ABSTRACT
Royal jelly (RJ) is used as a dietary supplement for human health promotion. Recently, a clinical trial has reported that RJ improved mental health. The present study was conducted to experimentally support the clinical effect of RJ on mental health and to further elucidate the mechanisms of action of RJ. RJ and an ethanol extract of RJ, which contains fatty acids but not proteins, inhibited an unpredictable chronic mild stress (UCMS)-induced increase in immobility time, a depression-like behavior, in the tail suspension test. DNA microarray analysis of the adrenal grand revealed that the expression of genes involved in cholesterol metabolism was up-regulated in response to UCMS exposure and that RJ suppressed expression of genes related to cholesterol synthesis and transport. These results suggested that RJ improves stress-induced depression-like behavior by regulating adrenal steroidogenesis and that fatty acids contained in RJ partly contribute to the antidepressant effect of RJ.
Graphical abstract
RJ inhibited an unpredictable chronic mild stress (UCMS)-induced increase in immobility time, a depression-like behavior, in the tail suspension test.
Royal jelly (RJ) is secreted from the hypopharyngeal and mandibular glands of worker honey bees to develop queen honey bees (Apis mellifera) and contains proteins, sugars, lipids, vitamins, minerals, amino acids, and other component [Citation1]. RJ has various bioactivities such as anti-oxidant [Citation2], anti-inflammatory [Citation3], anti-aging [Citation4,Citation5], lifespan extension [Citation6], and neurogenesis [Citation7] activities, and thus, it has been widely consumed as a nutritional supplement.
Recently, a randomized, double-blind, placebo-controlled trial of RJ was conducted in healthy elderly volunteers [Citation8]. Interestingly, RJ potentiated the vitality and mental health using the SF-36v2TM test, which is a general health status questionnaire of the Medical Outcomes Study 36-Item Short-form Health Survey (SF-36) [Citation9,Citation10], in this clinical test. Previous study have reported that oral administration of RJ suppresses elevated blood levels of corticosterone associated with stress in rats [Citation11] and that intraperitoneal administration of RJ or 10-hydroxy-2-decenoic acid (10-HDA), a fatty acid unique to RJ, improves stress-induced depressive symptoms in mice [Citation12]. These experimental findings appear to support the effects of RJ on human mental health in the clinical trial.
With regard to the physiological stress response, exposure to stress activates the hypothalamic–pituitary–adrenal (HPA) axis in the form of endocrine responses in adaptation to and recovery from stress. The corticotropin-releasing factor (CRF) is secreted from the parvocellular neurons of the hypothalamic paraventricular nucleus and activates the synthesis of pro-opiomelanocortin (POMC), a precursor of adrenocorticotropin hormone (ACTH), in the anterior pituitary. ACTH stimulates the synthesis and secretion of glucocorticoids (i.e., cortisol in humans and corticosterone in rodents) from the adrenal cortex [Citation13]. The secreted glucocorticoids are well known to exert negative feedback regulation of the HPA axis loop on the hypothalamus and pituitary, and excessive activation of the HPA axis has been considered to be one of the causes of depression [Citation14–Citation17]; for example, Cushing’s syndrome, which is caused by excessive ACTH secretion or by autonomous release of cortisol, is often associated with depression as a mental disorder [Citation18], and excessive activation of the HPA axis has been observed in approximately half of patients with depression [Citation19].
Forced swimming and tail suspension tests have been widely used to evaluate depression-like behaviors in experimental animals. However, these animal experiments have clinical problem that antidepressant is effective not only for chronic administration but also for acute administration. The “unpredictable chronic mild stress (UCMS)” rodent model of depression has been widely used for investigating the pathophysiology of depression [Citation20–Citation22]. Chronic, but not acute, treatment with antidepressants reversed the UCMS-induced depression-like behavior in rodents, indicating that the UCMS model of depression may reflect the pathology of depression in humans [Citation23].
