ABSTRACT
miR-29a-3p has been reported to function as a tumor suppressor in several cancers. However, the biological function role of miR-29a-3p in colorectal carcinoma (CRC) has not been well investigated. In this study, we found that miR-29a-3p was at lower level expression in CRC tissues and cell lines. Experimental up-regulation miR-29a-3p with mimic could inhibit cell proliferation, but induced cell cycle arrest at G0/G1 phase and apoptosis in CRC cells. MiR-29a-3p overexpression significantly down-regulated the expression levels of CDK4, Cyclin D1, and Bax, but up-regulated the expression levels of p21 and Bcl-2 in DLD-1 cells. Moreover, ribosomal protein S15A (RPS15A) was predicted and confirmed as a direct target gene of miR-29a-3p. Furthermore, restoration of RPS15A could rescue the phenotypic changes caused by miR-29a-3p. The findings demonstrate miR-29a-3p inhibits CRC cell function possibly by targeting RPS15A, which might be exploited therapeutically in CRC.
Graphical abstract

Downregulation of RPS15A by miR-29a-3p attenuates cell proliferation and induced cell cycle G0/G1 phase arrest and apoptosis in CRC.
With increasing incidence and mortality in economically developing countries, colorectal carcinoma (CRC) has been the third frequently diagnosed cancer and considered as the fourth cause of cancer-related death [Citation1]. In 2017, 27,150 men and 23,110 women are expected to die from this disease in the United States [Citation2]. The onset and progression of CRC are commonly ascribed to a heterogeneous process with distinct sets of epigenetic and genetic changes, which is often affected by age, chronic disease history and inappropriate lifestyle [Citation3]. Treatment options for CRC include surgical resection, cytotoxic therapy, angiogenesis-targeting drugs, as well as agents that target the epidermal growth factor receptor (EGFR) [Citation4]. However, the median survival duration was not more than 10 months in patients diagnosed with CRC [Citation5]. Therefore, it is important to broaden our knowledge concerning the biological behaviors of CRC cells so as to improve the treatment strategies.
For patients with solid tumors and hematologic maganancies, there is a growing interest in “microRNA (miRNA) regulatory networks” that emphasize their critical roles in controlling cancer initiation and progression [Citation6]. As a family of small non-coding RNAs (20–24 nucleotides in length), miRNAs are reported to regulate a wide variety of biological processes in multicellular eukaryotes including mammals [Citation7]. Emerging evidence indicates that abnormal expression of a single miRNA has a profound impact on cancer cell proliferation, apoptosis, and carcinogenesis via regulating mRNA translation and stability [Citation8]. It is now apparent that miR-29a-3p is an important feature in several types of malignancies. Zhao et al. [Citation9] have revealed that miR-29a-3p modulates cells proliferation, migration, and invasion in gastric cancer through altering the expression of CDK2, CDK4, CDK6, and CyclinD1. Wang et al. [Citation10] recently showed that the expression of miR-29a-3p is low in hepatocellular carcinoma and overexpression of miR-29a-3p attenuates proliferative and migratory phenotype of cancer cells. It is worth noting that miR-29a-3p is deregulated in CRC tissues, as compared with normal colon tissues by high-throughput real-time PCR method [Citation11]. However, the functional role of miR-23a-3p in CRC cells remains unclear. Ribosomal protein s15a (RPS15A), as a highly conserved protein, locates on chromosome 16p12.3 and has been shown to promote the formation of the mRNA-ribosome complex in translation [Citation12]. Related studies showed that suppression of RRPS15A can make human glioblastoma cells and acute myeloid leukemia cells more susceptible to apoptosis [Citation13,Citation14]. Knockdown of RPS15A is an anti-oncogenic event that leads to inhibition of proliferation in breast cancer [Citation15], gastric [Citation16], and lung cancer cells [Citation17]. Interestingly, high-level expression of RPS15A was found to be associated with a more aggressive CRC disease, such as higher primary pN stage, slightly more synchronous distant metastasis, and shorter overall survival in a cohort of CRC patients [Citation18].
In the present study, we first analyzed the expression of miR-29a-3p in CRC tissues and cell lines. By performing gain-of-functional assays, we further explore the biological function of miR-29a-3p in CRC cells. Furthermore, we confirmed whether RPS15A is a direct target gene of miR-29a-3p and affecting CRC cell proliferation, cell cycle distribution, and apoptosis. Our results might highlight the potential effect of miR-29a-3p/RPS15A axis in CRC cell proliferation.
