ABSTRACT
Long noncoding RNA (lncRNA) has emerged as a pivotal regulator improving neural regeneration in the progression of spinal cord injury (SCI). However, whether lncRNAs can be targeted for therapeutic intervention of SCI remains unclear. In this study, we found that LINC00707 expression was significantly up-regulated in lipopolysaccharide (LPS)-treated PC-12, a model that mimics nerve cell injury in an inflammatory environment after SCI. Suppression of LINC00707 alleviated LPS-induced inflammation and apoptosis in PC-12 cells. Furthermore, we found that LINC00707 adsorbed miR-30a-5p and silenced miR-30a-5p or overexpressed Neurod 1 reversed the effect of LINC00707 on the inflammation and apoptosis of LPS-treated PC-12 cells. These findings revealed that LINC00707 alleviates LPS-induced inflammation and apoptosis in PC-12 cells by targeting miR-30a-5p/Neurod 1, providing a preliminary theoretical basis for the clinical application of LINC00707 in SCI.
Graphical abstract
Suppression of LINC00707 alleviates lipopolysaccharide-induced inflammation and apoptosis in PC-12 cells by regulated miR-30a-5p/Neurod 1.
Spinal cord injury (SCI) may lead to the impairment of motor function, paralysis, and even death, which not only increases economic burdens, but also directly affects the quality of life of patients [Citation1,Citation2]. Although numerous studies have examined the therapeutic treatment of SCI [Citation3–Citation5], the therapeutic effects are not satisfactory. The pathological process of SCI is typically divided into two phase, i.e. primary and secondary injury. Suppressing secondary injury contributes to preventing the deterioration of SCI and improving motor functions [Citation6]. Secondary SCI is mainly promoted by inflammatory response caused by the excretion of proinflammatory cytokines, which promote nerve injury and apoptosis. Studies have shown that interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 levels are increased in the early stage of SCI [Citation7,Citation8]. Inhibition of proinflammatory cytokine excretion plays a central role in protecting nerve cells in SCI [Citation4]. Therefore, exploring the molecular mechanisms underlying inhibited excretion of inflammatory cytokines shows potential for effective treatment method of SCI.
Long noncoding RNA (lncRNA) has emerged as a pivotal regulator improving neural regeneration in the progression of SCI [Citation9]. LncRNAs suppress neuronal apoptosis and inflammatory responses to improve SCI [Citation10,Citation11]. Additionally, lncRNA TUSC7 and MALAT1 inhibited microglial activation and the secretion of inflammatory factors in SCI rats [Citation12,Citation13]. Moreover, lncRNAs plays a regulatory role by sponging micro RNAs (miRNAs). In SCI, lncRNA Sox2ot, BDNF-AS, and MALAT1, respectively, adsorbed miR-211, miR-130b-5p, and miR-199b to regulate the progression of SCI [Citation13–Citation15]. However, whether lncRNAs can be targeted for therapeutic intervention of SCI remains unclear.
Here, we measured the LINC00707 expression level in lipopolysaccharide (LPS)-induced inflammatory response in PC-12 cells, a model that mimics nerve cell injury in an inflammatory environment after SCI [Citation16,Citation17]. Additionally, the function and mechanism of LINC00707 were investigated in LPS-treated PC-12, providing a preliminary theoretical basis for the clinical application of LINC00707 in SCI.
Materials and methods
Cell culture and treatment
PC-12 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) (Thermo Scientific, Waltham, MA, USA) at 37°C in a humidified incubator with 5% CO2. Upon reaching 80% confluence, the cells were treated with 0.25% trypsin and passaged. In order to evaluate the LPS effect on the growth and inflammation of PC-12 cells, PC-12 cells (3 × 104 cells/mL) were seeded in 96-well plates at 100 µL/well and incubated at 37 °C overnight, then, 0, 5, and 10 μg/mL LPS were added to the PC-12 cells for 24 h.
Proliferation assay
The proliferation of PC-12 cells was measured by MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay; Promega, Madison, WI, USA). Absorbance was measured at 490 nm using a microplate reader (R&D Systems, Minneapolis, MN, USA). Each experiment was repeated three times.