The purpose of the current study was to examine the effect of RJ on the stress-induced depression-like behavior and its possible mechanisms involved in the HPA axis response to stress in the UCMS study of mice to test the clinical effect of RJ on mental health. Furthermore, the identity of the RJ component which contributed to the antidepressant-like effect was also investigated.
Materials and methods
Animals
Six-week-old male BALB/c mice (Nihon SLC, Hamamatsu, Japan) were used. The animals were housed at 23°C ± 2°C and 55% ± 10% humidity with a 12-h light/dark cycle (lights on at 8:00 AM). Food and water were available ad libitum. All animal experiments were approved by the Animal Care and Use Committee of Research Laboratories of API Co., Ltd. (experiment number #42-004, 012, #43-007).
RJ and ethanol extract of RJ (EERJ)
Raw RJ was purchased from Anhui, Zhejiang and Jiangsu Provinces, the People’s Republic of China and was lyophilized for use in animal experiments. The lyophilized RJ contains approximately 5% 10-hydroxy-2-decenoic acid (10-HDA) and approximately 40% protein. In addition, a sample of the lyophilized RJ was extracted with approximately 6 times the volume of 99% ethanol at room temperature overnight to obtain a fraction of fatty acids including 10-HDA (hereafter referred to as ethanol extract of RJ, EERJ). After filtration and addition of saccharides as an excipient, the extract was concentrated by heating at 60°C followed by lyophilization. The concentrations of 10-HDA and proteins in the EERJ were >10% and <5%, respectively.
Administration of RJ and EERJ
RJ and EERJ was each administered independently as part of a mixed food diet throughout the UCMS exposure period. Lyophilized powders of RJ and EERJ were added to the standard rodent diet CE-2 (CLEA Japan, Tokyo, Japan) at concentrations of 4% and 2%, respectively, so that they included almost equal amounts of 10-HDA. The daily intake amounts of RJ and EERJ were approximately 4.5 g/kg and 2.4 g/kg, respectively.
Unpredictable chronic mild stress (UCMS) procedure
Animals were randomly allocated into 1 of the 3 groups (i.e., non-UCMS, UCMS, and UCMC+ RJ or EERJ). The UCMS procedure was performed for approximately 3 weeks (22 days), according to previously described methods [Citation20–Citation22] with minor modifications. Briefly, the animals were exposed to the UCMS procedure, which included exposure to various stressors, namely, wet bedding, no bedding, tilted cage, the light/dark cycle inversion (light off at 8.00 AM), illumination during dark phase, food deprivation, water deprivation, restraint for 1 h, and forced swimming (at 23°C), each for 6 min. These stressors were applied in a random sequence every week to prevent animals from adapting to the stressors. The non-UCMS group (non-UCMS animals) was housed without receiving UCMS and was fed the CE-2 diet. On day 21, the tail suspension test was performed, and on the next day (day 22), mice were sacrificed to obtain blood, pituitary gland, and adrenal gland samples. Serum was separated by centrifugation at 3,000 × g for 10 min. Serum and tissue samples were stored at −80°C until assayed.
Tail suspension test
The tail suspension test was performed using a previously described method [Citation24]. Briefly, mice were individually suspended by the tail for 6 min, and immobility times were recorded manually using a stopwatch to evaluate depression-like behavior.
Locomotor activity
On day 21, mice were placed individually in a transparent acrylic cage (31.5 × 21.0 × 13.5 cm) to measure locomotor activity for 60 min using digital counters with infrared sensors (BrainScience Idea, Osaka, Japan).