Materials and methods
Clinical tissue samples
Human primary tumor tissues and matched adjacent normal tissues were collected from 10 patients histologically confirmed as CRC who underwent routine surgical resection at the Department of Oncology, Zhangjiakou First Hospital (Hebei, China) between October 2017 and July 2018. All patients received neither chemotherapy nor radiotherapy and provided the informed written consent before enrollment. All fresh tissues were immediately stored in a − 80°C refrigerator prior to subsequent experimentation. This study obtained the approval from the Ethics Committee of Zhangjiakou First Hospital.
Cell culture and transfection
Human CRC cell lines (DLD-1, RKO, SW480, and HCT116) and normal colon epithelial cell line (FHC) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). All the cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) with 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 10% fetal bovine serum (FBS, Gibco, USA) in a humidified incubator containing 5% CO2 at 37°C.
MiR-29a-3p mimic and corresponding negative control (miR-NC) were purchased from RiboBio (Guangzhou, China). DLD-1 or SW480 cells were incubated in the same environment and transfected with 50 nM miR-29a-3p or miR-NC with riboFECTTM CP Transfection Kit (RiboBio, China), followed by validating transfection efficiency. In the rescue experiments, the coding sequences of RPS15A were synthesized by RiboBio (Guangzhou, China) and then cloned into pcDNA3.1 to construct RPS15A expression vector. Empty pcDNA3.1 vector was served as a negative control. Subsequently, miR-29a-3p mimic and either RPS15A expression vector or empty vector were co-transfected into DLD-1 cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. After 48 h transfection, the cells were harvested and the transfection efficiency was confirmed.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA from tissues or cell lines was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized with a TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) both according to the manufacturer’s protocols. Subsequently, qRT-PCR was performed Applied Biosystems 7500 Sequence Detection system (Applied Biosystems) using a miRNA-specific TaqMan MicroRNA Assay kit (Applied Biosystems) according to the following thermocycling conditions: Denaturation at 95°C for 10 min; followed by 40 cycles of denaturation at 95°C for 15 sec and elongation at 60°C for 1 min. The relative expression of miR-29a-3p was normalized to U6 small nuclear RNA using quantification (2− ΔΔCt) method [Citation19]. The primer sequences used were designed based on a study from Wang et al. [Citation10] as follows: miR-29a-3p forward: 5ʹ-AGCACCAUCUGAAAUCGGUUA-3ʹ and reverse: 5ʹ-GTGCAGGGTCCGAGGT-3ʹ; U6 forward: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and reverse: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ.
Cell proliferation assay
Cell proliferation ability was accessed by Cell Counting Kit-8 (CCK-8, KeyGen Biotech, China). Briefly, transfected cells were seeded into 96-well plates (approximately 3, 000 cells per well) and cultured for 24, 48, 72 and 96 h in complete media at 37°C. At these indicated time points, 10 µL CCK-8 solution was added to each well and incubated for another 2 h. Next, the optical density at 450 nm was detected using a microplate reader (Bio-Rad Laboratories, Richmond, CA, USA).
Flow cytometry analysis
Transfected cells were harvested via trypsinization, washed with cold PBS twice. For cell cycle analysis, cells were stained with 20 µg/mL propidium iodide and 10 µg/mL RNase A for 30 min. Deoxyribonucleic acid (DNA) content and cell cycle distribution were analyzed using a flow cytometer (FACSCalibur; BD Biosciences) equipped with cell cycle analysis software (Modifit 2.0). For cell apoptosis, cells were resuspended and 250 µL of binding buffer and stained with 20 µL each of Annexin V-FITC and propidium iodide (KeyGen Biotech, Nanjing, China). Finally, the apoptotic cells were detected by flow cytometry (BD Biosciences).
Luciferase reporter assay
The potential target genes of miR-29a-3p were predicted using online tools, including TargetScan (http://www.targetscan.org/vert_71/) and miRDB (http://mirdb.org/). For luciferase reporter assay, the wild type (WT, including the predicted miR-29a-3p target sites) and a mutant sequences of the binding site of miR-29a-3p (MUT) 3ʹUTR of target RPS15A were inserted into pmirGLO plasmids (Promega Corporation, Madison, WI, USA) to construct RPS15A-3ʹUTR-WT and RPS15A-3ʹUTR-MUT, respectively. Subsequently, DLD-1 and SW480 cells were co-transfected with either miR-29a-3p mimic or miR-NC and double luciferase reporter gene (RPS15A-3ʹUTR-NC (pmirGLO), RPS15A-3ʹUTR-WT or MUT) using Lipofectamine 2000 (Invitrogen, USA). After 48 h transfection, the luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA). The relative luciferase activity was calculated.
