?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Kuji amber is fossilized tree resin of the Late Cretaceous in Japan. In this study, new biological activities of ethanol extract of Kuji amber (EtOH ext.) and supercritical carbon dioxide fluid extract of Kuji amber (scCO2 ext.) were examined. Both EtOH ext. and scCO2 ext. inhibited melanin production in B16 mouse melanoma cells and promoted collagen production in human skin fibroblast SF-TY cells. The scCO2 ext. had more potent activity than that of EtOH ext. and may depend on the efficiency of the extraction. The main new biologically active compound in Kuji amber, kujigamberol had no activities against melanin production, however, it promoted collagen production at low concentrations. A biologically active compound having a different structure, spirolactone norditerpenoid, showed both the inhibition activity against melanin production and the promotion activity of collagen production in a dose dependent manner. EtOH ext. and scCO2 ext., which include both kujigamberol and spirolactone norditerpenoid, have not only anti-allergy activity, but also inhibit melanin production and promote collagen production.
Graphical abstract
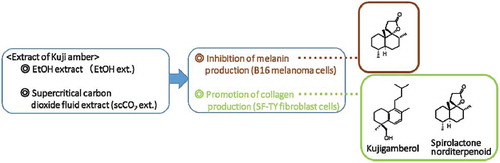
The extracts of Kuji amber had inhibition activity of melanin production and promotion activity of collagen activity, with almost the same activity as β-arbutin and L-ascorbic acid. Biologically active compounds were idetified as spirolactone norditerpenoid and kujigamberol.
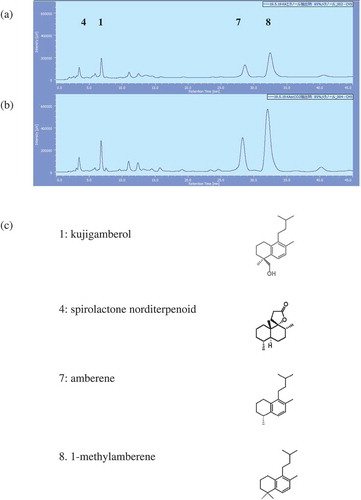
Amber is fossilized tree resin and consists of organic polymers derived through complex maturation processes of the original plant resin and has been used mainly for ornamental purposes. Kuji-city is located at the northern Pacific coast of Japan (141° E 40°N) and Kuji amber [90–86 million years ago (Late Cretaceous)] is buried in that region [Citation1]. Many kinds of medicines from modern plants such as Salix alba Linne [aspirin (modification of salicylic acid)], Ephedra sinica STAPF (ephedrine) and Catharanthus roseus (vinblastine) have already been developed. Our group has focused on the biological activity from methanol extracts of ambers (ancient plants) worldwide and isolated and identified many new compounds from Kuji amber by activity-guided fractionation using the mutant yeast, Saccharomyces cerevisiae (zds1Δ erg3Δ pdr1/3Δ), involving Ca2+-signal transduction [Citation2,Citation3]. They are kujigamberol [15,20-dinor-5,7,9-labdatrien-18-ol (1)] () [Citation1,Citation4,Citation5], kujiol A [13-methyl-8,11,13-podocarpatrien-19-ol (2)] [Citation1,Citation6], kujigamberol B [15,20-dinor-5,7,9-labdatrien-13-ol (3)] [Citation1,Citation6], spirolactone norditerpenoid [(4R*, 5S*, 8R*, 9R*, 10S*)-14,15,16,19-tetranor-labdan-13,9-olide (4)] [Citation1,Citation7], kujigamberol C [15-nor-8-labden-13-ol (5)] [Citation1,Citation8] and kujigamberoic acid A [15,20-dinor-5,7,9-labdatrien-18-oic acid (6)] [Citation1,Citation9]. Although they have no known biological activity, biomarkers of Cretaceous amber, amberene [15,19,20-trinor-5,7,9-labdatriene (or 1,6-dimethyl-5-isopentyltetralin) (7)] and 1-methylamberene [15,20-dinor-5,7,9-labdatriene (or 1,1,6-trimethyl-5-isopentyltetralin) (8)] were also isolated in greater quantity than the biologically active compounds and identified () [Citation1,Citation10]. The methanol extract of Kuji amber (MEKA) and kujigamberol have anti-allergy activity against rat basophilic leukemia-2H3 (RBL-2H3) cells and the rhinitis model of guinea pigs [Citation1,Citation11]. As the biological activities of MEKA are almost the same as those of kujigamberol, it suggested to us that MEKA include a variety of compounds with new structures and/or biological activities. In fact, kujigamberol has another biologically activity which interferes with the pro-inflammatory cytokine-induces expression of and N-glycan modifications to cell adhesion molecules in human umbilical vein endothelial cells (HUVEC) [Citation12].
