ABSTRACT
Endolichenic fungi, nonobligate microfungi that live in lichen, are promising as new bioresources of pharmacological compounds. We found that norlichexanthone isolated from the endolichenic fungus in Pertusaria laeviganda exhibited high antioxidant activity. Norlichexanthone produced by endolichenic fungus had the antioxidant activity with same level of ascorbic acid. This is the first report of high antioxidant activity of norlichexanthone.
Abbreviations: AAPH: 2,2ʹ-azobis (2-methylpropionamidine) dihydrochloride; DPPH: 2,2-diphenyl-1-picrylhydrazyl; FL: fluorescein sodium salt; HPLC-PDA: high-performance liquid chromatography with photodiode array; LC-ESI-MS: liquid chromatography with electrospray ionization mass spectrometry; ORAC: oxygen radical absorbance capacity; PB: phosphate buffer; ROS: reactive oxygen species; TLC: thin-layer chromatography
Antioxidant compounds inhibit the effects of reactive oxygen species (ROS), protecting against damage to biomolecules (e.g. DNA, membrane lipids, and enzymes) associated with the pathogenesis of chronic diseases such as cancer, arteriosclerosis, and age-related neurodegenerative diseases. ROS overproduction leads to a state of cellular oxidative stress, during which endogenous antioxidant systems cannot overcome the damaging effect of ROS such as superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (HO•), and singlet oxygen (1O2) [Citation1]. Phenolic compounds (e.g. flavonoids, tocopherols, and phenolic acids), nitrogen compounds, carotenoids, and ascorbic acid contained in fruits, vegetables, and medicinal plants are widely known natural antioxidant compounds [Citation2].
Lichens are symbiotic organisms consisting of a mycobiont and photobiont, and they can grow in extreme environments such as polar regions, deserts, seashores, and high altitudes. In addition to the mycobiont, many nonobligate microfungi live in natural lichen thalli in close relationships with algae, referred to as endolichenic fungi. These fungi have been recognized as new bioresources of pharmacological compounds. In fact, some secondary metabolites such as xanthones, terpenoids, and chromanone were discovered from endolichenic fungi, and these compounds have some noteworthy pharmacological activities and anticancer, antimicrobial, and antioxidant activities [Citation3]. However, investigations targeting these fungi have been limited.
To discover new antioxidant compounds, we assayed the antioxidant activity of 60 extracts obtained from cultured mycobionts or endolichenic fungi using the oxygen radical absorbance capacity (ORAC) method. In particular, a methanol extract obtained from the endolichenic fungus (PtsLa) derived from Pertusaria laeviganda exhibited relatively high ORAC values. Here, we demonstrated that PtsLa produced norlichexanthone, and its antioxidant activity was high as with known active compounds.
Initial endolichenic fungus was induced from thallus of Pertusaria laeviganda (collected from Mt. Sefuri, Saga, Japan) using the Yamamoto method [Citation4] and maintained by subculture using solid medium containing 2% malt extract, 0.2% yeast extract, and 1.5% agar in the dark at 15°C. The voucher specimen of this endolichenic fungus was classified in Dothideomycetes by identification based on base sequencing of the ITS region and deposited at Akita Prefectural University, Akita, Japan (registration no. 0215M). To increase the amount of cultured PtsLa, 2 g of this fungus was transferred to 200 mL of liquid medium containing 2% malt extract and 0.2% yeast extract in a 500-mL Erlenmeyer flask and incubated for 3 weeks.
To extract active compounds, dried fungus (10.7 g) was immersed in methanol at room temperature overnight. The methanol extract (2.5 g) was separated using Wakogel® C-200 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) eluted with 200 mL solution mixed chloroform and methanol in a stepwise gradient (chloroform-methanol, 19:1 [Fr. 1], 9:1 [Fr. 2], 4:1 [Fr. 3], 7:3 [Fr. 4], 3:2 [Fr. 5], 1:1 [Fr. 6], 2:3 [Fr. 7], 3:7 [Fr. 8], 1:4 [Fr. 9], 1:9 [Fr. 10], and 0:1 [Fr. 11]), yielding Fr. 1 (418 mg), Fr. 2 (21 mg), Fr. 3 (74 mg), Fr. 4 (193 mg), Fr. 5 (321 mg), Fr. 6 (106 mg), Fr. 7 (162 mg), Fr. 8 (123 mg), Fr. 9 (195 mg), Fr. 10 (224 mg), and Fr. 11 (113 mg). Fr. 1, which had a high ORAC value, was further separated using DIAION® HP20 resin (Mitsubishi Chemical Co., Tokyo, Japan) and eluted with 100 mL solution mixed water and methanol in a stepwise gradient (water-methanol, 1:0 [Fr. 1-1], 9:1 [Fr. 1-2], 4:1 [Fr. 1-3], 7:3 [Fr. 1-4], 3:2 [Fr. 1-5], 1:1 [Fr. 1-6], 2:3 [Fr. 1-7], 3:7 [Fr. 1-8], 1:4 [Fr. 1-9], 1:9 [Fr. 1-10], and 0:1 [Fr. 1-11]), yielding Fr. 1-1 (125 mg), Fr. 1-2 (10 mg), Fr. 1-3 (3 mg), Fr. 1-4 (4 mg), Fr. 1-5 (4 mg), Fr. 1-6 (6 mg), Fr. 1-7 (3 mg), Fr. 1-8 (2 mg), Fr. 1-9 (3 mg), Fr. 1-10 (5 mg), and Fr. 1-11 (37 mg). Fr. 1-11, which possessed high antioxidant activity, was subjected to thin-layer chromatography (TLC Silica gel 60 RP-18 F254s, Merck, Tokyo, Japan) with water-methanol (1:9), subsequently isolating compound 1 (1 mg).