Western blotting
Total cellular protein was extracted with a RIPA buffer composed of phosphate inhibitor and protease inhibitor and protein concentrations were determined using a BCA protein assay kit (both from KeyGen Biotech, Nanjing, China). Total 30 µg protein was separated by the 10% SDS-PAGE gel and then transferred onto polyvinylidene difluoride (PVDF) membranes at 4°C. Membranes were subsequently blocked with Tris-buffered saline containing 0.1% Tween-20 (TBST) at 25°C for 1 h, and then incubated at 4°C overnight with primary antibodies against RPS15A, CDK4, Cyclin D1, p21, Bax, Bcl-2 and GAPDH. After washing with cold PBS twice, the membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:5000) secondary antibody, followed by enhanced chemiluminescence system (ECL) detection.
Statistical analysis
Statistical analyses and graphing were performed using GraphPad Prism 6.01 (GraphPad Software, Inc., Chicago, IL, USA). All data from three independent experiments were expressed as the mean ± standard deviation (SD). Significant statistical differences were evaluated using Student’s t test (two groups) or one-way analysis of variance and Tukey test (three or above groups). Those p values less than 0.05 were considered to be statistically significant.
Results
The expression of mir-29a-3p was decreased in CRC
To explore the potential role of miR-29a-3p in CRC, qRT-PCR analysis was performed to determine its expression status in clinical tumor samples and matched adjacent normal tissues. As displayed in ), miR-29a-3p expression was obviously decreased in 10 pairs of CRC tissues compared to the matched adjacent tissues (p < 0.05, p< 0.001). Furthermore, miR-29a-3p expression was assessed in CRC cell lines, and lower expression was detected in DLD-1, RKO, SW480, and HCT116 cells compared with the FHC cell lines (), p < 0.01, p< 0.001). Notably, DLD-1 and SW480 presented lower miR-29a-3p expression (nearly one-fifth relative to FHC cells) than RKO and HC116 cells. We thus selected DLD-1 and SW480 cell lines for the subsequent functional assays.
Figure 1. MiR-29a-3p was down-expression in CRC.
Quantitative reverse transcription PCR was performed to examine the expression level of miR-29a-3p in ten pairs of CRC tissue samples compared with matched adjacent tissues (a, *p < 0.05, ***p < 0.001) and different cell lines (b, **p < 0.01, ***p < 0.001).
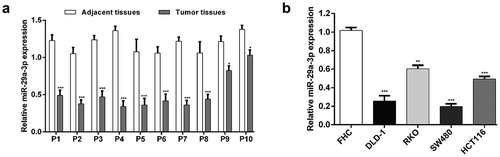
mir-29a-3p inhibited cell proliferation, induced cell cycle arrest and apoptosis in CRC cells
Since miR-29a-3p was down-regulated in CRC, we thus speculated it might negatively influence CRC cell behaviors. To confirm our hypothesis, miR-29a-3p mimic was transfected into DLD-1 and SW480 cells. After 48 h transfection, qRT-PCR analysis showed that miR-29a-3p expression was increased approximately fourfold in miR-29a-3p mimic-transfected group compared with miR-NC-transfected group in both DLD-1 and SW480 cells (), p < 0.001). There was no significant change in the miR-29a-3p expression between control and miR-NC vector-transfected cells. Then, the effects of miR-29a-3p overexpression on cell proliferation, cell cycle distribution and apoptosis were evaluated. As shown in ), the growth rate of DLD-1 (p < 0.001) and SW480 (p < 0.01, p < 0.001) cells was apparently suppressed in miR-29a-3p mimic-transfected group, in comparison with miR-NC-transfected group. Moreover, we found a significant increase in G0/G1 and G2/M phase fractions, but a decrease in S phase fraction in DLD-1 cells after miR-29a-3p overexpression (), p < 0.01, p < 0.001). Similarly, the percentage of cells in G0/G1 phase was obviously elevated and those in G2/M phase was reduced in miR-29a-3p mimic-transfected group compared with miR-NC-transfected group in SW480 cells (), p < 0.05, p < 0.01). In addition, we used flow cytometry to conduct cell apoptosis assay. The results ()) showed that miR-29a-3p overexpression caused a significantly elevated apoptotic rate in DLD-1 (p < 0.001) and SW480 (p < 0.05, p < 0.01) cells. Compared with SW480 cells, DLD-1 cells produced more obvious cell apoptosis after miR-29a-3p overexpression. These results revealed that up-regulated miR-29a-3p suppressed cell proliferation, facilitated cell cycle arrest and apoptosis in CRC cells.
