ABSTRACT
Phosphoenolpyruvate carboxylase (PEPC) is a carbon-fixing enzyme with critical roles in seed development. Previously we observed a positive correlation between PEPC activity and protein content in mature seeds among soybean cultivars and varietal differences of PEPC activity in immature seeds, which is concordant with seed protein accumulation. Here, we report a PEPC isoform (Gmppc2) which is preferentially expressed in immature soybean seeds at the late maturation stage. Gmppc2 was co-expressed with enzyme genes involved in starch degradation: α-amylase, hexokinase, and α-glucan phosphorylase. Gmppc2 was developmentally induced in the external seed coats, internal seed coats, hypocotyls, and cotyledons at the late maturation stage. The expression of Gmppc2 protein was negatively regulated by the application of a nitrogen fertilizer, which suppressed nodule formation. These results imply that Gmppc2 is involved in the metabolism of nitrogen originated from nodules into seeds, and Gmppc2 might be applicable as a biomarker of seed protein content.
Abbreviations: PEP: phosphoenolpyruvate; PEPC: phosphoenolpyruvate carboxylase; RNA-Seq: RNA sequencing; PCA: principal component analysis; SE: standard error
GRAPHICAL ABSTRACT
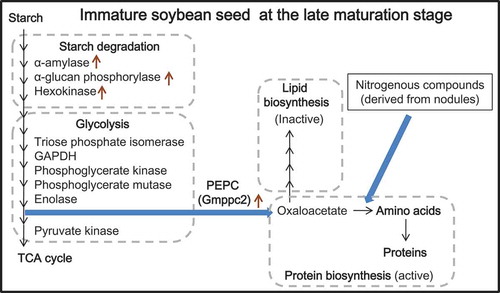
A hypothetical model which represents the metabolism in Immature soybean seeds at the late maturation stage. In this model, PEPC (Gmppc2) acts on provision of oxaloacetate for biosynthesis of amino acids that were coordinately originated from vegetative organs.
Phosphoenolpyruvate carboxylase (E.C.4.1.1.31, PEPC) catalyzes the β-carboxylation of phosphoenolpyruvate (PEP) into oxaloacetate (OAA) [Citation1]. PEPC is widely distributed across higher plants and is encoded by a small multigene family [Citation1–Citation3]. PEPC is recognized as a multi-functional enzyme: it plays a pivotal role in the provision of carbon skeletons in amino acid biosynthesis, fatty acid biosynthesis, and respiration [Citation4–Citation7]. In general, plant seeds exhibit high levels of PEPC activity [Citation8–Citation11], which highlights the probable contribution of PEPC in seed cell maturation [Citation12–Citation16]. The structure and function of plant PEPC are divergent among the PEPC isoforms [Citation2,Citation17]. The plant-type PEPC includes photosynthetic isoforms in C4 plants and crassulacean acid metabolism plants, and non-photosynthetic isoforms in C3 plants [Citation2]. The bacterial-type PEPC is monophyletic and lacks the phosphorylation site in the N-terminus. Plant-type isoforms have shown different gene expression and biochemical characteristics [Citation7,Citation18,Citation19]. In rice, mainly C3-type isogenes seem to be expressed in developing seeds, and their expression patterns are different from each other [Citation16]. In castor oil seeds, the plant-type and the bacterial-type PEPC are expressed and interact in the hetero-oligomeric complex [Citation17].
The plant-type PEPC plays an auxiliary role in nitrogen metabolism and often exhibits a link to the nitrogen supply. Zhang et al. [Citation20] reported the response of PEPC activity in detached wheat ears to ammonium nitrate. We observed an increase of PEPC activity in developing seeds under the application of nitrogen to wheat and rice at the flowering stage [Citation11,Citation21]. PEPC’s anaplerotic function complements OAA consumed in ammonium assimilation in roots [Citation3,Citation22]. It was also observed that PEPC activity in non-nodulated alfalfa roots was in response to nitrate, rather than ammonium [Citation23]. Transgenic studies further supported the involvement of PEPC in nitrogen metabolism. For example, a maize transcription factor, DOF1 (which promotes nitrogen assimilation under nitrogen-deficient conditions) controls the transcription of PEPC isogenes in rice [Citation24]. In tobacco, the overexpression of aspartate aminotransferase in proso millet enhanced the accumulation of PEPC protein [Citation25].