The 1H and 13C NMR spectra of compound 1 were recorded using a JEOL-ECS 400 (1H 400 and 13C 100 MHz) spectrometer in methanol-d4 using TMS as the internal standard. This compound was also analyzed by high-performance liquid chromatography with photodiode array (HPLC-PDA) and liquid chromatography with electrospray ionization mass spectrometry (LC-ESI-MS) and MS/MS. HPLC-PDA analysis was performed using the Shimadzu 10A-DP system (Shimadzu, Kyoto, Japan), and 10 µL of sample solution was injected onto a YMC-Pack ODS-A C18 column (column size, 4.6 × 150 mm; particle size, 5 µm; YMC Co., Ltd., Kyoto, Japan) maintained at 40°C and eluted at a flow rate of 1.0 mL min−1. The absorbance was monitored at 254 nm. The mobile phase consisted of methanol-water-phosphoric acid (80:20:1). Spectra were recorded from 200 to 400 nm. LC-ESI-MS analysis was performed using a TSQ Quantum Ultra mass spectrometer equipped with the Accela 600 HPLC system (Thermo Fisher Scientific, MA, USA), and 10 µL of sample solution was injected onto an InertSustainSwift C18 column (column size, 2.1 × 100 mm; particle size, 3 µm; GL Sciences, Tokyo, Japan) maintained at 35°C and eluted at a flow rate of 0.2 mL min−1. The mobile phase consisted of a linear gradient system of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile), 0–35 min, B 50%–70%; 35–40 min, B 70%–95%. Collision energy of MS/MS was set at 25 eV.
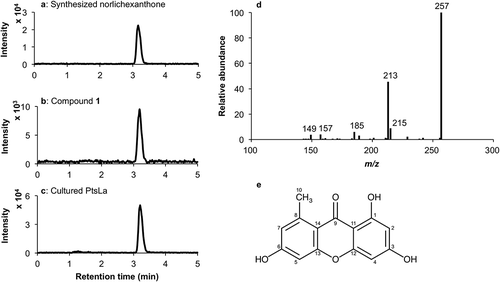
The 1H NMR spectra of compound 1 showed one methyl group [δH, 2.77 (s)] and four meta-coupled aromatic protons [δH, 6.10 (1H, d, J = 1.6 Hz), 6.22 (1H, d, J = 1.6 Hz), and 6.60 (2H, bs)]. The 13C NMR spectra of compound 1 indicated one methyl group [δC, 23.6 (C-10)], twelve aromatic carbons [δC, 166.2 (C-1), 98.8 (C-2), 164.9 (C-3), 94.3 (C-4), 100.5 (C-5), 162.5 (C-6), 116.0 (C-7), 142.7 (C-8), 111.0 (C-11), 158. 6 (C-12), 156.6 (C-13), and 102.2 (C-14)] and one carboxylic carbon [δC, 183.5 (C-9)]. Compound 1 eluted at 3.0 min and showed UV λmax absorbance values of 208, 240, and 311 nm in HPLC-PDA analysis. LC-ESI-MS analysis of the molecular ion peak of compound 1 detected at 3.1 min and m/z 257 ([M-H]−) produced fragment ions at m/z 149 (4), 157 (4), 185 (7), 213 (46, [M-CO2-H]−), 215 (9), and 257 (100, [M-H]−) (). Analytical features of compound 1, such as NMR data, retention time, UV spectrum, molecular ion peak, and fragmentation pattern, were consistent with the features of norlichexanthone, synthesized by methods reported by Santesson and Sundholm in 1968 [Citation5], and confirmed by comparison with literature values [Citation6]. Based on these results, compound 1 was identified as norlichexanthone.
Figure 1. LC-ESI-MS analysis of synthesized norlichexanthone, compound 1 isolated from PtsLa, and methanol extracts of PtsLa.