Figure 2. MiR-29a-3p regulated CRC proliferation, cell cycle progression and apoptosis.
(a) Relative expression levels of miR-29a-3p were measured by quantitative reverse transcription PCR in DLD-1 and SW480 cells transfected with miR-29a-3p mimic or miR-NC. (b) Cell proliferation was analyzed via a CCK-8 assay. (c) Flow-cytometric determination of the proportion of cells in each cell cycle phase in DLD-1 and SW480 cells transfected with miR-29a-3p mimic or miR-NC. (d) The percentages of apoptosis cells were measured by flow cytometry in DLD-1 and SW480 cells transfected with miR-29a-3p mimic or miR-NC. *p < 0.05, **p < 0.01, ***p < 0.001 vs. miR-NC
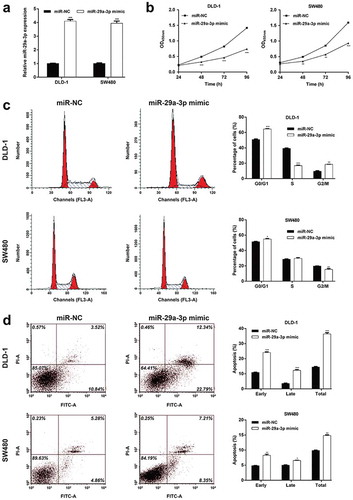
RPS15A was a direct target of mir-29a-3p
To further reveal the molecular mechanisms underlying miR-29a-3p regulating CRC cellular behaviors, the target genes of miR-29a-3p was predicted using online bioinformatics tools. Among these targets, RPS15A has been reported to promote the development of CRC, thus was selected as a potential target of miR-29a-3p in CRC. The potential binding site of miR-29a-3p in the 3ʹUTR of RPS15A is displayed in ). Subsequently, luciferase reporter assay was performed to verify whether miR-29a-3p directly targeted RPS15A. The results demonstrated that only miR-29a-3p mimic and pmirGLO-RPS15A 3′-UTR-WT co-transfection resulted in a significant decrease of the luciferase activity compared to the other controls in DLD-1 (), p < 0.01) and SW480 cells (), p < 0.05). Western blot analysis further confirmed that RPS15A protein content was obviously reduced in DLD-1 and SW480 cells with miR-29a-3p mimic transfection which suggested that miR-29a-3p inhibited RPS15A protein expression in CRC cells ()). In addition, we found the protein expression of RPS15A was obviously elevated in all the four CRC cell lines (DLD-1, RKO, SW480, and HCT116 cells), in comparison with the normal colon epithelial cells FHC. These results indicated that miR-29a-3p could be a direct negative regulator of RPS15A in CRC cells.
Figure 3. MiR-29a-3p targets RPS15A in CRC cells.