Apoptosis and cell cycle assay
PC-12 cells were evaluated by an apoptosis assay using the Annexin V-FITC/PI Apoptosis detection kit (KeyGEN, Nanjing, China) by BD Accuri C6 Plus flow cytometer (BD Biosciences, San Jose, CA, USA). After LPS-treatment for 24 h, PC-12 cells (1 × 106) were harvested and fixed in 500 μL 70% ice-cold ethanol for 2 h at 25°C. PC-12 cells were incubated in 5 μL of Annexin-V fluorescein isothiocyanate and 5 μL of propidium iodide in the dark for 15 min at 25°C. Cell cycle assays were performed using the Cell Cycle Detection kit (Keygen) by BD Accuri C6 Plus flow cytometer (BD). After LPS-treatment for 24 h, 1 × 106 cells were collected and fixed with 70 % precooled ethanol at 4 °C overnight, digested with 200 μg/mL Ribonuclease A at 37 °C for 30 min, then 100 μl propidium iodide (PI) was added at 4 °C in the dark for 30 min. Each experiment was repeated three times.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-1β, IL-6, and TNF-α in the culture supernatant were measured by ELISA according to the manufacturer’s instructions (Multi Sciences, Hangzhou, China), and absorbance at 490 nm was measured using a microplate reader (R&D Systems). Each experiment was repeated three times.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNAs were extracted using Trizol (Life Technologies, Carlsbad, CA, USA). To analyze LINC00707, total RNA was reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara, Shiga, Japan) and SYBR GREEN qPCR Super Mix (Invitrogen, Carlsbad, CA, USA) was used for qRT-PCR. GAPDH was used for the normalization of LINC00707. The primer sequences used in the experiments were as follows: LINC00707 forward: 5′-CCAACAGGGTATCAGAATTCTC-3′, reverse: 5′-TGCTGACAATAGCCATTAGG-3′; Neurod 1 forward: 5′-GTTATTGTACCCATGCCG-3′, reverse: 5′-GTCTCTAAGGCAACACAAC-3′; GAPDH forward: 5′-TGGTATCGTGGAAGGACTCAT-3′, reverse: 5′-GTGGGTGTCGCTGTTGAAGTC-3′. To analyze miR-30a-5p, total RNA was reverse-transcribed with Taqman MicroRNA Reverse Transcription Kit and real-time PCR was performed using Taqman Universal Master Mix II according to the manufacturer’s protocols (Applied Biosystems, Foster City, CA, USA). Small nuclear RNA U6 was used for the normalization of miR-30a-5p. The primer sequences used in the experiments were as follows: miR-30a-5p forward: 5′- TGTAAACATCCTCGACTGGAAG −3′, reverse: 5′-TGCGTGTCGTGGAGTC-3′; U6 forward: 5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCACGAATTTGCGT-3′. qRT-PCR was carried out using an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems). Relative fold-changes in the transcripts were calculated using the 2−ΔΔCT method. All samples were analyzed in triplicate.
Transfection
LINC00707 small interfering RNAs (siRNAs), negative control siRNA (si-NC), miR-30a-5p inhibitor, and negative control of miR-30a-5p inhibitor (NC inhibitor) were purchased from GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 3000 Reagent (Invitrogen) in LPS-treated PC-12 cells. The transfection efficiency was measured by qRT-PCR. LINC00707 siRNA-1: 5′-GGCUUUCCAUGACCCAUAATT-3′; siRNA-2: 5′-GCAGGAACAUCACCAUCUUTT-3′; siRNA-3: 5′-GGAAGCCACUCCUGCAUUUTT-3′. si-NC: 5′-UUCUCCGAACGUGUCACGUTT-3′. miR-30a-5p inhibitor: 5′-CUUCCAGUCGAGGAUGUUUACA-3′. negative control of miR-30a-5p inhibitor (NC inhibitor): 5′-CAGUACUUUUGUGUAGUACAA-3′. The complete open reading frame of Neurod 1 was synthesized by GENEWIZ (Suzhou, Jiangsu, China) and inserted into the pcDNA3.1 plasmid (ov- Neurod 1). The pcDNA3.1 plasmid was used as negative control (ov-NC). The transfection efficiency was measured by qRT-PCR and western blot.
