were assayed in the presence of 10 mM Mg2+. We added high concentration of Mg2+ to repress the basal PhoQ activity and to, hence, afford better comparison of SafA-mediated PhoQ activation. Western blot analyses of the mutants confirmed PhoQ and SafA accumulation. As shown in , SafA-mediated activation of PhoQ was not observed in D179R or D179K, indicating that the introduction of a positive charge rendered PhoQ insensitive to SafA. Repulsion between the side-chains of Lys179 and Lys186 was anticipated for D179K, as seen between Arg179 and Lys186 ()). Substituting Asp179 to a negatively charged Glu did not alter SafA-mediated activation. Furthermore, substituting Asp179 to hydrophilic residues such as Gly, Gln, and Asn also showed SafA-mediated activation similar to that of the wild-type level, indicating that the negative charge of D179 was not essential for activation by SafA. However, substituting Asp179 to hydrophobic residues such as Ala, Leu, and Pro did not lead to SafA-mediated activation (compare left and right panels of )). SafA-mediated activation was also reduced in the D179W mutant. Our results indicate that the introduction of positively charged or hydrophobic residues at the D179 position represses SafA-mediated activation.
Although the loading controls showed similar amount of protein among the samples, SafA and PhoQ accumulation varied among the mutants ()). Further studies are necessary to elucidate SafA stability. However, we have previously shown that only very low levels of SafA expression were necessary for PhoQ activation [Citation13]. Because SafA saturated at a low level against PhoQ, we consider that the variation in SafA accumulation among the mutants does not affect the SafA-mediated PhoQ activation.
In the absence of SafA, D179A, L, and P mutants showed enhanced PhoQ activity in the presence of 10 mM Mg2+ (). A previous report has shown that the activities of PhoQ D179A and D179L mutants were not repressed by high concentration of divalent cations. These mutants were termed as “locked-on mutants” [Citation36]. From our results, D179P may also be included in the group of “locked-on mutants”. Furthermore, because these PhoQ mutants are not activated by SafA, they may also be in a locked-on state against SafA.
Figure 3. Effect of mutations in PhoQ D179. (a) The effect of D179 substitutions was assayed by measuring reported β-galactosidase activities under the mgtA promoter. Analyzed strains were: MC4100 ΔphoQ PmgtA-lacZ transformed with pBAD-phoQ or pBAD-phoQ bearing D179 mutations; and with pHSG576 (vector) or pHSG576 ha-safA. Cells were proliferated in LB medium containing 50 µg/mL ampicillin, 25 µg/mL chloramphenicol, 1 mM IPTG, 1 mM cAMP, 0.002% L-arabinose, and 10 mM MgCl2. Columns represent the means of the results of at least two independent experiments with standard errors. (b) Accumulation of PhoQ, PhoQ with D179 mutations, and HA-SafA. Immunoblotting analyses using anti-PhoQ antiserum for PhoQ detection and anti-HA antibody for HA-SafA detection are shown. Samples are from the same culture as those subjected to β-galactosidase assay. Asterisks (* and **) indicate nonspecific bands detected by anti-PhoQ, and the panels indicated by ** serve as the loading control. The complete scanned gels for western blots are shown in Figure S1.
Amino acid residues involved in PhoQ activation surround the SD pocket
The location of the SD pocket is adjacent to the N- and C-termini of PhoQ-SD, which are the two ends connected to the two transmembrane helices TM1 and TM2 of PhoQ. When the periplasmic sensor domain of PhoQ (PhoQ-SD) receives a signal for activation and changes its conformation, this signal is transduced to the transmembrane four-helix bundle (two TM1 and two TM2 helices of the PhoQ dimer) by moving the periplasmic ends of the two TM1 helices closer to each other and pushing the two TM2 helices farther apart, accompanied by TM2 rotation [Citation34]. This rotation combined with a scissoring movement is proposed to mediate signal transmission through the TM domain to the cytoplasmic domain [Citation37–Citation39]. This suggested that the conformational change of PhoQ-SD should be integrated at the N- and C-termini of PhoQ-SD, where the SD pocket is located. The different PhoQ activities of the D179 mutants shown in support this notion: The presence of the SD pocket at the core of PhoQ can provide a space for conformational changes of PhoQ for controlling activity.