PEPC in soybean seeds is a possible key factor in relation to seed chemical compositions. Soybean seeds exhibit a high protein content (typically approx. 40%) in accordance with the high PEPC activity (~3.0 U/g F.W.) in immature seeds [Citation26]. The PEPC activity and the total protein content in mature soybean seeds among 11 cultivars were positively correlated [Citation13], and the PEPC activity was increased from the middle to late maturation stage [Citation26], at which time soybean seeds prominently accumulate nitrogen [Citation27,Citation28].
In two soybean cultivars the seed protein contents of which were contrasted, concordant patterns of PEPC activity with seed protein accumulation were observed, implying that PEPC can be a biomarker for seed protein content [Citation29]. At the molecular level, some PEPC isogenes such as Gmppc1 and Gmppc16 were isolated and analyzed [Citation17,Citation30–Citation33], but the expression patterns of the genes in developing soybean seeds are unknown, and the relevant isogenes involved in amino acid biosynthesis in seeds have not been identified.
Soybean genomes were sequenced in 2010 [Citation34,Citation35], and some transcriptome data in developing soybean seeds then became publicly available. A genome-wide survey in soybean revealed ten PEPC isogenes, GmPEPC1 to GmPEPC10 [Citation36]. In the present study, we utilized the soybean omics resources to isolate a PEPC isogene, GmPEPC3 (denoted Gmppc2), which is likely to be relevant to the accumulation of seed protein. We analyzed the gene sequence, the gene expression patterns in immature seeds, and the protein expression characteristics of Gmppc2 in a high-protein soybean cultivar under different nitrogen supply conditions. Our results suggest that Gmppc2 underlies the key nitrogen metabolism in immature soybean seeds, and they imply that Gmppc2 is a candidate protein biomarker for seed protein content.
Materials and methods
Re-mining of PEPC isogenes in the soybean genome
We searched for PEPC sequences from all soybean proteins by using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Automatic Annotation Server (KAAS) [Citation37]. All of the soybean proteins (Glyma 2.0) sequences were downloaded from the Phytozome platform [Citation38] and queried against the KEGG metabolic pathway database (http://www.genome.jp/kegg/) by the assignment method of KAAS searches as a bi-directional best hit. Proteins with the KEGG Orthology identifier K01595 (phosphoenolpyruvate carboxylase) were designated as PEPC isoforms.
Molecular evolutionary analysis
We constructed a phylogenetic tree of PEPC from soybean, six other dicotyledonous plant species, and rice. PEPC protein sequences were collected from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/), the KEGG pathway database (Brassica rapa, Phaseolus vulgaris, Ricinus communis), miyakogusa.jp (Lotus japonicus, http://www.kazusa.or.jp/lotus/), MtGDB (Medicago truncatula, http://www.plantgdb.org/MtGDB/), and Oryza sativa (MSU Rice Genome Annotation Project) [Citation39]. The deduced amino acid sequences were aligned by CLUSTALW ver. 2.1 [Citation40] and applied to construct a phylogenetic tree with the neighbor-joining (NJ) method [Citation41]. A bootstrap test was conducted with 1,000 resamplings [Citation42]. This phylogenetic analysis was conducted using MEGA6 software [Citation43].
RNA-Seq data analysis
We downloaded 38 profiles of RNA sequencing (RNA-Seq) data in soybean seeds from the U.S. National Center for Biotechnology Information (NCBI) Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra). The data set was comprised of 11 whole seed profiles and five cotyledon profiles with experimental duplicates at different developmental stages (Suppl. Material 1). The data files were transformed into fastq files by using the SRA toolkit provided by the NCBI. Adapter sequences, poly (A), and poly (T) sequences were trimmed by using a software cutadapt [Citation44].
Low-quality sequences with values less than the quality value (QV) of 10 at both ends of the reads were then trimmed by a hand-made Perl script as described [Citation45]. Briefly, sequence reads that satisfied either of the following criteria were filtered: (1) containing the ambiguous sequence “N”, (2) reads shorter than 20 bp, (3) reads with <17 as the average QV, and (4) reads that included >10% of sequences with QVs <10. Resultant high-quality reads were mapped on the soybean genome sequence (ver. Glyma2.0: http://www.phytozome.net/soybean.php) by using TopHat [Citation46] with the default parameter setting. Mapped reads were counted using Cufflinks [Citation47].