Measurement of serum corticosterone concentrations
Serum corticosterone concentrations were measured using the corticosterone enzyme immunoassay EIA Kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
Gene expression microarrays
Total RNA was extracted from the adrenal gland and was purified using the RNeasy micro kit (Qiagen, Hilden, Germany). DNA microarray analysis was performed at the genetic testing laboratory Cell Innovator Co. Ltd. (Fukuoka, Japan). cRNA was amplified, labeled from 50 ng of total RNA samples with Agilent Low-Input Quick Amp Labeling Kit and hybridized to Agilent 60-mer oligo microarray SurePrint G3 Mouse GE 8x60K Microarray slides (Agilent Technologies Japan, Ltd., Tokyo, Japan) according to the manufacturer’s instructions. All microarray slides were scanned by the Agilent microarray scanner. Relative hybridization intensities and background hybridization values were calculated using the Agilent Feature Extraction Software (10.7.1.1).
Absolute signal intensities and flags for each probe were calculated from the hybridization intensities (gProcessedSignal) and spot information (gIsSaturated, etc.) according to the procedures recommended by Agilent (Flag criteria on GeneSpring Software) as follows: Absent (A): “Feature is not positive and significant” and “Feature is not above background;” Marginal (M): “Feature is not uniform,” “Feature is saturated,” and “Feature is a population outlier;” and Present (P): others. The absolute signal intensities of 9 samples were log2-transformed and normalized by quantile algorithms with the “preprocessCore” library package [Citation25] on the Bioconductor software [Citation26]. The criteria used to find differences in gene expression were the detection call of “P” in at least 3 samples, a fold change of >1.5 (ratio of ≥1.5 ≤ 0.667), limma p-value of <0.05 and signal value beyond background. The differentially expressed genes were classified into functional categories using the database for annotation, visualization, and integrated discovery, which is a freely available bioinformatics resource at the web-site https://david.ncifcrf.gov/. The relative mRNA expression level was determined by quantitative reverse transcriptase-polymerase chain reactions.
Quantitative RT-PCR
Total RNA was isolated from the pituitary gland using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified by precipitation with lithium chloride. The relative levels of mRNAs were determined by the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), ABI 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). First-strand cDNA was synthesized from total RNA of each sample using the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen, Carlsbad, CA, USA). Gene-specific primers were designed with Primer-BLAST at the web-site https://www.ncbi.nlm.nih.gov/tools/primer-blast/, wherein the sequences were as follows: 5′-AAGCCGGTGGGCAAGAAA-3′ and 5′-GCCGACTCGTTCTCAGCAA-3′ for proopiomelanocortin (POMC) mRNA (NCBI Refseq accession no. NM_001278581.1), 5′-CAACATGCCTGCCGTCAAAG-3′ and 5′-CTGGCAGTTGGGAAGGTTCT-3′ for farnesyl-diphosphate farnesyl transferase 1 (Fdft1) mRNA (NM_010191), 5′-ACTGTGGGCTATGAAGAAGGA-3′ and 5′-TCGTCTCACTCCACTCAACAC-3′ for StAR-related lipid transfer (START) domain containing 4 (StarD4) mRNA (NM_133774), and 5′-GGCTCCTAGCACCATGAAGA-3′ and 5′-AGCTCAGTAACAGTCCGCC-3′ for β-actin (Actb) mRNA (NM_007393.3). The relative mRNA expression level was normalized to Actb mRNA expression.
Statistical analysis
Data were expressed as the mean ± SEM. Statistical differences between the two groups, UCMS-exposed and UCMS-exposed, RJ-fed groups, were analyzed using Student’s t-test or Welch’s t-test. As a reference, differences between UCMS-exposed and non-UCMS-exposed groups were also statistically compared in order to evaluate stress responses to UCMS exposure. All analyzes were performed using the Microsoft Excel-Toukei 2012 software (Social Survey Research Information, Tokyo, Japan). A p-value of <0.05 was considered to be statistically significant.