Figure 1. HPLC profiles of the extracts of Kuji amber and structures of each peak.
(a) HPLC profile of the EtOH ext. of Kuji amber, (b) HPLC profile of the scCO2 ext. of Kuji amber.Injection: 50 μg, Flow rate: 1.0 ml/min, Solvent: 85% MeOH, Detector: 205 nm, Pump: PU-2080 (Nihon Bunko Co. Ltd.), Detector: PDA (MD-2018), Column: CAPCELL PAK C18(ODS 4.6 mmφ ×150 mm, TYPE UG 120 Å, 5 μm). (c) Structures of each peak.
Supercritical carbon dioxide (scCO2) has been used as a substitute for organic solvents such as hexane, ethanol and methanol, etc., for the extraction of biologically active compounds and has advantages that it is environmental friendly, nontoxic, nonflammable, and inexpensive [Citation13,Citation14]. In addition, it can be easily removed from the extract. Although this technology has been used in the food and/or cosmetic industries, it was applied to Kuji amber extraction as a substitute for methanol and/or ethanol for the first time.
In this study, we examined new biological activities involving cosmetics (melanin production in B16 mouse melanoma cells and the promotion of collagen production in human fibroblast SF-TY cells) of Kuji amber extracts, the ethanol extract of Kuji amber (EtOH ext.) and the supercritical carbon dioxide fluid extraction of Kuji amber (scCO2 ext.). Both extracts showed inhibition activity against melanin production and promotion activity for collagen production. We identified one of inhibitors against melanin production as spirolactone norditerenoid and identified two kinds of compounds that promote collagen production as kujigamberol and spirolactone norditerpenoid. These results suggested Kuji amber extracts are important not only for a medicine, but also for cosmetics.
Materials and methods
Kuji amber and preparation of the extract
Kuji amber powder (150–180 μm) was purchased from Kuji Kohaku Co. Ltd. (Kuji, Japan). Melanin was purchased from MP Biomedicals, LLC (CA, USA). β-arbutin was purchased from API Co. (Tokyo, Japan). Unless otherwise stated, chemicals used were of the best commercially available grade. Ethanol extractions of Kuji amber were performed as described in the literature [Citation4]. Briefly, three volumes of ethanol was added to Kuji amber powder and it was extracted for two days at room temperature. The extract was filtered by filter paper and followed by 0.45 μm filtration (Merck KGaA, Darmstadt, Germany). It was then evaporated and weighed.
Supercritical CO2 extractions of Kuji amber were performed as previously described [Citation13,Citation14]. Briefly, carbon dioxide was pumped into the extractor by a positive displacement controlled volume metering-pump of the Super Critical Fluid Extraction System (JASCO co., Tokyo, Japan) (The internal volume of extractor was 10 cm3). Extractions were performed at 80°C, 20 MPa using 3 mL CO2/min and after evaporation of CO2 at room temperature, the extracts were weighed. Both extracts were dissolved in ethanol at 10 mg/mL for HPLC analysis and biological studies with the melanoma cells, and in dimethyl sulfoxide (DMSO) at 10 mg/mL for biological studies using fibroblasts and for the biochemical analysis of tyrosinase.
HPLC analysis
Both extracts (50 μg) dissolved in EtOH were analyzed using HPLC. The HPLC conditions were as follows; solvent: MeOH:H2O (85:15), flow rate: 1 mL/min, column: Capcell Pak C18 column [4.6 mm i.d. × 150 mm (Shiseido, Tokyo, Japan)], equipment: PU-2080 HPLC pump and MD-2018 photo-diode array detector (JASCO). The quantification of each compound in EtOH ext. and scCO2 ext. was calculated from each standard curve [kujigamberol: 0.31 μg–5 μg (R2=0.9876), amberene: 0.31 μg-5 μg (R2=0.9777), 1-methylamberene: 0.63 μg-10 μg (R2=0.9582)] using HPLC (n=4).