The fragments per kilobase of transcript per million mapped (FPKM) values were calculated as gene expression levels for individual loci [Citation48]. A principal component analysis (PCA) was conducted for these expression data using the R statistical package (https://www.r-project.org/). In order to observe co-expressed genes, a correspondence analysis was conducted for these expression data and additional RNA-Seq profiles in soybean seeds (SRA accession: SRR1174207- SRR1174209, SRR1174211, SRR1174212, SRR1174214, SRR1174233, SRR203037, SRR203038, SRR389188- SRR389192, SRR639176, SRR639177, SRR827653- SRR827671, SRR827673- SRR827698, SRR830182- SRR830211, SRR887342, SRR891238, SRR891247, SRR891260, SRR891284, SRR891292, SRR923882, SRR923884- SRR923887, SRR923889- SRR923894, SRR923896- SRR923897, SRR923899- SRR923901, SRR923903- SRR923904, SRR955406) to obtain similarities of expression patterns of genes (distance) by the method of Yano et al. and Hamada et al. [Citation49,Citation50].
Plant materials
The soybean (Glycine max L.) cultivar Enrei, which is one of the main soybean varieties cultivated in Japan, was grown in 2014 in an experimental field at Kobe University (34° 72ʹ 48.68” N, 135° 23ʹ 24.49” E) with the application of 20 g m−2 of a basal compound fertilizer [N:P:K = 8:8:8]. Seeds were sowed into vermiculite on June 6, and the seedlings were transplanted into the field on June 24. Two experimental-condition plots with different nitrogen supplies were set: a low-nitrogen plot (LN) and a high-nitrogen plot (HN). In the LN, no additional fertilizer was applied whereas in the HN, 166 g m−2 of a slow-release nitrogen fertilizer (MEISTER-15, JCAM AGRI. CO., LTD., Tokyo, Japan) was applied. This fertilizer was designed to release urea linearly for 100 days at 25°C.
Flowering started at the end of July. Pods containing immature seeds were collected on August 20, September 1, 11, 16, and 25 and immediately frozen in liquid nitrogen to store at −80°C until use. The developmental stages (DSs) of the soybean immature seeds were designated according to the study by Yamamoto et al. [Citation29] as follows: DS1 (100–250 mg), DS2 (250–400 mg), DS3 (400–600 mg), DS4 (600–700 mg), DS5 (700–900 mg), DS6 (900–600 mg), and DS7 (600–300 mg) from the early stage to the late stage of maturation in turn. Much less number of nodules in HN than that in LN was confirmed when sampling was carried out.
cDNA cloning
Total RNA from developing soybean seed tissues (with approx. 200 mg F.W.) was extracted by using RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) with the use of the RNase-Free DNase set (Qiagen) to eliminate contaminated genomic DNA by following the manufacturer’s instruction. The obtained total RNA was applied to a first-strand cDNA synthesis by using oligo-dT primers and ReverTra Ace reverse transcriptase (Toyobo, Osaka, Japan). A polymerase chain reaction (PCR) was conducted using the cDNA fraction, KOD Plus High Fidelity DNA polymerase (Toyobo), and a pair of gene-specific primers (Suppl. Material 2). The PCR products were then cloned into the entry vector pENTR/D-TOPO (Life Technologies, Tokyo, Japan). The cDNA inserts of three independent clones were sequenced by primer-walking (Suppl. Material 3).
Quantitative RT-PCR analysis
Frozen immature soybean seed tissues were defrost on ice and external layer of seed coat, internal layer of seed coat, hypocotyls, and cotyledons were quickly dissected using a razor and tweezers for total RNA extraction. First-strand cDNA was prepared by the method described above. The cDNA fraction was diluted at 50-fold with TE buffer and applied to real-time reverse transcription (RT)-PCR using KOD SYBR qPCR Mix (Toyobo) and gene-specific primers (Suppl. Material 2). An actin gene was selected as a reference, and the relative expression levels were quantified by the ΔΔCt-method.
Extraction of soluble proteins
All procedures for PEPC protein extraction were carried out at 4°C. Frozen seed samples were quickly homogenized with a motor and pestle in five volumes of an extraction buffer [100 mM Tris-HCl, pH 7.8, 1 mM EDTA, 1 mM 2-mercaptoethanol, 10% (w v−1) glycerol, 25 mM sodium fluoride, and 5 mM sodium pyrophosphate decahydrate] with Complete Protease Inhibitor Cocktail (Hoffmann-La Roche, Basel, Switzerland). The homogenate was centrifuged at 13,000 g for 30 min, and the supernatant was collected for the PEPC assay and western blot analysis.