Dual luciferase reporter assay
The wild-type and mutant fragments from LINC00707 containing the predicted miR-30a-5p binding site were amplified and cloned into the psi-CHECK2 vector (Promega), which was named to wt LINC00707 and mut LINC00707, respectively. The mutant fragment was mutated at the putative binding site. The plasmids were respectively co-transfected into PC-12 cells with negative control of miR-30a-5p mimic (NC mimic, 5ʹ‑UUC UCC GAA CGU GUC ACG UTT‑3ʹ) or miR-30a-5p mimic (5ʹ‑UGUAAACAUCCUCGACUGGAAG‑3ʹ). Luciferase activity ratio of Renilla and firefly was determined using a Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
The data were analyzed using SPSS version 19.0 (SPSS, Inc., Chicago, IL) statistical software. All data were presented as means ± standard deviation (SD). Analysis of variance was used for the overall comparison of the measurement indices between more than 2 groups followed by least significant difference analysis between 2 groups. Independent t test was used to compare 2 groups. P < 0.05 was considered statistically significant.
Results
LPS induced inflammatory injury and promoted LINC00707 expression in PC-12 cells
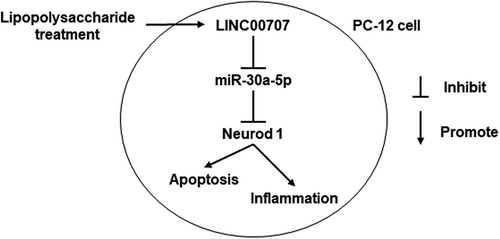
Proliferation, cycle, and apoptosis were measured by MTS assay and flow cytometry, respectively, in PC-12 cells treated with LPS at 0, 5, and 10 μg/mL. The results showed that LPS significantly inhibited proliferation, prevented the transition from G1 phase to S phase, and promoted apoptosis in a dose-dependent manner (–)). Additionally, the ELISA results showed that LPS significantly enhanced the levels of IL-1β, IL-6, and TNF-α in a dose-dependent manner ()). The qRT-PCR results showed that LPS significantly up-regulated LINC00707 expression in a dose-dependent manner ()).
Figure 1. LPS significantly inhibited proliferation and promoted apoptosis, the levels of IL-1β, IL-6, and TNF-α, and LINC00707 expression in PC-12 cells in a dose-dependent manner. (a) Proliferation was measured by MTS assay and apoptosis and cycle was measured by flow cytometry in PC-12 cells at 24 h after 0, 5, and 10 μg/mL LPS treatment. (b and c) Representative images of apoptosis and cell cycle. (d) The levels of IL-1β, IL-6, and TNF-α were measured by ELISA in PC-12 cells at 24 h after 0, 5, and 10 μg/mL LPS treatment. (e) LINC00707 expression were measured by qRT-PCR in PC-12 cells at 24 h after 0, 5, and 10 μg/mL LPS treatment. ***P < 0.001.
Suppression of LINC00707 alleviated LPS -induced inflammation and apoptosis in PC-12 cells
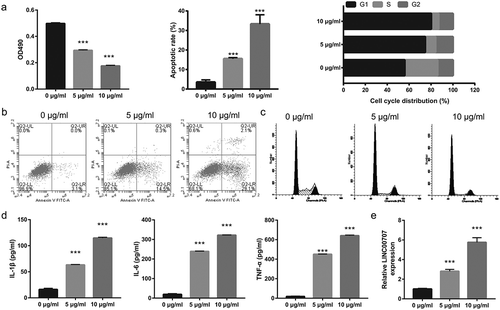
Next, LINC00707 expression was measured by qRT-PCR after transfection of siRNAs for 48 h. Compared to transfected si-NC, LINC00707 expression was significantly suppressed by the transfected siRNAs without or with LPS (5 μg/mL), particularly following transfection with siRNA-2 (,)). According to the results, siRNA-2 (named si-LINC00707) was used for further analysis. Subsequent experiments showed that suppression of LINC00707 promoted proliferation, enhanced the transition from G1 phase to S phase, inhibited apoptosis, and reduced the levels of IL-1β, IL-6, and TNF-α in LPS-treated PC-12 cells (–)).
Figure 2. Suppression of LINC00707 reduced inflammation, promoted proliferation, and inhibited apoptosis of LPS (5 μg/mL)-treated PC-12 cells. (a and b) LINC00707 expression was measured by qRT-PCR after transfection of siRNAs at 48 h and then treatment without or with 5 μg/mL LPS at 24 h. (c) Proliferation was measured by MTS assay. (d and e) Apoptosis and cycle was measured by flow cytometry. (f-h) The levels of IL-1β, IL-6, and TNF-α were measured by ELISA. (i and j) Representative images of apoptosis and cell cycle. ***P < 0.001.