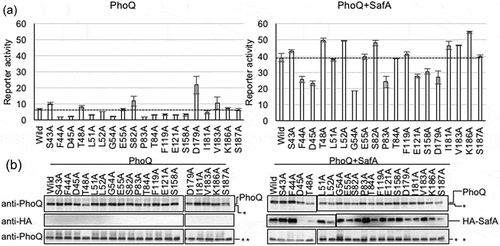
In order to investigate the effects of amino acid residues surrounding the SD pocket on PhoQ activity, we selected 19 residues that constitute (S43, F44, D45, T48, F119, E121, S158, D179, I181, V183, and K186) or were adjacent (L51, L52, G54, E55, S82, P83, T84, and S187) to the SD pocket. Mutations were induced in pBAD-phoQ plasmid and were then transformed into MC4100 ΔphoQ PmgtA-lacZ. These transformants were subjected to reporter assays to confirm their PhoQ activity in the presence of 10 mM Mg2+ (). As a result, S43A, T48A, E55A, S82A, I181A, V183A, K186A, and S187A showed similar activity to that of wild-type PhoQ under high concentration of Mg2+. However, F44A, D45A, L51A, L52A, G54A, P83A, T84A, F119A, E121A, and S158A showed depressed activity, and D179A enhanced activity compared to that of wild-type PhoQ ()). Western blot analyses of the alanine mutants confirmed PhoQ accumulation ()). From our results, it was observed that as many as 11 of the 19 residues constituting or adjacent to the SD pocket are involved in PhoQ activity, and the SD pocket is indeed an important site within PhoQ for activity control. Structural differences that may be induced by alanine mutation can cause a movement of the TM1 and/or TM2 and activate or repress PhoQ activity.
Figure 4. Effect of alanine mutations of the residues constituting the SD pocket. (a) Activity of the mgtA promoter of alanine mutants was measured by β-galactosidase assay. Strain MC4100 ΔphoQ PmgtA-lacZ transformed with pBAD-phoQ or pBAD-phoQ with alanine mutations and with pHSG576 (vector) or pHSG576 ha-safA were assayed. Cells were proliferated in LB medium containing 50 µg/mL ampicillin, 25 µg/mL chloramphenicol, 1 mM IPTG, 1 mM cAMP, 0.002% L-arabinose, and 10 mM MgCl2. Columns represent the means of the results of at least two independent experiments with standard errors. (b) Accumulation of PhoQ, PhoQ with alanine mutations, and HA-SafA. Immunoblotting analyses using anti-PhoQ antiserum for PhoQ detection and anti-HA antibody for HA-SafA detection are shown. Samples are from the same culture as those subjected to β-galactosidase assay. Asterisks (* and **) indicate nonspecific bands detected by anti-PhoQ, and the panels indicated by ** serve as the loading control. The complete scanned gels for western blots are shown in Figure S1.
Some amino acid residues surrounding the SD pocket are necessary for SafA-mediated activation
SafA was co-expressed with PhoQ mutants to examine how the 19 PhoQ mutations affected SafA-mediated activation. Among the mutations that affected PhoQ activity, L51A, T84A, and F119A mutants showed reduced PhoQ activity compared to that of the wild-type in the absence of SafA but were activated to a level similar to that of the wild-type in the presence of SafA (). This suggested that the binding of SafA to PhoQ compensated for the negative effect of the conformational change caused by these mutations. Also, these residues were considered not to be involved in SafA-mediated activation. Similarly, S43A, T48A, L52A, E55A, S82A, I181A, V183A, K186A, and S187A showed activity similar to that of the wild-type in the presence of SafA, indicating that these mutations were not related to SafA-mediated activation.