PEPC antibodies
A rabbit polyclonal anti-peptide antibody was raised against an amino acid sequence of Gmppc2 (TKYEETKEFLLQ) (Eurofins Genomics, Tokyo, Japan). The antibody was purified by a peptide-affinity column. The plant-type PEPC antibody and the bacterial-type PEPC antibody raised in our previous study were re-used [Citation51].
Western blot analysis
We performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 7.5% of polyacrylamide gel by Laemmli’s method [Citation52]. As described by Yamamoto et al. [Citation16], we electrophoresed samples on a 6-cm-long gel for 3 h at 20 mA. Precision Plus Protein Standards (Bio-Rad, Hercules, USA) was used as the molecular weight marker. The separated proteins were transferred onto a polyvinylidene difluoride membrane, and targeted proteins were immunologically reacted with a polyclonal antibody for detection, using alkaline phosphatase. Polypeptide bands of PEPC were analyzed using ImageJ software [Citation53] for the quantification of the signal intensities.
Measurement of PEPC activity
Seed PEPC activity was measured by coupling with the malate dehydrogenase reaction by the method of Echevarria et al. [Citation54] with minor modifications by Yamamoto et al. [Citation7]. The assay was carried out three times for each sample, and the average of the measured values was used.
Results
Expression patterns of PEPC isogenes during soybean seed development
The genomic sequence of a soybean cultivar Williams 82 was released in 2010 [Citation34,Citation35], and PEPC isogenes in soybean were reported [Citation36]. In our in-house search, we confirmed the presence of ten PEPC isogenes in the genome assembly (Wm82. a2v1). To see how these isogenes are regulated in developing soybean seeds, we analyzed public RNA-Seq data for soybean seeds. These data sets allowed an analysis of the gene expression patterns in whole seeds and cotyledons (Suppl. Material 1).
Gene expressions of all the PEPC isogenes were detected in both whole seeds and cotyledons, but these isogenes exhibited different expression patterns and levels during seed maturation (; Suppl. Material 3). Among them, one isogene (Glyma.06g277500; we denoted it ‘Gmppc3ʹ and refer to it as such hereafter) showed the highest expression levels from the globular stage to the early-maturation stage in the whole seeds (); Suppl. Material 3). The expressions of two isogenes, Gmppc1 (Glyma.12g229400) and Gmppc16 (Glyma.12g161300), were expressed in the same manner with Gmppc3 at almost half the expression level of Gmppc3. Another isogene (Glyma06g229900; we denoted it ‘Gmppc2ʹ) showed the highest expression level in the mid-maturation-stage to dry-stage seeds, whereas this isogene was less expressed before these stages. High-level expression of Gmppc2 was distinctively presented even in dry-stage seeds. In the cotyledons, the expression patterns of PEPC isogenes differed from each other (). Gmppc2 exhibited the highest expression level at the late-maturation stage. Gmppc16 also exhibited the highest expression level at the late-maturation stage while its gene expression pattern was different from that of Gmppc2 at the 20 DAF sample. Gmppc1 showed the highest expression levels in the 400–500 mg F.W. cotyledon sample. Gmppc3 presented an expression pattern that was similar to that of Gmppc1 and exhibited the highest expression at the sample of 100–200 mg F.W.
Figure 1. The gene expression patterns of PEPC isogenes in RNA-Seq data of soybean seeds. (a) Heat map showing the gene expression patterns in whole seeds at different growth stages. (b) Heat map of the gene expression levels in cotyledons at different growth stages. (c) Score plot of the results of the PCA based on the expression patterns of Gmppc genes in the heatmap. White circles: Plant-type isogenes. Black circles: Bacterial-type isogenes. 1: Glyma.01G091000, 2: Glyma.02G130700, 3: Glyma.06G229900, 4: Glyma.06G277500, 5: Glyma.10G205500, 6: Glyma.12G161300, 7: Glyma.12G210600, 8: Glyma.12G229400, 9: Glyma.13G270400, 10: Glyma.13G290700. (d) Variance of the principal components in the PCA based on the expression patterns of Gmppc genes in the heatmap.