MiR-30a-5p was the direct target of LINC00707 in LPS-treated PC-12 cells
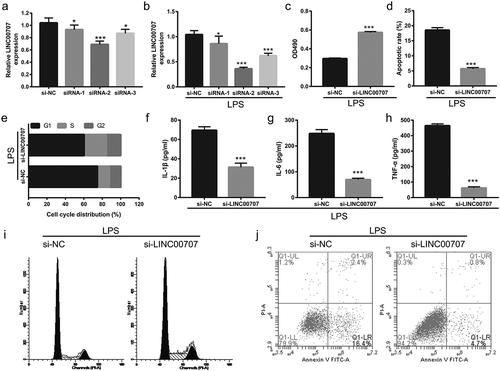
Next, we examined the underlying molecular mechanism of how LINC00707 exerted its effects on neuronal cell apoptosis and inflammation. We hypothesized that LINC00707 functions as a competing endogenous RNA to sponge miRNA. First, starBase tools [Citation18] were used to predict the potential miRNAs adsorbed by LINC00707, and miR-30a-5p was selected. The binding sites between LINC00707 and miR-30a-5p are shown in ). Next, the luciferase reporter assay results showed that transfection of miR-30a-5p mimic significantly decreased the luciferase activity of wt LINC00707, whereas no obvious changes were observed in the luciferase activity of mut LINC00707 ()). The qRT-PCR results showed that miR-30a-5p expression was significantly down-regulated in LPS-treated PC-12 cells in a dose-dependent manner ()), while it was significantly up-regulated in LPS-treated PC-12 cells after transfection with si-LINC00707 ()).
Figure 3. LINC00707 directly targeted miR-30a-5p in LPS-treated PC-12 cells. (a) Potential binding sites between LINC00707 and miR-30a-5p. (b) Luciferase activity ratio (Renilla/firefly) was measured in a luciferase reporter assay. (c) miR-30a-5p expression was measured by qRT-PCR in 0, 5, and 10 μg/mL LPS-treated PC-12 cells. (d) miR-30a-5p expression was measured by qRT-PCR after transfection with si-NC and si-LINC00707 in LPS (5 μg/mL)-treated PC-12 cells. ***P < 0.001.
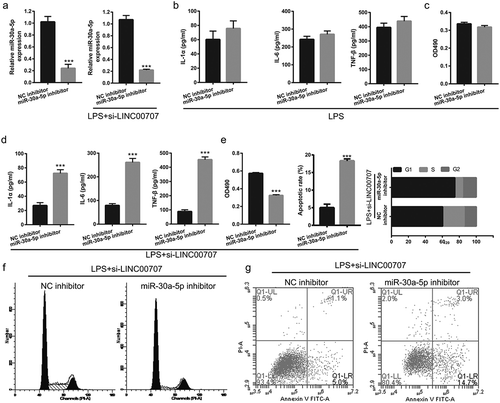
Suppression of miR-30a-5p reversed the effect of LINC00707 on LPS-induced inflammation injury in PC-12 cells
We next evaluate the effect of miR-30a-5p expression on LINC00707-regulated LPS-treated PC-12 injury by co-transfected si-LINC00707 and miR-30a-5p inhibitor. First, miR-30a-5p expression were significantly down-regulated after miR-30a-5p inhibitor transfection compared with NC inhibitor transfection in PC-12 cells treated without LPS treatment ()). We also found that co-transfected si-LINC00707 and miR-30a-5p inhibitor could down-regulate miR-30a-5p expression after LPS treatment ()). Compared with NC inhibitor group, proliferation and inflammation had no significant changed in miR-30a-5p inhibitor group in PC-12 cells treated with LPS alone (,)). Subsequent experiments showed that co-transfection of si-LINC00707 and miR-30a-5p inhibitor enhanced the levels of IL-1β, IL-6, and TNF-α, inhibited proliferation, prevented the transition from G1 phase to S phase, and promoted apoptosis in LPS-treated PC-12 cells (–)).