Results
Changes in body weight on UCMS exposure
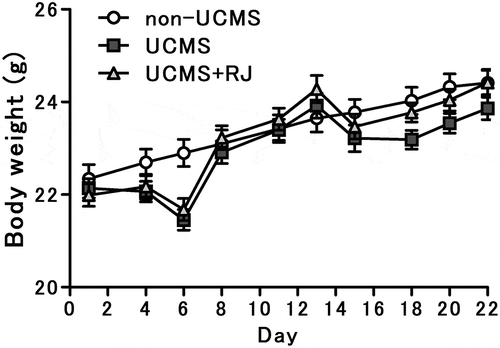
Changes in body weight during the experimental UCMS exposure period are shown in . There was no significant difference in body weight between the two groups, UCMS-exposed and UCMS-exposed, RJ-fed groups, during the 22-day experimental period, despite significant loss in body weight between UCMS-exposed and non-UCMS-exposed groups being found at the start of UCMS exposure on day 6.
Figure 1. Changes in body weight during exposure to unpredictable chronic mild stress (UCMS).
Data represent the means ± standard error of the mean (SEM) (non-UCMS: n = 20, UCMS: n = 20, UCMS+RJ: n = 20). A significant decrease in body weight was found between UCMS-exposed and non-UCMS-exposed groups at the start of UCMS exposure on day 6.
Effect of RJ on UCMS-induced depression-like behaviors
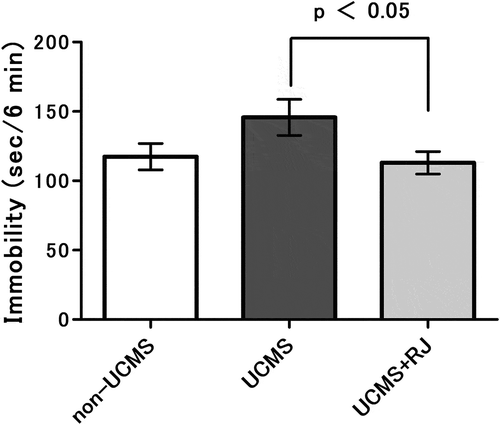
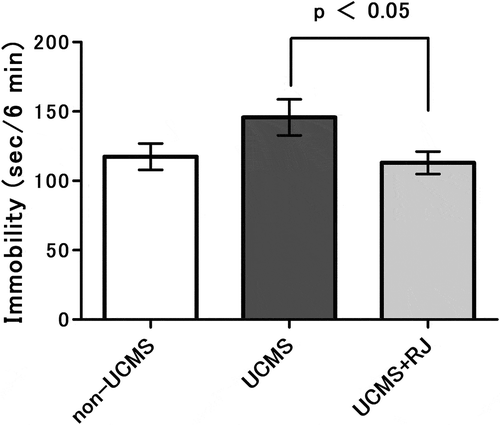
Immobility time was measured as a depression-like behavior using the tail suspension test after UCMS exposure for 3 weeks. As shown in , immobility time was obviously increased by UCMS exposure (UCMS vs. non-UCMS) and was significantly decreased in RJ diet-fed animals (UCMS vs. UCMS+RJ). In contrast, there was no clear difference in immobility time between animals with and without RJ diet under non-UCMS conditions (data not shown).
Figure 2. Effect of Royal jelly (RJ) on unpredictable chronic mild stress (UCMS)-induced increases in immobility times in the tail suspension test.
The test was performed one day after completion of the 3-week UCMS exposure. Data represent the means ± standard error of the mean (SEM) (non-UCMS: n = 17, UCMS: n = 19, UCMS + RJ: n = 18). p < 0.05 UCMS vs. UCMS+RJ by Welch’s t-test, and as a reference, P = 0.094 UCMS vs. non-UCMS by Student’s t-test.
In addition, no significant differences were found between the 3 groups in terms of locomotor activity (counts/60 min: non-UCMS group: 1697.9 ± 34.2; UCMS group: 1706.3 ± 25.3; UCMS + RJ group: 1744.0 ± 20.2). These results suggest that the immobility time was largely unaffected by motor function in these groups.