Cell culture and cytotoxicity
Mouse skin melanoma cell line, B16 (JCRB0202) and human normal skin fibroblast cell line, SF-TY (JCRB0075) were obtained from the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN) JCRB Cell Bank (Osaka, Japan). The B16 melanoma cells were cultured in Eagle’s minimum essential medium (EMEM) supplemented with 10% (v/v) FBS [fetal bovine serum, Biological Industries, CT, USA] and antibiotics [penicillin (100 units/mL)-streptomycin (100 µg/mL) (FUJIFILM Wako Pure Chemical Co., Osaka, Japan)]. The SF-TY fibroblast cells were cultured in EMEM with 10% FBS, antibiotics and non-essential amino acids (FUJIFILM Wako Pure Chemical Co.).
Cell viability was determined using the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (Dojindo Lab., Kumamoto, Japan). The B16 melanoma cells were plated in triplicate at a concentration of 2.4 × 105 cells/well in a 6 well-plate. The SF-TY cells were in quintuplicate at a concentration of 1 × 104 cells/well in a 96-well plate. They were incubated overnight prior to treatment with various concentrations of the samples and incubated for an additional 72 h. To the B16 melanoma cells, 100 μM IBMX [3-isobutyl-1-methylxanthine (FUJIFILM Wako Pure Chemical Co.)] was added in order to stimulate melanin production, except for the non-simulated group cells.
After the treatment, cells were washed with phosphate buffered saline (PBS) and incubated for 2 h at 37°C in the media containing 0.5 mg/mL MTT. DMSO was added to dissolve the MTT formazan and the absorbance was measured at 545 nm with a microplate reader (CHROMATE MODEL 4300, Awareness Technology Inc., FL) [Citation15–Citation17]. The absorbance at 630 nm was subtracted from it as a background.
Microscopic observation
After the treatment with IBMX and samples, the B16 melanoma cells were observed by photomicrography, using an inverted microscope (AE2000, SHIMADZU RIKA Co., Tokyo, Japan) without phase contrast.
Determination of melanin content
After the observation, the B16 melanoma cells were washed with PBS and dissolved in 1 M NaOH at room temperature. The absorbance at 450 nm was measured and the absorbance at 630 nm subtracted as a blank. The melanin content was measured using an authentic standard of synthetic melanin [Citation15,Citation16,Citation18–Citation20].
Measurement of tyrosinase inhibition activity
The reaction mixture contained 0.03% L-dopa [L-3,4-dihydroxyphenylalanine (FUJIFILM Wako Pure Chemical Co.)], 135 units/mL of mushroom tyrosinase (Sigma-Aldrich Co. LLC, UK) and the samples dissolved in 50 mM phosphate buffer (pH 6.5) in equal amounts. As a positive control, 100 μM kojic acid [KA (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan)] was used. After incubation at 25°C for 10 min, dopachrome formation was monitored by measuring the absorbance at 450 nm [Citation15,Citation19,Citation20]. The IC50 of each extract was calculated from the sigmoid curve using the equation below.
A: The concentration whose inhibitory ratio is just higher than 50%.
B: The concentration whose inhibitory ratio is just lower than 50%.
C: The inhibitory ratio of B.
D: The inhibitory ratio of A.
Determination of collagen content
After adding the sample to each plate and incubated for 3 days, the SF-TY fibroblast cells were washed with PBS and dissolved in the cell lysis buffer (Atto Co. Ltd., Tokyo, Japan). Using part of the lysates, the amount of collagen in the cells was measured using a Sirius Red Total Collagen Detection Kit (Chondrex Inc., WA, USA). The lysates were also collected for measurement of the total protein content with BCA Protein Assay Kit (Takara Bio Inc., Shiga, Japan). The collagen content was expressed as the amount per total protein content [Citation21].
Isolation of kujigamberol and spirolactone norditerpenoid
Kujigamberol and spirolactone norditerpenoid was isolated from MEKA by the same method as described in the literature [Citation4,Citation6,Citation7].
Statistical analysis
Homoscedasticity of sample variances were confirmed using Hartley tests. Differences of data were assessed by one-way analysis of variance. If there were significant differences among the data, then they were assessed by Tukey’s test.
Results
Ethanol extract of Kuji amber (EtOH ext.) and supercritical carbon dioxide fluid extract of Kuji amber (scCO2 ext.)