Figure 4. Co-transfection of si-LINC00707 and miR-30a-5p inhibitor increased inflammation, inhibited proliferation, and promoted apoptosis in LPS (5 μg/mL)-treated PC-12 cells. (a) The expression level of miR-30a-5p was measured by qRT-PCR after only transfection of miR-30a-5p inhibitor in PC-12 cells without LPS treatment and co-transfection of si-LINC00707 and miR-30a-5p inhibitor in PC-12 cells with LPS treatment at 48 h. (b) The levels of IL-1β, IL-6, and TNF-α were measured by ELISA in PC-12 cells with LPS treatment after transfection of miR-30a-5p inhibitor. (c) The proliferation was measured by MTS in PC-12 cells with LPS treatment after transfection of miR-30a-5p inhibitor. (d) The levels of IL-1β, IL-6, and TNF-α were measured by ELISA in PC-12 cells with LPS treatment after co-transfection of si-LINC00707 and miR-30a-5p inhibitor. (e) Proliferation, and apoptosis and cycle was measured by MTS assay and flow cytometer assay, respectively, in PC-12 cells with LPS treatment after co-transfection of si-LINC00707 and miR-30a-5p inhibitor. (f and g) Representative images of apoptosis and cell cycle measured by flow cytometry. ***P < 0.001.
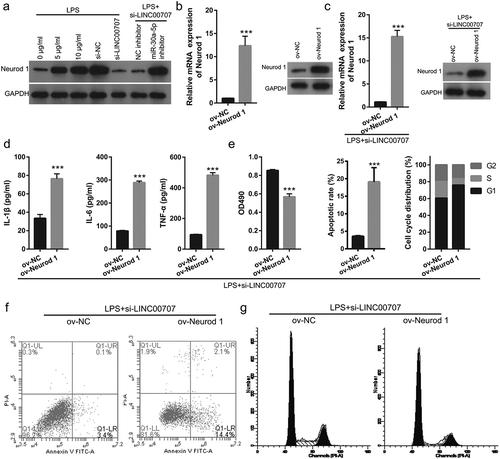
Upregulated Neurod 1 reversed the effect of LINC00707 on LPS-induced inflammation injury in PC-12 cells
Previous study found that miR-30a-5p ameliorated SCI-induced inflammatory responses by targeting Neurod 1 expression [Citation19]. First, we found that Neurod 1 expression was promoted in LPS-treated PC-12 cells while it was inhibited in LPS-treated PC-12 cells after transfected of si-LINC00707 ()). Co-transfected si-LINC00707 and miR-30a-5p inhibitor enhanced Neurod 1 expression in LPS-treated PC-12 cells ()). We next evaluated the effect of Neurod 1 expression on LINC00707-regulated LPS-treated PC-12 injury by co-transfected of si-LINC00707 and ov-Neurod 1. We found that transfected ov-Neurod 1- could promote Neurod 1 expression in PC-12 cells ()). We found that co-transfected si-LINC00707 and ov-Neurod 1- could promote Neurod 1 expression in LPS-treated PC-12 cells ()). Subsequent experiments showed that co-transfection of si-LINC00707 and Neurod 1-pcDNA 3.1 enhanced the levels of IL-1β, IL-6, and TNF-α, inhibited proliferation, prevented the transition from G1 phase to S phase, and promoted apoptosis in LPS-treated PC-12 cells (–)).
Figure 5. Co-transfection of si-LINC00707 and Neurod 1-pcDNA 3.1 increased inflammation, inhibited proliferation, and promoted apoptosis in LPS (5 μg/mL)-treated PC-12 cells. (a) Neurod 1 expression was measured by western blot. (b) Neurod 1 expression was measured by qRT-PCR and western blot after transfection Neurod 1-pcDNA 3.1 at 48 h in PC-12 cells. (c) Neurod 1 expression was measured by qRT-PCR and western blot after co-transfection of si-LINC00707 and Neurod 1-pcDNA 3.1 at 48 h in LPS-treated PC-12 cells. (d) The levels of IL-1β, IL-6, and TNF-α were measured by ELISA. (e) Proliferation, and apoptosis and cycle was measured by MTS and flow cytometer assay, respectively, in PC-12 cells with LPS treatment after co-transfection of si-LINC00707 and Neurod 1-pcDNA 3.1. (f) Representative images of apoptosis. (g) Representative images of cell cycle. ***P < 0.001.