(a) A diagram for the RPS15A 3′-UTR fragment containing the WT or MUT miR-29a-3p binding site was displayed. We constructed pmirGLO-RPS15A-3′UTR-WT and pmirGLO-RPS15A-3′UTR-MUT plasmids to perform luciferase reporter assay. Luciferase reporter assay indicated that miR-29a-3p could directly bind to RPS15A 3′UTR in (b) DLD-1 and (c) SW480 cells. *p < 0.05, **p < 0.01 vs. miR-NC; (d) Western blot results suggested that miR-29a-3p overexpression decreased RPS15A protein level in DLD-1 and SW480 cell lines. (e) Western blot analysis of RPS15A protein level in four CRC cell lines (DLD-1, RKO, SW480 and HCT116 cells) and the normal colon epithelial cells FHC
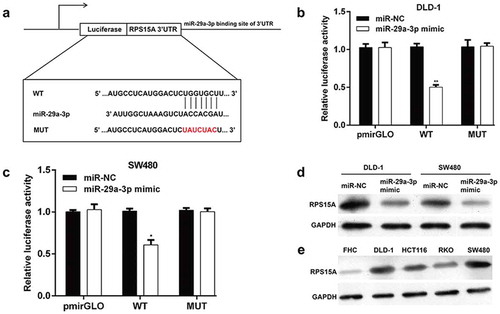
Restoration of RPS15A expression abolished the antitumor effects of miR-29a-3p
To further elucidate whether RPS15A was required for miR-29a-3p-mediated effects on CRC cells, rescue experiments were performed by constructing an overexpression plasmid of RPS15A (pcDNA3.1-RPS15A) to transfect into DLD-1 cells treated with miR-29a-3p mimic. As shown in ), the expression of RPS15A protein was notably increased in DLD-1 cells expressing the RPS15A overexpression vector compared with cells transfected with sole miR-29a-3p mimic transfected. Furthermore, the inhibitory effects on cell proliferation caused by miR-29a-3p mimic were partly abolished by overexpressing RPS15A (), p < 0.01, p < 0.001). Consistently, restoration of RPS15A significantly reversed the effects of miR-29a-3p on cell cycle arrest (), p < 0.01, p < 0.001) and apoptosis (), p < 0.05, p < 0.01) in DLD-1 cells. Collectively, these data suggested that miR-29a-3p regulated cell proliferation, cell cycle progression, and apoptosis partially by targeting RPS15A in CRC.
Figure 4. RPS15A can rescue the phenotypic changes caused by miR-29a-3p in DLD-1 cells.
(a) RPS15A protein expression was measured by western blot in DLD-1 cells co-transfected with miR-29a-3p mimic/miR-NC and with RPS15A overexpression plasmid/empty vector. GAPDH was used as an internal control. (b-d) Cell proliferation, cell cycle distribution and apoptosis were determined in DLD-1 cells co-transfected with miR-29a-3p mimic/miR-NC and with RPS15A overexpression plasmid/empty vector. *p < 0.05, **p < 0.01, ***p < 0.001 vs. miR-29a-3p mimic + Vector
Restoration of RPS15A expression counteracted the effects of miR-29a-3p on cell cycle and apoptotic markers
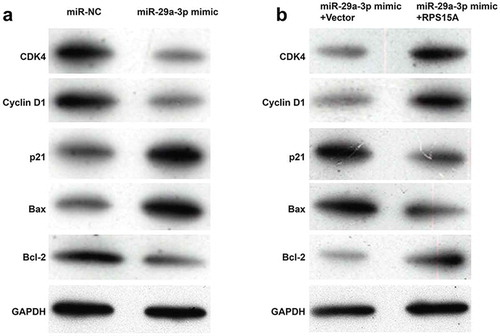
Using western blotting analysis, we further analyzed the expression alterations of some cell cycle regulators and apoptotic markers. As shown in ), the expression levels of CDK4 and Cyclin D1 were obviously decreased, while p21 was increased, associated with G0/G1-S transition in miR-29a-3p mimic-transfected group compared with miR-NC-transfected group in DLD-1 cells. Besides, pro-apoptotic Bax was up-regulated and anti-apoptotic Bcl-2 was down-regulated in DLD-1 cells after miR-29a-3p overexpression. Consistent with the effects of RPS15A on cell cycle and apoptosis, restoration of RPS15A reversed the effects of miR-29a-3p overexpression on the above cell cycle and apoptotic markers ()). These data confirm that RPS15A is a downstream effector of miR-29a-3p in CRC cells.
Figure 5. RPS15A counteracted the effects of miR-29a-3p on cell cycle and apoptotic markers.
(a) The cell cycle and apoptotic markers were detected in DLD-1 cells transfected with miR-29a-3p mimic or miR-NC using western blotting. (b) The cell cycle and apoptotic markers were detected in DLD-1 cells co-transfected with miR-29a-3p mimic/miR-NC and with RPS15A overexpression plasmid/empty vector using western blotting. GAPDH was used as an internal control.
Discussion
In the present study, we showed miR-29a-3p was significantly down-regulated in 10 pairs of CRC tissue samples in comparison to matched adjacent tissues, which was consistent with a previous paper [Citation11]. The similar expression profile was also observed in four malignant CRC cell lines compared with FHC normal colonic epithelial cell line. Similarly, several literatures presented miR-29a-3p to be remarkably down-regulated in hepatocellular carcinoma [Citation10], gastric cancer [Citation9] and laryngocarcinoma.