On the other hand, co-expression of SafA did not activate PhoQ to the level of wild-type in F44A, G54A, P83A, E121A, S158A, and D179A mutants (). D45A also showed depressed SafA-mediated activation, but we were unable to confirm SafA expression in this transformant ()). The aforementioned six residues are considered important for PhoQ activation by SafA. These residues may be involved in SafA binding and/or in the conformational change of PhoQ caused by SafA binding, supporting our view of the SD pocket serving as a site for PhoQ control.
The PhoQ-SD structure is similar to that of DcuS and CitA sensor domains and shares the PDC (PhoQ–DcuS–CitA) sensor fold [Citation31]. This fold consists of an N-terminal α-helix (H1) and a central five-stranded antiparallel β-sheet (S1–5) ()). The ligand binding sites of DcuS and CitA are positioned at the central core formed by the β-sheets [Citation34,Citation40]. However, in PhoQ, the cavity for ligand binding, which is present in DcuS and CitA, is occupied by H3. Furthermore, PhoQ is distinguished from DcuS and CitA with respect to harboring the H4–H5 α-helix pair and acidic cluster as an insertion. Thus, it has been interpreted that PhoQ uses its negatively charged surface (acidic cluster) for ligand binding instead of the central core [Citation34]. Divalent cations and cationic antimicrobial peptides bind to the highly negatively charged surface that is in close proximity to the inner membrane [Citation5,Citation34]. Acidic pH is reported to cause conformational changes in Η4 and 5, which is also included in the highly negatively charged surface [Citation41]. We have previously shown that SafA can activate an acid cluster neutralized mutation (EDDDDAE to QNNNNAQ) of the highly negatively charged surface of PhoQ, indicating that SafA activates PhoQ in a different manner from that by cationic antimicrobial peptides and acidic pH [Citation16].
Figure 5. Secondary structure of PhoQ-SD and alanine mutations involved in SafA-mediated PhoQ activation. (a) Secondary structure elements labeled in wild-type PhoQ-SD structure (PDB code: 6A8U). (b) Positions of alanine mutated residues in the secondary structure. H2 has a disordered structure (broken line). The red area indicate the location of the SD pockets. Colored arrows indicate the effect of the mutation against PhoQ activity (green, no significant change; blue, depressed; red, enhanced). (c) Mutations that affect SafA-mediated PhoQ activation are mapped onto the wild-type structure of PhoQ-SD. Color codes are as indicated in (b).
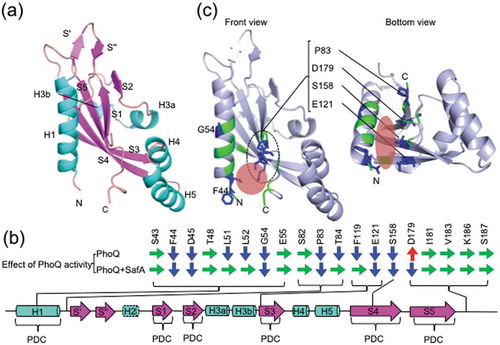
In our reporter assay, E121A and S158A mutants showed repressed PhoQ activation by SafA. These residues are positioned at S3 and S4 in the central core of PhoQ-SD ()) and their side-chains face the opposite side of the ligand-binding sites of DcuS and CitA. This suggests that SafA binds or affects the central core of PhoQ-SD at the side opposite from the ligand binding sites of DcuS and CitA and that the SD pocket enables SafA to approach the central core. Interestingly, Pro83, Glu121, Ser158, and Asp179 were positioned linearly when mapped onto our PhoQ-SD structure ()). These four residues are candidates as SafA binding sites. Attenuated PhoQ activation by SafA was also observed in F44A and G54A mutants. These two residues are located in helix H1, which is among the constituents of the PDC sensor fold and forms the dimer interface. The detailed mechanism of the interaction and the stoichiometry of PhoQ and SafA complex upon binding remains to be elucidated.