Discussion
Inflammatory responses play an important role in regulating secondary phase in SCI [Citation20]. The level of TNF-α and IL-6 were clearly increased in the injured area from 3 h after the onset of SCI [Citation7,Citation8]. Previous studies showed that LPS-treated PC-12 cell can be used as a model to mimic nerve cell injury in an inflammatory environment after SCI and that LPS inhibited proliferation, promoted apoptosis, and enhanced TNF-α, IL-1β, and IL-6 levels [Citation21,Citation22]. Similarly, we found that LPS treatment inhibited proliferation, prevented the transition from G1 phase to S phase, promoted apoptosis, and enhanced TNF-α, IL-1β, and IL-6 levels in a dose-dependent manner. Additionally, we found that LPS induced an increase in LINC00707 expression in a dose-dependent manner.
LINC00707, a 3087-base pair lncRNA, is located on chromosome 10p14. Recent studies showed that LINC00707 regulated cancer cell proliferation and migration [Citation23–Citation25]. However, the effect of LINC00707 on inflammation and SCI remains unclear. In this study, we found that suppression of LINC00707 reduced the levels of IL-1β, IL-6, and TNF-α, promoted proliferation, enhanced the transition from G1 phase to S phase, and inhibited apoptosis, and alleviated LPS-induced inflammation and apoptosis in PC-12 cells. These results showed that suppression of LINC00707 has anti-apoptotic and anti-inflammatory effects in LPS-treated PC-12 cells.
Additionally, the underlying molecular mechanism of how LINC00707 exerted its effects on LPS-treated PC-12 cell apoptosis and inflammation were evaluated. LncRNAs plays a regulatory role by adsorbing miRNAs. In hepatocellular carcinoma, LINC00707 promoted proliferation via sponging miR-206. Our mechanism studies indicated that miR-30a-5p is the target of LINC00707 by starBase analysis. miR-30a-5p plays an important role in anti-inflammation. In diabetes, miR-30a-5p functions in anti-inflammation by targeting IL-1α [Citation26]. In SCI, miR-30a-5p was down-regulated, and overexpression of miR-30a-5p inhibited TNF-α and IL-1β expression and reduced oxidative stress to ameliorate SCI-induced injury by targeting Neurod 1 through the MAPK/ERK signaling pathway, suggesting that miR-30a-5p plays protective roles in SCI [Citation19]. In this study, we found that miR-30a-5p binds to LINC00707. miR-30a-5p expression was down-regulated in LPS-treated PC-12 cells, and its expression was reversed by suppressing LINC00707. These results suggest that LINC00707 sponge miR-30a-5p to inhibit miR-30a-5p expression. Additionally, the effect of LINC00707 silenced on LPS-induced inflammation and apoptosis was reversed by miR-30a-5p silencing, suggesting that LINC00707 silencing inhibited apoptosis and inflammation by up-regulation of miR-30a-5p. Furthermore, we found that the effect of LINC00707 silencing on LPS-induced inflammation and apoptosis was reversed by Neurod 1 overexpression, suggesting that LINC00707 silencing inhibited apoptosis and inflammation by inhibiting Neurod 1. These results showed that LINC00707 regulates LPS-induced apoptosis and inflammation by miR-30a-5p/Neurod 1 system.
In conclusion, our results suggest that suppression of LINC00707 alleviates LPS-induced inflammation and apoptosis in PC-12 cells by regulating miR-30a-5p/Neurod 1, suggesting LINC00707 is a potential therapeutic target of SCI. We will validate these findings using SCI models in subsequent experiments.
Author contribution
SZ, ZZ, and YL conceived and designed the present study. ZL, JS, GJ, and YH developed the methodology. SZ, ZZ, ZL, and JS completed the experiment and collected the data. SZ, ZZ, GJ, YH, and YL analyzed and interpreted the data. SZ and ZZ drafted the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Yang R, Guo L, Huang L, et al. Epidemiological characteristics of traumatic spinal cord injury in Guangdong, China. Spine (Phila Pa 1976). 2017;42:E555–e561.
- Wang Y, Xie H, Zhao X. Psychological morbidities and positive psychological outcomes in people with traumatic spinal cord injury in Mainland China. Spinal Cord. 2018;56:704–711.
- Sun GD, Hu XJ, Zhang GM, et al. Arctigenin suppresses inflammation and plays a neuroprotective effect in mice with spinal cord injury. Int J Clin Exp Med. 2018;11:2100–2106.