HPLC analytical pattern of EtOH ext. and scCO2 ext. are shown in . Kujigamberol was found in EtOH ext. at 0.338 ± 0.017 μg/50 μg and in scCO2 ext. at 0.815 ± 0.031 μg/50 μg. Amberene was found in EtOH ext. at 0.564 ± 0.015 μg/50 μg and in scCO2 ext. at 2.29 ± 0.13 μg/50 μg. 1-Methylamberene was found in EtOH ext. at 1.870 ± 0.075 μg/50 μg and in scCO2 ext. at 6.80 ± 0.36 μg/50 μg. They showed a similar analytical pattern, however, the quantity of kujigamberol, amberene and 1-methylamberene was increased about 2.4, 4.1 and 3.6 times more than those of the EtOH ext, respectively. Several minor peaks from the retention time of 10 min to 25 min on the HPLC chromatogram were also increased. Spirolactone norditerpenoid without UV absorption was not observed on the HPLC chart as a peak.
Inhibition of melanin production by EtOH ext. and scCO2 ext
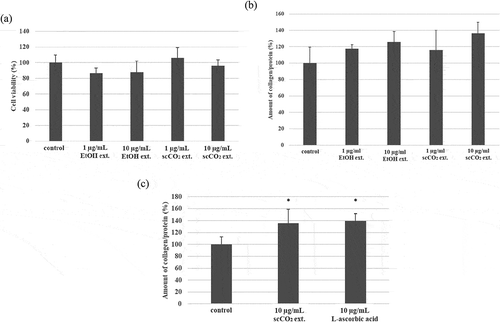
Cytotoxicity of EtOH ext. and scCO2 ext. against B16 melanoma cells at 1 and 10 μg/mL was not observed ()). However, both extracts inhibited melanin production in a dose dependent manner (). The scCO2 ext. had more potent inhibition activity of melanin production in B16 melanoma cells than that of EtOH ext. and significantly decreased melanin production at 10 μg/mL. Comparing the inhibition activity of scCO2 ext. (10 μg/mL) with that of 25 μM β-arbutin (6.81 μg/mL), both showed almost the same inhibition activity against B16 melanoma cells, although the melanin reduction by β-arbutin was not significant (). Although scCO2 ext. is not a pure compound, the potency is equal to that of a pure compound (β-arbutin) that is used as a cosmetic ingredient [Citation22,Citation23].
Figure 2. Effects of the EtOH ext. and the scCO2 ext. of Kuji amber against B16 mouse melanoma cells.
(a) Cytotoxicity of the EtOH ext. and the scCO2 ext. of Kuji amber against B16 mouse melanoma cells. (b) Microscopic observation of melanin production in B16 mouse melanoma cells treated by the EtOH ext. and the scCO2 ext. of Kuji amber. a) Without IBMX stimulation, b) with 100 µM IBMX, c) with 100 µM IBMX+ the EtOH ext. of Kuji amber (1 µg/mL), d) with 100 µM IBMX+ the EtOH ext. of Kuji amber (10 µg/mL), e) with 100 µM IBMX+ the scCO2 ext. of Kuji amber (1 µg/mL), f) with 100 µM IBMX+ scCO2 ext. of Kuji amber (10 µg/mL). Scale bar = 50 µm. (c) Quantification of melanin production in B16 mouse melanoma cells by the EtOH ext. and the scCO2 ext. of Kuji amber. * p < 0.05 (significant difference compared to the control). (d) Microscopic observation of melanin production in B16 mouse melanoma cells by the scCO2 ext. of Kuji amber and β- arbutin. a) Without IBMX stimulation, b) with 100 µM IBMX, c) with 100 µM IBMX+ the scCO2 ext. of Kuji amber (10 µg/mL), d) with 100 µM IBMX+ β-arbutin (25 µM). Scale bar = 50 µm. (e) Quantification of melanin production in B16 mouse melanoma cells by the scCO2 ext. of Kuji amber and β-arbutin. * p < 0.05 (significant difference compared to the control).
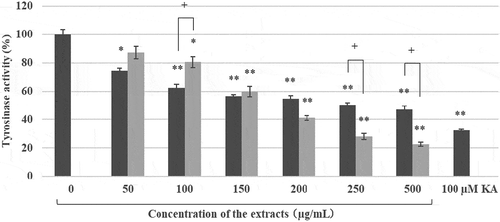
Tyrosinase inhibition activity
Both extracts showed dose dependent inhibition activity against mushroom tyrosinase. The scCO2 ext. (IC50 = 175 μg/mL) had more potent inhibition activity against mushroom tyrosinase than that of EtOH ext. (IC50 = 275 μg/mL) ().