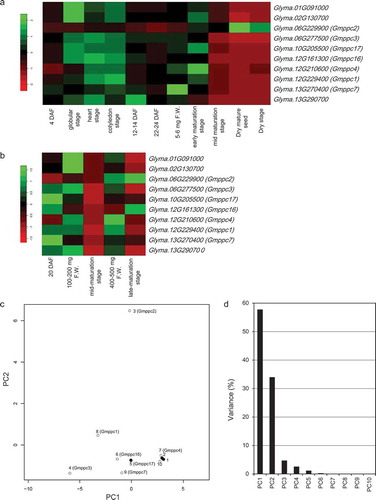
The results of the PCA of the whole seeds and cotyledon samples highlighted Gmppc2 because of its distinctive expression pattern among the isogenes (). These results indicate that there is selectivity of PEPC isogenes in developing soybean seeds, and they show the probable importance of the above-mentioned isogenes for seed maturation at each developmental stage. It was also notable that the expression pattern of Gmppc2 was totally different from those of the bacterial-type PEPC isogenes (Glyma.01G091000, Glyma.02G130700, and Glyma.10G205500), implying a unique biochemical regulation of Gmppc2.
Since the gene expression pattern of Gmppc2 seemed to be linked with the timing of starch breakdown and the continuous accumulation of soybean seed proteins [Citation29], we investigated whether the genes that were co-expressed with Gmppc2 included enzyme genes that are involved in starch degradation and the downstream metabolism. Interestingly, the correspondence analysis of the public RNA-Seq data in soybean seeds revealed expression patterns of α-amylase (Glyma.08G296800), hexokinase (Glyma.11G015800), α-glucan phosphorylase (Glyma.08G334000), glucosidase (Glyma.10G192400; Glyma.20G198000; Glyma.09G174500), sucrose phosphate synthase (Glyma.17G109700), and glyceraldehyde-3-phosphate dehydrogenase (Glyma.06G172600) that were similar to that of Gmppc2 (Suppl. Material 5). These enzyme genes could act in starch degradation [Citation55] and glycolysis, which are related to the production of PEP. This result implied that Gmppc2 is a candidate PEPC isoform contributing to seed protein accumulation.
cDNA cloning, molecular evolution, and the gene expression of Gmppc2 in Enrei
The Japanese soybean cultivar Enrei is one of the high-protein soybean cultivars, and its genome sequence is available [Citation56]. Enrei exhibits higher PEPC activity and protein content in mature seeds compared to several other varieties [Citation13], and the PEPC activity in developing Enrei seeds is concordant with seed protein accumulation [Citation29]. We therefore isolated Gmppc2 in Enrei and analyzed it. The cloned full-length cDNA was 2,904 bp long, and the cDNA sequence was completely identical to the coding sequence of Gmppc2 gene in the WIlliam 82 and Enrei genomes. The cDNA sequence was deposited to GenBank (LC006107).
We conducted a phylogenetic analysis of PEPC in soybean and six plant species of which the genomes have been sequenced. As expected, the phylogenetic tree presented two main clusters of plant-type and bacterial-type PEPC isoforms (Suppl. Material 6). Among the plant-type isoforms, there were four distinctive lineages in soybean. Except for Gmppc3, the soybean isoforms have similar paralogous isozymes. With regard to the bacterial type, we observed a pair of paralogous isoforms. Such duplicated paralogs might be a result of chromosomal-level evolution [Citation35]. Four plant-type isoforms in soybean were clustered into a legume-specific lineage, implying that these isoforms might be related to the characteristics of legumes.
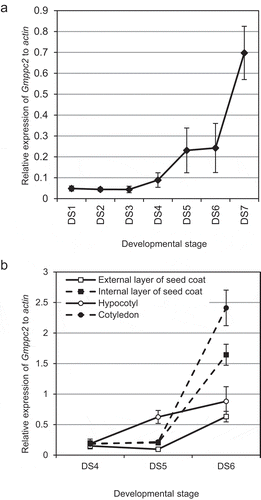
The gene expression patterns of Gmppc2 during seed maturation were monitored by quantitative RT-PCR. The gene expression of Gmppc2 in whole seeds was relatively low at DS1 to DS3 but started to rise at DS4, and the elevation continued to the mature-seed stage (). This expression pattern was consistent with the PEPC activity in developing soybean seeds reported by Sugimoto et al. [Citation26]. We also analyzed the gene expressions in separated seed organs at DS4 to DS6 (). Notably, the transcript level for Gmppc2 was increased in all four tissues: the external layer of the seed coat, the internal layer of the seed coat, hypocotyls, and cotyledons. These seed coats might play roles in temporal storage or conversion of organic chemicals transported from vegetative organs while hypocotyls and cotyledons are the storage tissues of seed proteins. The expression pattern from DS4 to DS6 was similar between the internal layer of the seed coat and the cotyledons. In contrast, the expression pattern in the hypocotyls at DS5 was distinct from those at other stages. These results suggested that Gmppc2 plays an important role in these seed organs, especially in cotyledons and the internal layer of seed coats at the late maturation stage of DS6. Physiological roles of Gmppc2 in seed coat and hypocotyls/cotyledons may be different from each other while in both cases Gmppc2 are likely to be related to nitrogen metabolism.