As the role of miR-29a-3p in malignant CRC in detail remains rarely elucidated, we aimed to carefully determine whether miR-29a-3p exerts effects on growth and apoptosis of CRC cells. Here, miR-29a-3p overexpressed DLD-1 and SW480 cells showed strongly decreased proliferation, increased G0/G1 arrest and apoptosis. In the last two decades, miRNAs have emerged as critical post-transcriptional modulators of gene expression exerting their function via binding to “seedless” miRNA recognition elements typically located in the 3ʹUTR of targeted mRNAs [Citation20]. In bioinformatics efforts, RPS15A was suggested to interact with miR-29a-3p, the binding of RPS15A “UGGUGCUU” with miR-29A-3P “ACCACGAU” was confirmed by luciferase reporter assay. We also demonstrated the role of miR-29a-3p as a negative regulator of RPS15A in DLD-1 and SW480 cells. The overexpressed expression of RPS15A accompanies with increased growth and reduced apoptosis of DLD-1 cells co-transfection with miR-29a-3p mimic and RPS15A expression vector. These results suggesting that miR-29a-3p exerts anti-oncogenic effects in CRC cells through directly targeting RPS15A.
In fact, we have preliminary data indicated that miR-29a-3p/RPS15A axis regulates expression of genes closely related to cell cycle progression and apoptosis. It is known that complex between D type Cyclins and CDK4 are essential components of the G1-S transition [Citation21]. Positive regulation of CDK4 and Cyclin D1, as well as negative regulation of the CDK inhibitor p21, could guarantee that G0/G1 arrest was not easily evaded. Activation of the mitochondrial pathway to apoptosis is known to be repressed by Bcl-2, but can be promoted by Bax [Citation22,Citation23]. Here, our results showed that restoration of RPS15A reversed the effects of miR-29a-3p overexpression on the cell cycle G1-S transition and apoptotic markers, which further confirmed RPS15A is a direct target of miR-29a-3p in CRC cells.
It is well established that ribosome plays an indispensable role in protein synthesis in all cells [Citation24]. RPS15A is identified as a component of the 40S ribosomal subunit [Citation17]. It serves to facilitate mRNA/ribosome interactions in translation and interacts with eukaryotic initiation factor 4F to promote translation in yeast [Citation25]. It is worth noting that RPS15A has been shown to trigger proliferation and colony formation in CRC HCT116 and DLD-1 cells through dysregulation of p53 signaling pathway [Citation18]. Furthermore, recent studies also suggest its pathological involvement in malignant transformation of gastric cancer and glioblastoma through activating AKT signaling pathway [Citation26,Citation27]. Here, our rescue experiments further demonstrated that RPS15A might be a functional regulator in miR-29a-3p inhibiting the cell proliferation.
In conclusion, we have confirmed the role of miR-29a-3p as a negative regulator of RPS15A in DLD-1 and SW480 cells. Impact of miR-29a-3p/RPS15A axis on growth and apoptosis of CRC cells are associated with abnormal protein levels of CDK4, Cyclin D1, p21, Bax and Bcl-2. We suggest that recovery of miR-29a-3p expressions may have therapeutic effects in CRC.
Authors’ contributions
YWL is responsible for experimental overall design and providing necessary conditions. ZZL mainly performed the experiments and collected data. CHT and WY mainly analyzed the experiment data and wrote the paper. All authors read and approved this paper.
Acknowledgement
The study was supported by Zhangjiakou First Hospital (Hebei, China).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017 Apr;66(4):683–691. PubMed PMID: 26818619.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017 May 6;67(3):177–193. PubMed PMID: 28248415.
- Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, et al. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017 Jan 19;18(1). PubMed PMID: 28106826; PubMed Central PMCID: PMCPMC5297828.
- Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015 Jun 1;33(16):1809–1824. . PubMed PMID: 25918280.
- Riihimaki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016 Jul 15;6:29765. PubMed PMID: 27416752; PubMed Central PMCID: PMCPMC4945942.
- Gao L, Jiang F. MicroRNA (miRNA) profiling. Methods Mol Biol. 2016;1381:151–161. PubMed PMID: 26667459.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017 Mar;16(3):203–222. PubMed PMID: 28209991.
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015 Jun;15(6):321–333. . PubMed PMID: 25998712; PubMed Central PMCID: PMCPMC4859809.
- Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015 Mar 12;13:101. PubMed PMID: 25889078; PubMed Central PMCID: PMCPMC4363339. doi: 10.1186/s12957-015-0513-x.
- Wang X, Liu S, Cao L, et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget. 2017 Oct 17;8(49):86592–86603. PubMed PMID: 29156819; PubMed Central PMCID: PMC5689709.
- Kara M, Yumrutas O, Ozcan O, et al. Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma. Gene. 2015 Aug 1;567(1):81–86. PubMed PMID: 25925209.
- Chan YL, Olvera J, Paz V, et al. The primary structure of rat ribosomal protein S15a. Biochem Biophys Res Commun. 1994 May 16;200(3):1498–1504. PubMed PMID: 8185605.
- Zhang C, Fu J, Xue F, et al. Knockdown of ribosomal protein S15A induces human glioblastoma cell apoptosis. World J Surg Oncol. 2016 Apr 29;14:129. PubMed PMID: 27130037; PubMed Central PMCID: PMCPMC4850639.doi: 10.1186/s12957-016-0891-8.
- Li G, Zhang L, Liu J, et al. shRNA-mediated RPS15A silencing inhibits U937 acute myeloid leukemia cell proliferation and enhances apoptosis. Mol Med Rep. 2016 may;13(5):4400–4406. PubMed PMID: 27035327.
- Feng W, Liang C, Wang C, et al. Knockdown of ribosomal protein S15A inhibits proliferation of breast cancer cells through induction of apoptosis in vitro. Cytotechnology. 2018 Oct;70(5):1315–1323. PubMed PMID: 29802490; PubMed Central PMCID: PMCPMC6214850. doi: 10.1007/s10616-018-0221-9.
- Shi D, Liu J. RPS15a silencing suppresses cell proliferation and migration of gastric cancer. Yonsei Med J. 2018 Dec;59(10):1166–1173. PubMed PMID: 30450850; PubMed Central PMCID: PMCPMC6240561.
- Zhao X, Shen L, Feng Y, et al. Decreased expression of RPS15A suppresses proliferation of lung cancer cells. Tumour Biol. 2015 Sep;36(9):6733–6740. PubMed PMID: 25833696. doi: 10.1007/s13277-015-3371-9.
- Chen J, Wei Y, Feng Q, et al. Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int J Oncol. 2016 Apr;48(4):1628–1638. PubMed PMID: 26847263.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods. 2001 Dec;25(4):402–408. PubMed PMID: 11846609.
- Rouleau S, J-PS G, Brumwell A, et al. 3ʹ UTR G-quadruplexes regulate miRNA binding. RNA (New York, NY). 2017 Aug;23(8):1172–1179. PubMed PMID: 28473452.
- Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. PubMed PMID: 9338094.
- Khodapasand E, Jafarzadeh N, Farrokhi F, et al. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75. PubMed PMID: 25864810; PubMed Central PMCID: PMCPMC4412916.
- Renault TT, Floros KV, Elkholi R, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol Cell. 2015 Jan 8;57(1):69–82. PubMed PMID: 25482509; PubMed Central PMCID: PMCPMC4289414.
- Uechi T, Nakajima Y, Chakraborty A, et al. Deficiency of ribosomal protein S19 during early embryogenesis leads to reduction of erythrocytes in a zebrafish model of diamond-blackfan anemia. Hum Mol Genet. 2008 Oct 15;17(20):3204–3211. PubMed PMID: 18653748.
- Xu M, Wang Y, Chen L, et al. Down-regulation of ribosomal protein S15A mRNA with a short hairpin RNA inhibits human hepatic cancer cell growth in vitro. Gene. 2014 Feb 15;536(1):84–89. PubMed PMID: 24334120.
- Liu C, He X, Liu X, et al. RPS15A promotes gastric cancer progression via activation of the Akt/IKK-beta/NF-kappaB signalling pathway. J Cell Mol Med. 2019 Mar;23(3):2207–2218. PubMed PMID: 30661291; PubMed Central PMCID: PMCPMC6378197.
- Yao Y, Liu Y, Lv X, et al. Down-regulation of ribosomal protein S15A inhibits proliferation of human glioblastoma cells in vivo and in vitro via AKT pathway. Tumour Biol. 2016 Apr;37(4):4979–4990. PubMed PMID: 26537582. doi: 10.1007/s13277-015-4323-0.