The aforementioned UgtL, a Salmonella-specific functional homologue of SafA, activates PhoQ via its small-sized (24 residues) periplasmic region [Citation23]. The structure of PhoQ-SD from S. enterica [Citation34] also shows an internal cavity (SD pocket) at the same position as PhoQ-SD from E. coli (Figure S2). Therefore, we suspect a conserved mechanism involving the SD pocket for the activation of PhoQ by UgtL.
Conclusion
The SD pocket found in our structural analysis of PhoQ-SD is an internal cavity of the PDC sensor fold. The structure-guided site-directed mutagenesis and the reporter assay confirmed that the residues around the SD pocket are involved in controlling PhoQ activity. Activation of PhoQ by SafA is also exerted via the SD pocket. The SD pocket region is distinct from the previously recognized PhoQ controlling site (the highly negatively charged surface). This result is consistent with the previous data in which the activation of PhoQ by SafA is independent from other signals that control the activity of PhoQ [Citation16]. Since the SD pocket controlling PhoQ activity may become a potent candidate for drug development against pathogens, further studies are necessary to find whether PhoQs of other pathogens also possess sites similar to the SD pocket and how these pockets may control PhoQ activity.
Author contributions
K.Y., E.I., and Y.E conceived and designed the experiments. K.Y., K.T., and H.S. performed the experiments. K.Y., E.I., H.S. and Y.E. wrote the paper. K.Y., E.I., H.S., Y.S., Y.A., A.K., R.U, and Y.E. discussed the results, commented on the manuscript, and approved the manuscript submission.
Supplementary_Figures__Yoshitani_et_al_181005.pdf
Download PDF (5.3 MB)Acknowledgments
We thank A. Doi for guidance in protein crystallization, and H. Mori and R. Miyazaki for helpful comments. The authors would like to thank Editage (www.editage.jp) for the English language review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this aricle can be accessed here.
Additional information
Funding
References
- Stock AM, Robinson VL, Goudrear PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215.
- Gotoh Y, Eguchi Y, Watanabe T, et al. Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr Opin Microbiol. 2010;13:232–239.
- Véscovi EG, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174.
- Véscovi EG, Ayala YM, Di Cera E, et al. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+. J Biol Chem. 1997;272:1440–1443.
- Bader MW, Sanowar S, Daley ME, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472.
- Prost LR, Daley ME, Le Sage V, et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174.
- Lippa AM, Goulian M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009;5:e1000788.
- Minagawa S, Ogasawara H, Kato A, et al. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J Bacteriol. 2003;185:3696–3702.
- Zwir I, Latifi T, Perez JC, et al. The promoter architectural landscape of the Salmonella PhoP regulon. Mol Microbiol. 2012;84:463–485.
- Choi J, Groisman EA. Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol Microbiol. 2016;101:1024–1038.
- Chakraborty S, Mizusaki H, Kenney LJ. A FRET-based DNA biosensor tracks OmpR-dependent acidification of Salmonella during macrophage infection. PLoS Biol. 2015;13:e10002116.
- Liu Y, Rose J, Huang S, et al. A pH-gated conformational switch regulates the phosphatase activity of bifunctional HisKA-family histidine kinases. Nat Commun. 2017;8:2104–2113.
- Eguchi Y, Itou J, Yamane M, et al. B1500, a small membrane protein, connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc Natl Acad Sci USA. 2007;104:18712–18717.
- Masuda N, Church GM. Regulatory network of acid resistance genes in Escherichia coli. Mol Microbiol. 2003;48:699–712.
- Eguchi Y, Utsumi R. Alkali metals in addition to acidic pH activate the EvgS histidine kinase sensor in Escherichia coli. J Bacteriol. 2014;196:3140–3149.