Figure 2. The results of the gene expression analysis in developing seeds by quantitative RT-PCR. (a) The gene expression pattern of Gmppc2 in whole seeds. Horizontal axis: The relative expression level of Gmppc2. Vertical axis: The sample stage. Error bar: Standard Standard error (SE). (b) The gene expression of Gmppc2 in separated seed tissues. Error bar: SE.
Developmentally expressed Gmppc2 protein in developing seeds
We monitored the protein expression of Gmppc2 during seed development by using an antipeptide polyclonal antibody. First, we raised a Gmppc2-specific antibody using the unique peptide sequence for this isogene ()). The specificity of the raised antibody was tested against two isoforms (Glyma.12G161300 and Glyma.13G270400) which have peptide sequences close to that of Gmppc2; the dot blot analysis showed that the antibody could detect Gmppc2 specifically (). By using this Gmppc2 antibody, we detected PEPC protein of 113.4 kDa in developing whole seeds. A slight amount of Gmppc2 protein was present from DS1 to DS4, and the level was increased at DS5, reaching the maximum level at DS6 and DS7 (). This result indicated that Gmppc2 was developmentally regulated at the translational level. Additional polypeptides were detected at 75 kDa and 60 kDa at DS6 and DS7, suggesting proteolysis or a cross-immune reaction with other proteins. Such proteolyzed polypeptides were observed in various plant seeds [Citation9,Citation16].
Figure 3. The expression of PEPC in developing soybean seeds. (a) Partial sequence alignment of the ten PEPC isoforms of the C-termini. Bold letters: The region that was used to raise a Gmppc2-specific polyclonal antibody. (b) Dot blot assay to determine the specificity of the Gmppc2 antibody. (c) The protein expression patterns of Gmppc2, plant-type PEPC, and bacterial-type PEPC in developing whole seeds during seed maturation from DS1 to DS7. (d) The effect of nitrogen application on the expression of Gmppc2 protein. The measurement was duplicated, and the average of the values is shown. Error bar: Standard error (SE). Significance at *10% and **5% by Student’s t-test. e: The effect of nitrogen application on PEPC activity. Error bar: SE.
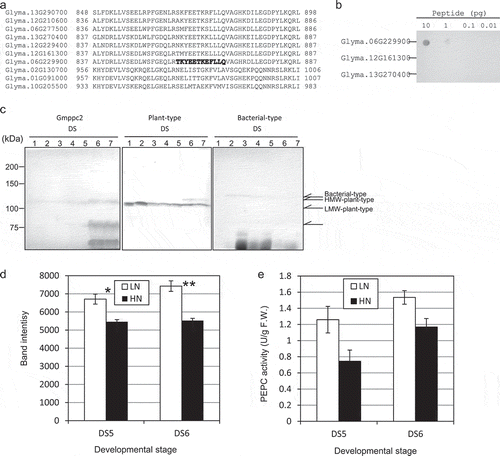
However, when we conducted the western blot analysis using the plant-type PEPC antibody, we could not detect such polypeptides around 75 kDa and 60 kDa (). Therefore, these would not be proteolyzed polypeptides of Gmppc2. Interestingly, Gmppc2 protein exhibited an apparent higher molecular weight compared to that of the main PEPC polypeptide band detected by the plant-type PEPC antibody. Since the deduced amino acid sequences of the plant-type PEPC isogenes were very similar (966–967 amino acid residues), the difference in the apparent molecular weight might be due to a post-translational modification of PEPC.