Effect of RJ on the HPA axis response to UCMS
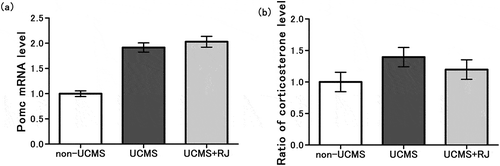
We measured Pomc mRNA level in the pituitary gland, corticosterone level in the serum, and Crf mRNA level in the hypothalamus to examine the effect of RJ on the HPA axis response to the UCMS exposure. As shown in ), a marked increase in the Pomc mRNA level of the pituitary gland was observed in the UCMS-exposed group relative to the non-UCMS group, and there was no significant difference between the standard diet-fed group (UCMS) and RJ diet-fed group (UCMS+RJ). No clear differences were observed between the 3 groups (non-UCMS, UCMS and UCMS + RJ groups) with respect to the Crf mRNA level in the hypothalamus (data not shown). As for corticosterone production in the HPA axis response to stress, serum corticosterone concentrations were modestly increased by exposure to UCMS, and the increase was lower, although not significantly (p = 0.370), in the RJ-fed group than in the standard diet fed group ()).
Figure 3. Effect of Royal jelly (RJ) on the HPA axis response to unpredictable chronic mild stress (UCMS) exposure.
(a) Expression level of Pomc mRNA in the pituitary gland were expressed relative to the mean value of the non-UCMS group. Data represent the means ± standard error of the mean (SEM) (non-UCMS: n = 10, UCMS: n = 9, UCMS + RJ: n = 10). As a reference, p < 0.01 UCMS vs. non-UCMS by Student’s t-test.(b) Serum corticosterone level were expressed relative to the mean value of the non-UCMS group. Data represent the means ± SEM (non-UCMS: n = 20, UCMS: n = 20, UCMS + RJ: n = 20). P = 0.370 UCMS vs. UCMS+RJ by Student’s t-test, and as a reference, p = 0.077 UCMS vs. non-UCMS by Student’s t-test.
Effects of RJ on gene expression in the adrenal gland
We performed DNA microarray analysis to assess global gene expression profiles of the adrenal gland after exposure to UCMS. First, 3 representative animals from each experimental group were used for microarray analysis. Genes with significantly different expressions (p-value < 0.05, ratio ≥ 1.5 or ratio ≤ 0.667) were analyzed using the DAVID functional annotation tool to identify gene functional categories. presents the top 5 Gene Ontology (GO) functional categories in order of p values, which were obtained using data from the non-UCMS and the UCMS-exposed groups. Expression of genes associated with the cholesterol biosynthetic process and sterol biosynthetic process were affected by exposure to UCMS.
Table 1. Gene Ontology (GO) functional category of genes differentially expressed in the adrenal gland following exposure to unpredictable chronic mild stress (UCMS).
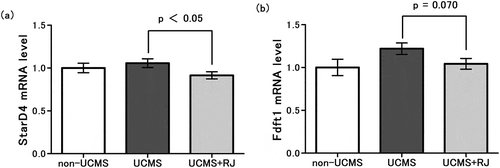
From the results of DNA microarray analysis, the START domain containing 4 (StarD4) gene, which encodes a protein that transports cholesterol to the inner mitochondrial membrane [Citation27], and farnesyl-diphosphate farnesyltransferase 1 (Fdft1) gene, which encodes the first specific enzyme in cholesterol biosynthesis [Citation28], were selected for further analysis. In the RJ-fed mice, the StarD4 mRNA level was found to decrease slightly but significantly ()), whereas the Fdft1 mRNA level also decreased but not significantly ()) compared with those of the standard diet-fed mice under the UCMS conditions.
Figure 4. Changes in gene expression related to cholesterol metabolism in the adrenal grand.