Promotion activity of collagen production
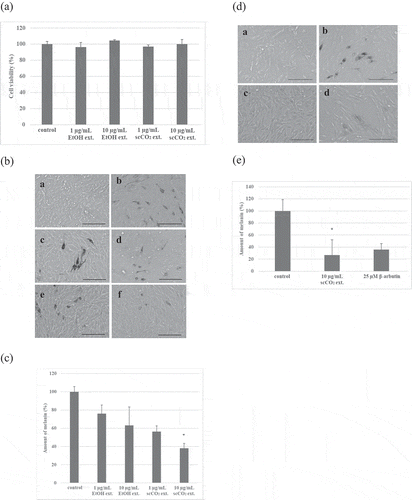
The EtOH ext. and scCO2 ext. had no effect on cell viability ()). Both extracts promoted collagen production in SF-TY fibroblast cells dose dependently ()). The promotion activity of scCO2 ext. was more potent than that of EtOH ext. although the increase was not significant. Comparing the promotion activity of scCO2 ext. (10 μg/mL) with that of 10 μg/mL of L-ascorbic acid, both showed almost the same promotion activity against collagen production in the cells ()). Although scCO2 ext. is not a pure compound, the potency was equal to that of a pure compound (L-ascorbic acid) that is used as a cosmetic ingredient [Citation24,Citation25].
Figure 4. Effect of the EtOH ext. and the scCO2 ext. of Kuji amber against human normal fibroblast SF-TY cells.
(a) Cytotoxicity of the EtOH ext. and the scCO2 ext. of Kuji amber against human fibroblast SF-TY cells. (b) Effect on collagen production in SF-TY cells by the EtOH ext. and the scCO2 ext. of Kuji amber. (c) Quantification of the collagen production in SF-TY cells by the scCO2 ext. and L-ascorbic acid(n = 5). *p < 0.05 (significant difference compared to the control).
Biologically active compounds in the extract of Kuji amber
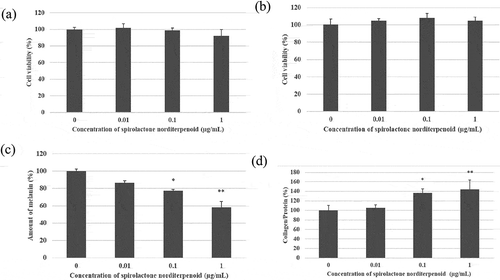
We tried to identify the biologically active compounds against melanin and collagen production in the isolated compounds from MEKA. The main biologically active compound having potent anti-allergy activity against cells and animals, kujigamberol, did not affect cell viability of both B16 melanoma cells ()) and SF-TY fibroblast cells ()) at 0.01 μg/mL. It had no inhibition activity against melanin production in B16 melanoma cells ()). However, it promoted collagen production in SF-TY fibroblast cells at low concentrations dose dependently ()) although the increase was not significant. Spirolactone norditerpenoid also did not affect cell viability of both B16 melanoma cells ()) and SF-TY fibroblast cells ()) at 1 μg/mL. It had both inhibition activity of melanin production ()) and promotion activity of collagen production dose dependently ()).
Figure 5. Inhibition of melanin production in B16 melanoma cells and the promotion of collagen production in SF-TY cells by kujigamberol.
(a) Cytotoxicity of kujigamberol against B16 melanoma cells. (b) Cytotoxicity of kujigamberol against SF-TY fibloblast cells. (c) Inhibition of melanin production in B16 melanoma cells by kujigamberol. (d) Promotion of collagen production in SF-TY fibroblast cells by kujigamberol..
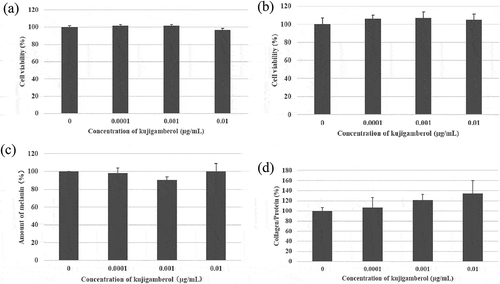
Figure 6. Inhibition of melanin production in B16 melanoma cells and the promotion of collagen production in SF-TY cells by spirolactone norditerpenoid.