- Eguchi Y, Ishii E, Yamane M, et al. The connector SafA interacts with the multi-sensing domain of PhoQ in Escherichia coli. Mol Microbiol. 2012;85:299–313.
- Montagne M, Martel A, Le Moual H. Characterization of the catalytic activities of the PhoQ histidine protein kinase of Salmonella enterica serovar typhimurium. J Bacteriol. 2001;183:1787–1791.
- Ishii E, Eguchi Y, Utsumi R. Mechanism of activation of PhoQ/PhoP two-component signal transduction by SafA, and auxiliary protein of PhoQ histidine kinase in Escherichia coli. Biosci Biotechnol Biochem. 2013;77:814–819.
- Eguchi Y, Ishii E, Hata K, et al. Regulation of acid resistance by connectors of two-component signal transduction systems in Escherichia coli. J Bacteriol. 2011;193:1222–1228.
- Gerken H, Misra R. MzrA-EnvZ interactions in the periplasm influence the EnvZ/OmpR two-component regulon. J Bacteriol. 2010;192:6271–6278.
- Kox LF, Wosten MM, Groisman EA. A small protein that mediates the activation of a two-component system by another two-component system. Embo J. 2000;19:1861–1872.
- Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004;18:2302–2313.
- Choi J, Groisman EA. Activation of master virulence regulator PhoP in acidic pH requires the Salmonella-specific protein UgtL. Sci Signal. 2017;10:eaan6284.
- Baba T, Ara T, Hasegawa M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the keio collection. Mol Syst Biol. 2006;2:0008.
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326.
- Winn MD, Ballard CC, Cowtan KD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242.
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674.
- Adams PD, Afonine PV, Bunkóczi G, et al. PHENIX: a comprehensive python-based system for macromlecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221.
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132.
- Mori H, Sakashita S, Ito J, et al. Identification and characterization of a translation arrest motif in VemP by systematic mutational analysis. J Biol Chem. 2018;293:2915–2926.
- Cheung J, Bingman CA, Reyngold M, et al. Crystal structure of a functional dimer of the PhoQ sensor domain. J Biol Chem. 2008;283:13762–13770.
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797.
- Waldburger CD, Sauer RT. Signal detection by the PhoQ sensor-transmitter – characterization of the sensor domain and a response-impaired mutant that identifies ligand-binding determinatnts. J Biol Chem. 1996;271:26630–26636.
- Cho US, Bader MW, Amaya MF, et al. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206.
- Tian W, Chen C, Lei X, et al. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 2018;46:W363–W367.
- Minagawa S, Okura R, Tsuchitani H, et al. Isolation and molecular characterization of the locked-on mutant of Mg2+ sensor PhoQ in Escherichia coli. Biosci Biotechnol Biochem. 2005;69:1281–1287.
- Lemmin T, Soto CS, Clinthorne G, et al. Assembly of the transmembrane domain of E. coli PhoQ histidine kinase: implications for signal transduction from molecular simulations. PLoS Comput Biol. 2013;9:E1002878.
- Goldberg SD, Clinthorne GC, Goulian M, et al. Transmembrane polar interactions are required for signaling in the Escherichia coli sensor kinase PhoQ. Proc Natl Acad Sci USA. 2010;107:8141–8146.
- Molner KS, Bonomi M, Pellarin R, et al. Cys-scanning disulfide crosslinking and bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ. Structure. 2014;22:1239–1251. 31.
- Gerharz T, Reinelt S, Kaspar S, et al. Identification of basic amino acid residues important for citrate binding by the periplasmic receptor domain of the sensor kinase CitA. Biochemistry. 2003;42:5917–5924.
- Hicks KG, Delbecq SP, Sancho-Vaello E, et al. Acidic pH and divalent cation sensing by PhoQ are dispensable for systemic salmonellae virulence. eLife. 2015;4:e06792.