Expression level of StarD4 mRNA (a) and Fdft1 mRNA (b) were expressed relative to the mean value of the non-UCMS group. Data represent the means ± standard error of the mean (SEM) (n = 10). The level of mRNA expression was normalized to that of the housekeeping gene β-actin. (a) p < 0.05 UCMS vs. UCMS+RJ by Student’s t-test; (b) p = 0.070 UCMS vs. UCMS+RJ by Student’s t-test, and as a reference, p = 0.078 UCMS vs. non-UCMS by Student’s t-test.
Effect of ethanol extract of RJ (EERJ) on UCMS-induced depression-like behavior
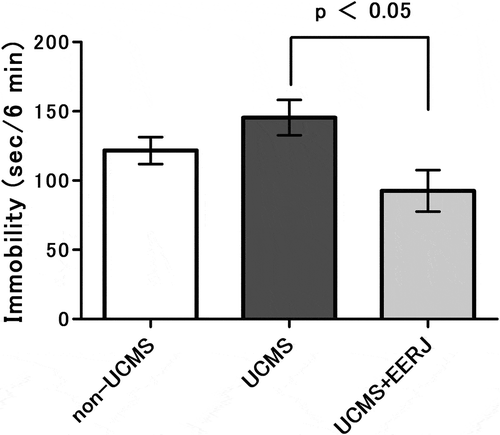
RJ contains several fatty acids including 10-HDA, a medium-chain fatty acid, unique to RJ. To clarify whether the fatty acid components contributed to the relieving effect of RJ on the UCMS-induced depression-like behavior, we evaluated the effect of EERJ using the same UCMS-experimental protocol as that for RJ, that is, a tail suspension test after UCMS exposure for 3 weeks. The EERJ diet-fed mice exhibited a significant decrease in immobility time compared with the standard diet-fed mice under the UCMS conditions ().
Figure 5. Effects of ethanol extract of Royal jelly (RJ) (EERJ) on unpredictable chronic mild stress (UCMS)-induced increases in immobility time in the tail suspension test.
The test was performed one day after completion of the 3-week UCMS exposure. Data represent the means ± standard error of the mean (SEM) (non-UCMS: n = 17, UCMS: n = 19, UCMS + EERJ: n = 17). p < 0.05 vs. UCMS UCMS+EERJ by Student’s t-test, and as a reference, p = 0.15 UCMS vs. non-UCMS by Student’s t-test.
Discussion
As described previously, the effect of RJ on mental health was reported after long-term (6 months) intake in healthy elderly individuals [Citation8]. The clinical trial was performed in healthy persons, rather than in melancholic patients; health foods are obviously less potent than antidepressant drugs on depression symptoms. Thus, we need to establish an experimental method by which mild effects of RJ can be evaluated and/or the effects of RJ on moderate depressive-like behavior can be modulated in animals. In this study, we used the UCMS method to induce a depressive state in rodents, corresponding to mild depression in humans, and demonstrated that UCMS-exposed mice showed an evident, although statistically not significant, increase in immobility time, a severity marker of depression-like behavior, compared with non-UCMS-exposed mice. Under these UCMS conditions, mice fed with RJ did not exhibit such an increase at all. These findings suggest that RJ suppresses the pathogenesis of depression, supporting the reported clinical effects on mental health of RJ.
Second, we elucidated the mechanisms underlying the effect of RJ on depression-like behavior in the UCMS study to support the clinical use of RJ for human mental health. We examined whether RJ acts on the regulation of the HPA axis by comparing changes in gene expression levels between RJ-fed and non-RJ-fed mice under the UCMS conditions. A marked increase in Pomc mRNA level of the pituitary gland, but not in Crf mRNA level of the hypothalamus (data not shown), was observed in the UCMS-exposed groups; no significant effect of RJ was detected on either Crf mRNA or Pomc mRNA levels, suggesting that RJ may not be involved in the response of these stress hormones to the UCMS used.