LC-ESI-MS chromatograms of synthesized norlichexanthone (a), compound 1 isolated from PtsLa (b), and methanol extracts of PtsLa (c), which were detected at m/z 257 in negative ionization mode. Mass spectrum of norlichexanthone (d). Chemical structure of norlichexanthone (e).
Norlichexanthone, 6-O-methylnorlichexanthone, and griseoxanthone C were isolated from culture of endolichenic fungus Ulocladium sp. induced from lichen Everniastrum sp [Citation7]. Moreover, it was reported that it is possible to produce active compounds using cultured mycobionts, for example, anthraquinones from Cladonia [Citation8], Caloplaca, and Xanthoria lichens [Citation9], nitrogen-containing substances such as bostrycoidin derivatives from Haematomma lichen [Citation10], and xanthones from Pyrenula lichens [Citation11,Citation12]. It was reported that cultured mycobiont of Pyrenula japonica contains 1,5,8-trihydroxy-3-methylxanthone, 1,8-dihydroxy-5-methoxy-3-methylxanthone, 1,7-dihydroxy-3-methylxanthone, 1,8-dihydroxy-3-hydroxymethyl-5-methoxyxanthone, emodin, and sclerotiorin and that P. pseudobufonia produces two xanthones, 1,5,8-trihydroxy-3-methylxanthone and 1,2,8-trihydroxy-5-methoxy-3-methylxanthone [Citation11,Citation12]. However, norlichexanthone was not produced in this lichen mycobiont. In this study, norlichexanthone was newly found in endolichenic fungi cultured from natural lichen thalli of Pertusaria laeviganda.
The antioxidant activity of norlichexanthone was compared with that of known active compounds such as ascorbic acid, catechin, gallic acid, and quercetin dihydrate by the ORAC method using fluorescein sodium salt (FL; Sigma-Aldrich, Tokyo, Japan) as a fluorescent probe, as described by Ou et al. in 2001 [Citation13]. These five compounds were diluted with methanol. Measurements were made in black, 96-well, flat-bottom plates (Nunc F96 MicroWell, Thermo Fisher Scientific). To prepare 75 mM phosphate buffer (PB; pH 7.2), 27.2 g of potassium dihydrogen phosphate (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 34.8 g of dipotassium hydrogen phosphate (Wako Pure Chemical Industries, Ltd.) were diluted in 1 L of water. Stock solutions of 1.2 mM FL and 1 mM Trolox (Calbiochem, San Diego, CA, USA) diluted in PB were stored in complete darkness at −20°C. Working solutions were prepared daily by diluting 33.4 µL of 1.2 mM FL in 10 mL PB. Working reference standard solutions of 100, 10, 1, and 0.1 µM Trolox were prepared daily by dilution in methanol. The following were added to each well of the 96-well plate: 50 µL of 78 nM FL and 50 µL of sample, Trolox solution, or methanol (blank), followed by 50 µL of 132 mM 2,2ʹ-azobis (2-methylpropionamidine) dihydrochloride (AAPH; Wako Pure Chemical Industries, Ltd.). After adding AAPH, the plate was incubated at 37°C for 15 min. Fluorescence was measured immediately after incubation and at 5-min intervals for 90 min at an excitation wavelength of 485 nm and emission wavelength of 535 nm. The final results (ORAC value) were calculated using the differences of areas under the FL decay reaction between a blank and a sample and expressed as moles of Trolox equivalents (TE) per gram of pure compounds.
As the results, the antioxidant activity of norlichexanthone was the same level with those of ascorbic acid and lower than those of gallic acid, catechin and quercetin dihydrate (). Manojlovic et al. [Citation14] examined extracts containing antioxidant activity obtained from natural lichen thalli of 2 chemo-types of Laurera benguelensis using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay and reported that these lichens contain xanthones such as lichexanthone and norlichexanthone or other anthraquinones. Moreover, the antioxidant activity of norlichexanthone contained in fungus of genus Arthrinium was suggested by Rosa et al. in 2010 [Citation15]. Additionally, extracts obtained from leaves of Eryngium foetidum, a traditional medicinal herb, exhibited antioxidant activity as determined by a DPPH scavenging assay; this activity was attributed to quinones such as norlichexanthone [Citation16]. However, these reports did not identify compounds showing antioxidant activity. Conversely, Wang et al. [Citation7] demonstrated that the antioxidant activity of norlichexanthone (IC50 > 200 µM) was lower than that of ascorbic acid (IC50 51.7 ± 2.74 µM) determined by a DPPH scavenging assay. In this study, the antioxidant activity of norlichexanthone was higher than that of ascorbic acid () evaluated by the ORAC method. Taken together, the antioxidant activity value of norlichexanthone analyzed by DPPH scavenging assay was low, whereas it was high as determined by the ORAC method. A similar phenomenon was noted in antioxidant analysis of genistein, an isoflavone [Citation17]. Therefore, it is important to compare the antioxidant activity by multiple methods to determine the correct value of active compounds. Norlichexanthone exhibits pharmacologic activity, including inhibition of growth of methicillin-resistant Staphylococcus aureus [Citation10], toxicity, biofilm formation and aggregation of S. aureus [Citation18] and monoamine oxidase [Citation19], antimalarial [Citation20] and adiponectin secretion–enhancing activity [Citation21]. However, excepted to our knowledge, there are no reports describing the high antioxidant activity of norlichexanthone.