(a) Cytotoxicity of spirolactone norditerpenoid against B16 melanoma cells. (b) Cytotoxicity of spirolactone norditerpenoid against SF-TY fibloblast cells. (c) Inhibition of melanin production in B16 melanoma cells by spirolactone norditerpenoid. *p < 0.05, **p < 0.01 (significant difference compared to the control). (d) Promotion of collagen production in SF-TY fibroblast cells by spirolactone norditerpenoid. *p < 0.05, **p < 0.01 (significant difference compared to the control).
Discussion
Kuji amber is a very unique amber in the world because it contains new biologically active compounds having various structures. However, we could not identify any new compounds and isolated only known compounds from Baltic, Dominican and Burmese ambers using the same mutant yeast, Saccharomyces cerevisiae (zds1Δ erg3Δ pdr1/3Δ) involving Ca2+-signal transduction [Citation1].
Although, MEKA and kujigamberol had potent anti-allergy activity against cells, a variety of new biologically active compounds in Kuji amber suggested to us that they may also have a variety of biological activities [Citation1,Citation11,Citation12]. The scCO2 ext. is advantageous for efficient extract of nonpolar compounds (amberene and 1-methylamberene were isolated in greater quantity than kujigamberol), compared to alcohol extracts. As indicated, nonpolar compounds, amberene (4.1 times) and 1-methylamberene (3.6 times) (), were more efficiently extracted by scCO2 than that of EtOH ext. This technology has also been used in the food and cosmetic industries [Citation13,Citation14]. In this study, we focused on the inhibition activity against melanin production and the promotion activity against collagen production in each cell as these are useful biological activities for the cosmetic industry.
As expected, both extracts of Kuji amber had both activities. Surprisingly, the extract consisting of various mixtures had almost the same activity as those of pure compounds, β-arbutin and L-ascorbic acid [Citation11,Citation22–Citation25]. Inhibition potency of EtOH ext. against tyrosinase was gradually decreased at high concentration (). It may be due to the low solubility of EtOH ext. than scCO2 ext. in the reaction mixture. Inhibition activities of both extracts against mushroom tyrosinase were weaker than those against B16 melanoma cells ( and ). It may depend on the molecular difference between mushroom tyrosinase and mammalian (human or mouse) tyrosinase [Citation26]. It is necessary to measure the inhibition activities of both extracts against mammalian enzymes at the next stage.
Finally, we examined what compound in the extracts had each activity. The main new anti-allergy compound identified to date, kujigamberol, had no activities against melanin production even at 1 μg/mL (data not shown), however, it promoted collagen production at low concentrations. Spirolactone norditerpenoid, that has different structure from other biological compounds in Kuji amber, is a candidate as an inhibitor against melanin production and a promotor of collagen production in both extracts. The extract of Kuji amber containing kujigamberol and spirolactone norditerpenoid will therefore have good potential as an ingredient for cosmetics. We are continuing to isolate other biologically active compounds from the extract of Kuji amber.
Authors contribution
K. K. and S. S. designed the research and supervised manuscript preparation. S. S., J. A., Y. K. and M. S. performed the experiments. All authors reviewed the manuscript.
Acknowledgments
We are grateful to Emeritus Professor Don R Phillips, La Trobe University for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kimura K Studies of novel bioprobes isolated from rare natural sources using mutant yeasts. J Antibiot (Rev). 2019;72:579–589.
- Shitamukai A, Mizunuma M, Hirata D, et al. A positive screening for drugs that specifically inhibit the Ca2+-signaling activity on the basis of the growth promoting effect on a yeast mutant with a peculiar phenotype. Biosci Biotechnol Biochem. 2000;64:1942–1946.
- Ogasawara Y, Yoshida J, Shiono Y, et al. New eremophilane sesquiterpenoid compounds, eremoxylarins A and B directly inhibit calcineurin in a manner independent of immunophilin. J Antibiot. 2008;61:496–502.
- Kimura K, Minamikawa Y, Ogasawara Y, et al. Kujigamberol, a new dinorlabdane diterpenoid isolated from 85 million years old Kuji amber using a biotechnological assay. Fitoterapia. 2012;83:907–912.