Exposure to stress causes CRF secretion, which, in turn, activates the synthesis of POMC, a precursor of ACTH, in the anterior pituitary gland. ACTH finally stimulates steroid neosynthesis in the adrenal gland [Citation13]. Cholesterol, a starting material for steroid synthesis, is transported to mitochondria, where cholesterol is converted to pregnenolone, a precursor of all steroid hormones, including corticosterone, by the cholesterol side-chain cleavage enzyme, P450scc (CYP11A1) [Citation29]. In general, steroid hormones are biosynthesized from cholesterol in steroidogenic tissues, such as those of the adrenal glands, and regulate various physiological functions [Citation29–Citation31]. Interestingly, as revealed by microarray analysis, the RJ diet decreased the expression of genes involved in important components of cholesterol homeostasis, namely, the cholesterol transport-related gene, the START domain containing 4 (StarD4) gene, and the cholesterol synthesis-related gene, farnesyl-diphosphate farnesyltransferase 1 (Fdft1) mRNA, in the adrenal gland. In particular, the change in StarD4 mRNA was significant (p < 0.05). The delivery of cholesterol to mitochondria is mainly mediated by StarD4 [Citation27], and the concentration of cholesterol in the adrenal gland temporarily decreases when blood corticosterone concentrations, an output of the stress-induced HPA axis activation, increase [Citation32]. Thus, RJ may counteract the UCMS-induced depression by suppressing the production of the steroid hormone corticosterone in mitochondria of the adrenal gland because the transport of cholesterol to mitochondria is decreased by treatment with RJ. However, in fact, serum corticosterone levels were modestly increased by UCMS exposure, and this increase was slightly, but not significantly, inhibited by treatment with RJ. It was recently reported that RJ suppressed the elevation in blood corticosterone levels in rats following exposure to restraint and cold stress for 7 days [Citation11]. However, in some cases, an elevation in the blood corticosterone concentration has not been observed in UCMS-exposed animals [Citation33]. Thus, it is likely that blood corticosterone levels in response to UCMS are affected by the period of and/or timing of the stress exposure, which could explain the variable change in the serum corticosterone levels in the current study. Currently, the suppression of de novo biosynthesis of corticosterone in the adrenal gland is thought to be a part of the mechanism of the effects of RJ on the UCMS-induced depression-like behavior.
Ito et al. reported that intraperitoneal administration of RJ or 10-HDA ameliorates depression-like behaviors induced by chronic mild stress [Citation12]. In the present study, we found that oral administration of EERJ, an ethanol extract of RJ, did not cause an increase in immobility time in the UCMS experiment. EERJ contains 10-HDA and various fatty acids such as 10-hydroxy decanoic acid and sebacic acid. Therefore, it is suggested that fatty acids unique to RJ, especially 10-HDA, contribute to the anti-depressive effect of RJ in UCMS-exposed animals.
Furthermore, elevations in the glucocorticoid levels in response to stress may damage the hippocampal neuron [Citation34]. Sustained administration of corticosterone reduces neurogenesis in the hippocampus and causes depression-like behaviors in rodents [Citation35]. Moreover, chronic antidepressant treatment increases neurogenesis in the hippocampus of adult rat [Citation36]. Our previous studies have shown that 10-HDA promotes neurogenesis from neural stem/progenitor cells in in vitro studies [Citation7]. Therefore, RJ is thought to act on the corticosterone synthesis in the adrenal gland and on hippocampal neuronal degeneration in UCMS-exposed animals, which may be involved in potentiation of human mental health.
In conclusion, RJ reduced depression-like behavior induced by UCMS in mice, at least in part, through the suppression of biosynthesis of corticosterone in the adrenal gland. The present study may allow us to understand the positive effects of RJ on mental health in human.
Author contributions
K. Ichihara and Y. Hirata designed studies. N. Iegaki, N. Hattori and Y. Narita performed experiments and analyzed data. N. Iegaki wrote the manuscript under the guidance of K. Ichihara, and all authors have agreed with its submission to the journal.