- Sun GD, Shao JL, Deng DJ, et al. A chitosan scaffold infused with neurotrophin-3 and human umbilical cord mesenchymal stem cells suppresses inflammation and promotes functional recovery after spinal cord injury in mice. Int J Clin Exp Med. 2017;10:11672–11679.
- Kingwell K. Spinal cord injury: clamping down on calpains to treat injury-induced spasticity. Nat Rev Drug Discov. 2016;15:310.
- Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356.
- Saxena T, Loomis KH, Pai SB, et al. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 2015;9:1492–1505.
- Pan JZ, Ni L, Sodhi A, et al. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res. 2002;68:315–322.
- Chandran R, Mehta SL, Vemuganti R. Non-coding RNAs and neuroprotection after acute CNS injuries. Neurochem Int. 2017;111:12–22.
- Zhang H, Wang W, Li N, et al. LncRNA DGCR5 suppresses neuronal apoptosis to improve acute spinal cord injury through targeting PRDM5. Cell Cycle. 2018;17:1992–2000.
- Jiang ZS, Zhang JR. LncRNA SNHG5 enhances astrocytes and microglia viability via upregulating KLF4 in spinal cord injury. Int J Biol Macromol. 2018;120:66–72.
- Yu Y, Zhu M, Zhao Y, et al. Overexpression of TUSC7 inhibits the inflammation caused by microglia activation via regulating miR-449a/PPAR-gamma. Biochem Biophys Res Commun. 2018;503:1020–1026.
- Zhou HJ, Wang LQ, Wang DB, et al. Long noncoding RNA MALAT1 contributes to inflammatory response of microglia following spinal cord injury via the modulation of a miR-199b/IKKbeta/NF-kappaB signaling pathway. Am J Physiol Cell Physiol. 2018;315:C52–c61.
- Yin D, Zheng X, Zhuang J, et al. Downregulation of long noncoding RNA Sox2ot protects PC-12 cells from hydrogen peroxide-induced injury in spinal cord injury via regulating the miR-211-myeloid cell leukemia-1 isoform2 axis. J Cell Biochem. 2018;119:9675–9684.
- Zhang H, Li D, Zhang Y, et al. Knockdown of lncRNA BDNF-AS suppresses neuronal cell apoptosis via downregulating miR-130b-5p target gene PRDM5 in acute spinal cord injury. RNA Biol. 2018;15:1071–1080.
- Zhang G, Liu Y, Xu L, et al. Resveratrol alleviates lipopolysaccharide-induced inflammation in PC-12 cells and in rat model. BMC Biotechnol. 2019;19:10.
- Li R, Yin F, Guo Y, et al. Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother. 2018;108:1320–1327.
- Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–97.
- Fu X, Shen Y, Wang W, et al. MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting Neurod 1 through MAPK/ERK signalling. Clin Exp Pharmacol Physiol. 2018;45:68–74.
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137.
- Zhang Z, Wan F, Zhuang Q, et al. Suppression of miR-127 protects PC-12 cells from LPS-induced inflammatory injury by downregulation of PDCD4. Biomed Pharmacother. 2017;96:1154–1162.
- Li G, Chen T, Zhu Y, et al. MiR-103 alleviates autophagy and apoptosis by regulating SOX2 in LPS-injured PC12 cells and SCI rats. Iran J Basic Med Sci. 2018;21:292–300.
- Ma T, Ma H, Zou Z, et al. The long intergenic noncoding RNA 00707 promotes lung adenocarcinoma cell proliferation and migration by regulating Cdc42. Cell Physiol Biochem. 2018;45:1566–1580.
- Xie M, Ma T, Xue J, et al. The long intergenic non-protein coding RNA 707 promotes proliferation and metastasis of gastric cancer by interacting with mRNA stabilizing protein HuR. Cancer Lett. 2018;443:67–79.
- Wang J, Luo Z, Yao T, et al. LINC00707 promotes hepatocellular carcinoma progression through activating ERK/JNK/AKT pathway signaling pathway. J Cell Physiol. 2019;234:6908–6916.
- Jiang X, Xu C, Lei F, et al. MiR-30a targets IL-1alpha and regulates islet functions as an inflammation buffer and response factor. Sci Rep. 2017;7:5270.