- Ye YQ, Koshino H, Hashizume D, et al. Synthesis and biological activity of both enantiomers of kujigamberol isolated from 85-million-years-old Kuji amber. Bioorg Med Chem Lett. 2012;22:4259–4262.
- Uchida T, Koshino H, Takahashi S, et al. Ca2+-Signal transduction inhibitors, kujiol A and kujigamberol B, isolated from Kuji amber using a mutant yeast. J Nat Prod. 2018;81:1070–1074.
- Shimizu E, Koshino H, Noro A, et al. Isolation of a spirolactone norditerpenoid as a yeast Ca2+ signal transduction inhibitor from Kuji amber and evaluation of its effects on PPM1A activity. Fitoterapia. 2019;134:290–296.
- Takahashi H, Koshino H, Maruyama M, et al. A novel Ca2+-signal transduction inhibitor, kujigamberol C, isolated from Kuji amber. Biosci Biotechnol Biochem. 2019; 83: 1630–1634.
- Takahashi H, Shimoda N, Koshino H, et al.Kujigamberoic acid A, a carboxylic acid derivative of kujigamberol, has potent inhibitory activity against the degranulation of RBL-2H3 cells. Biosci Biotechnol Biochem 2019;83:1193–1196.
- Kawamura T, Koshino H, Nakamura T, et al. Amberene and 1-methylamberene, isolated and identified from Kuji amber (Japan). Org Geochem. 2018;120:12–18.
- Maruyama M, Kobayashi M, Uchida T, et al. Anti-allergy activities of Kuji amber extract and kujigamberol. Fitoterapia. 2018;127:263–270.
- Fukuhara S, Tanigaki R, Kimura K, et al., Kujigamberol interferes with pro-inflammatory cytokine-induced expression of and N-glycan modifications to cell adhesion molecules at different stages in human umbilical vein endothelial cells. Int Immunopharmacol. 2018;62:313–325.
- Choi JS, Jeon MH, Moon WS, et al. In vivo hair growth-promoting effect of rice bran extract prepared by supercritical carbon dioxide fluid. Biol Pharm Bull. 2014;37:44–53.
- Khaw KY, Parat MO, Shaw PN, et al. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules. 2017;22:1186.
- Chung SY, Seo YK, Park JM, et al. Fermented rice bran downregulates MITF expression and leads to inhibition of alpha-MSH-induced melanogenesis in B16F1 melanoma. Biosci Biotechnol Biochem. 2009;73:1704–1710.
- Yoshida I, Ito C, Matsuda S, et al. Alisol B, a triterpene from Alismatis rhizoma (dried rhizome of Alisma orientale), inhibits melanin production in murine B16 melanoma cells. Biosci Biotechnol Biochem. 2017;81:534–540.
- Deglesne PA, Arroyo R, Fidalgo López J, et al. In vitro study of RRS® Silisorg CE class III medical device composed of silanol: effect on human skin fibroblasts and its clinical use. Med Devices (Auckl). 2018;11:313–320.
- Ohguchi K, Ito M, Yokoyama K, et al. Effects of sesquiterpene lactones on melanogenesis in mouse B16 melanoma cells. Biol Pharm Bull. 2009;32:308–310.
- Kim Y, Lee S, Ryu JH, et al. Effect of aurea helianthus stem extract on anti-melanogenesis. Biosci Biotechnol Biochem. 2018;82:1871–1879.
- Ochiai A, Tanaka S, Tanaka T, et al. Rice bran protein as a potent source of antimelanogenic peptides with tyrosinase inhibitory activity. J Nat Prod. 2016;79:2545–2551.
- Fang QQ, Wang XF, Zhao WY, et al. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-β1 pathways. Sci Rep. 2018;8:3332.
- Lee SY, Baek N, Nam TG Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzyme Inhib Med Chem. 31;2016:1–13.
- Seo DH, Jung JH, Lee JE, et al. Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives, Appl Microbiol Biotechnol. 2012;95:1417–1425.
- Luck MR, Jeyaseelan I, Scholes RA Ascorbic acid and fertility. Biol Reprod. 1995;52:262–266.
- Nabzdyk CS, Bittner EA Vitamin C in the critically ill - indications and controversies, World J Crit Care Med. 2018;7:52–61.
- Mann T, Gerwat W, Batzer J, et al. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J Invest Dermatol. 2018;138:1601–1608.