Table 1. ORAC values for norlichexanthone and known antioxidant compounds.
Author contributions
H.K. cultured fungi and isolated and analyzed active compounds, and wrote this manuscript. C.S., and H.Y. cultured fungi and isolated and analyzed active compounds. K.H. identified cultured fungi by performing base sequencing of the ITS region. M.K. and Y.Y. provided support.
Acknowledgments
We especially thank Prof. John A. Elix for his generous gift of compounds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Halliwell B, Gutteridge JMC. Redox chemistry: the essentials: free radicals in biology and medicine. Oxford (UK): Oxford University Press; 2015. p. 30–76.
- Velioglu YS, Mazza G, Gao L, et al. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46(10):4113–4117.
- Singh BN, Upreti DK, Gupta VK, et al. Endolichenic fungi: a hidden reservoir of next generation biopharmaceuticals. Trends Biotech. 2017;35:808–813.
- Santesson J, Sundholm G. Chemical studies on lichens 14. Syntheses and chlorinations of norlichexanthone. Ark Kemi. 1969;30(40):427–431.
- Yamamoto Y, Mizuguchi R, Yamada Y. Tissue cultures of Usnea rubescens and Ramalina yasudae and production of usnic acid in their cultures. Agric Biol Chem. 1985;49(11):3347–3348.
- Yamamoto Y, Matsubara H, Kinoshita Y, et al. Naphthazarin derivatives from cultures of the lichen Cladonia cristatella. Phytochem. 1996;43(6):1239–1242.
- Nakano H, Komiya T, Shibata S. Anthraquinones of the lichens of Xanthoria and Caloplaca and their cultivated mycobionts. Phytochem. 1972;11(12):3505–3508.
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626.
- Huneck S, Yoshimura I. Identification of lichen substances. Berlin: Springer-Verlag Berlin Heidelberg; 1996.
- Wang QX, Bao L, Yang XL, et al. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia. 2012;83:209–214.
- Moriyasu Y, Miyagawa H, Hamada N, et al. 5-Deoxy-7-methylbostrycoidin from cultured mycobionts from Haematomma sp. Phytochem. 2001;58(2):239–241.
- Tanahashi T, Takenaka Y, Ikuta Y, et al. Xanthones from the cultured lichen mycobionts of Pyrenula japonica and Pyrenula pseudobufonia. Phytochem. 1999;52:401–405.
- Takenaka Y, Tanahashi T, Nagakura N, et al. Production of xanthones with free radical scavenging properties, emodin and sclerotiorin by the cultured lichen mycobionts of Pyrenula japonica. Z Naturforsch C. 2000;55:910–914.
- Manojlovic NT, Vasiljevic PJ, Gritsanapan W, et al. Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res. 2010;43(2):169–176.
- Rosa LH, GonçAlves VN, Caligiorne RB, et al. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz J Microbiol. 2010;41:420–430.
- Singh S, Singh DR, Banu S, et al. Determination of bioactives and antioxidant activity in Eryngium foetidum L.: A traditional culinary and medicinal herb. Proc Natl Acad Sci I S B Biol Sci. 2013;83:453–460.
- Yamada J, Akahori Y, Matsuda H, et al. Correlation between the DPPH radical scavenging activity and ORAC for dried bonito and other stock types. J Cookery Sci Jpn. 2010;43(3):201–205.
- Baldry M, Nielsen A, Bojer MS, et al. Norlichexanthone reduces virulence gene expression and biofilm formation in Staphylococcus aureus. PLoS One. 2016;11:e0168305.
- Suzuki O, Katsumata Y, Oya M, et al. Inhibition of type A and type B monoamine oxidases by naturally occurring xanthones. Planta Medi. 1981;42(1):17–21.
- Fotie J, Nkengfack AE, Rukunga G, et al. In-vivo antimalarial activity of some oxygenated xanthones. Ann Trop Med Parasitol. 2003;97:683–688.
- Ikeda M, Kurotobi Y, Namikawa A, et al. Norlichexanthone isolated from fungus P16 promotes the secretion and expression of adiponectin in cultured ST-13 adipocytes. Med Chem. 2011;7:250–256.