Acknowledgments
A part of this work was supported by MEXT (Ministry of Education, Culture, Sports, Science and Science and Technology) for a project entitled, “The Initiative for the implementation of diversity research environment (Collaboration Type).”
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Takenaka T. Chemical composition of royal jelly. Honeybee Sci. 1982;3:69–74.
- Nakajima Y, Tsuruma K, Shimazawa M, et al. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9:4–10.
- Kohno K, Okamoto I, Sano O, et al. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem. 2004;68(1):138–145.
- Park HM, Cho MH, Cho Y, et al. Royal jelly increases collagen production in rat skin after ovariectomy. J Med Food. 2012;15(6):568–575.
- Narita Y, Ohta S, Suzuki KM, et al. Effects of long-term administration of royal jelly on pituitary weight and gene expression in middle-aged female rats. Biosci Biotechnol Biochem. 2009;73(2):431–433.
- Honda Y, Fujita Y, Maruyama H, et al. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS One. 2011;6(8):e23527.
- Hattori N, Nomoto H, Fukumitsu H, et al. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res. 2007;28(5):261–266.
- Morita H, Ikeda T, Kajita K, et al. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr J. 2012;11:77.
- Fukuhara S, Bito S, Green J, et al. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51(11):1037–1044.
- Fukuhara S, Ware JE Jr, Kosinski M, et al. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1045–1053.
- Teixeira RR, de Souza AV, Peixoto LG, et al. Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neurosci Lett. 2017;655:179–185.
- Ito S, Nitta Y, Fukumitsu H, et al. Antidepressant-like activity of 10-hydroxy-trans-2-decenoic acid, a unique unsaturated fatty acid of royal jelly, in stress-inducible depression-like mouse model. Evid Based Complement Alternat Med. 2012;2012:139140.
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475.
- Belanoff JK, Kalehzan M, Sund B, et al. Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry. 2001;158(10):1612–1616.
- Keller J, Flores B, Gomez RG, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60(3):275–281.
- Posener JA, DeBattista C, Williams GH, et al. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry. 2000;57(8):755–760.
- Arana GW, Baldessarini RJ, Ornsteen M. The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Commentary and Review Arch Gen Psychiatry. 1985;42(12):1193–1204.
- Murphy BE, Dhar V, Ghadirian AM, et al. Response to steroid suppression in major depression resistant to antidepressant therapy. J Clin Psychopharmacol. 1991;11(2):121–126.
- Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25.
- Farley S, Apazoglou K, Witkin JM, et al. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int J Neuropsychopharmacol. 2010;13(9):1207–1218.
- Detanico BC, Piato AL, Freitas JJ, et al. Antidepressant-like effects of melatonin in the mouse chronic mild stress model. Eur J Pharmacol. 2009;607(1–3):121–125.
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175(1):43–50.
- Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):625–631.
- Miyamoto Y, Iegaki N, Fu K, et al. Striatal N-Acetylaspartate synthetase Shati/Nat8l regulates depression-like behaviors via mGluR3-mediated serotonergic suppression in mice. Int J Neuropsychopharmacol. 2017;20(12):1027–1035.
- Bolstad BM, Irizarry RA, Astrand M, et al. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193.
- Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80.
- Miller WL. StAR search–what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21(3):589–601.
- Trapani L, Segatto M, Ascenzi P, et al. Potential role of nonstatin cholesterol lowering agents. IUBMB Life. 2011;63(11):964–971.
- Hu J, Zhang Z, Shen WJ, et al. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47.
- Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165:18–37.
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46.
- Djordjević J, Cvijić G, Davidović V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol Res. 2003;52(1):67–72.
- Salomons AR, Kortleve T, Reinders NR, et al. Susceptibility of a potential animal model for pathological anxiety to chronic mild stress. Behav Brain Res. 2010;209(2):241–248.
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935.
- Sawamoto A, Okuyama S, Yamamoto K, et al. 3,5,6,7,8,3ʹ,4ʹ-Heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules. 2016;21(4):541.
- Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110.