Our western blot analyses using the plant-type PEPC antibody and bacterial-type PEPC antibody provided an insight into the functional partitioning of PEPC isoforms (). The plant-type PEPC antibody immunologically reacted with three types of PEPC proteins with different gel mobilities (at least); high-molecular-weight (HMW-) PEPC, middle-molecular-weight (MMW-) PEPC, and low-molecular-weight (LMW-) PEPC. HMW-PEPC, which was expressed at DS6 and DS7, showed mobility in the SDS-PAGE that was similar to that of Gmppc2, implying that Gmppc2 corresponds to HMW-PEPC. MMW-PEPC was detected across all of the samples tested, but a high-level accumulation of it was observed at DS2. LMW-PEPC was expressed from DS1 to DS5 and not detected in DS6 and DS7. Bacterial-type PEPC was detected mainly from DS2 to DS4, and it was faint after DS5. These results suggest that Gmppc2 is one of the main isoforms acting at the late-maturation stage in developing soybean seeds. Isoforms of MMW-PEPC might play a role in soybean seed development but was not likely to be directly related to regulation of seed storage compound biosynthesis.
Effect of nitrogen application on the expression of Gmppc2 protein
Soybean plants have two nitrogen sources – nodule nitrogen and soil-derived nitrogen – and both are required to satisfy the nitrogen demand in seeds [Citation57]. Zapata et al. [Citation58] stated that nitrogen fixed in nodules seems to be the predominant source of nitrogen in pods and seeds at the end of the R7 stage, which is the beginning of maturity and corresponds to DS5 to DS6. Fixed nitrogen in nodules was transported as allantoic acid and allantoin and converted into various amino acids in seeds [Citation59]. It appeared that nitrogen application suppresses nodule formation in soybean plants via our multi-year field experiments.
Here, we analyzed the response of the Gmppc2 protein in developing seeds of soybean plants in the HN plot, i.e. under constant nitrogen application throughout the soybeans’ growth for the suppression of nodule formation. We expected that this nitrogen application would decrease the nodule formation [Citation60–Citation65], resulting in an alteration of the nitrogen supply to the developing seeds. The nitrogen application showed a significantly negative effect on the accumulation of Gmppc2 protein ()). In concordance with that finding, we observed a clear decrease in the PEPC activity under nitrogen application ()), which suggests that Gmppc2 could be involved in the metabolism of nitrogen transported from nodules.
Discussion
Soybean seeds are an important source of protein and oil in our society, and the elucidation of genetic factors that control the seed protein content is a major issue in soybean cultivation. Our present analyses revealed a candidate PEPC isogene Gmppc2 that promotes the accumulation of seed protein in soybean. Gmppc2 was highly expressed at the late stage of seed maturation, at which time the seeds exhibited high-level PEPC activity in the high-protein variety [Citation29]. The PEPC activity at this developmental stage seems important for nitrogen accumulation, because the high-protein variety showed higher PEPC activity in seeds compared to that of the low-protein variety [Citation29]. The response of Gmppc2 to the nitrogen application suggests that Gmppc2 could be responsible for the transportation of nitrogen from the nodules. Although we isolated two PEPC isogenes from an expression library of developing soybean seeds by using a polyclonal antibody against soybean seed PEPC [Citation30,Citation31], we have obtained no evidence of the involvement of these PEPC in the biosynthesis of storage compounds. Our present findings indicate that Gmppc2 might be applicable as a biomarker of the soybean seed protein content in soybean breeding and/or the assessment of cultivation conditions.
The relationship between PEPC and nitrogen metabolism has been well documented in various plants and organs. Melzer and O’Leary [Citation66] demonstrated that CO2/HCO3− in the atmosphere fixed by tobacco leaves was used to produce aspartate. In the roots of alfalfa and wheat, PEPC was increased at the protein and enzymatic activity levels in response to the nitrogen supply [Citation22,Citation23]. A few studies of rice and wheat reported a relationship between nitrogen accumulation and PEPC in developing seeds [Citation7,Citation20,Citation21]. In soybean, there are two pathways for transporting nitrogen from vegetative organs to seeds [Citation59]. One is the phloem, which transports aspartic acid from leaves, and the other is the vessels, which transport ureides from the nodules. The nitrogen supply from leaves has almost stopped at DS6 and DS7 (corresponding to growth stage R7 in the definition by Pedersen [Citation67]), but the nitrogen uptake in the seeds is still active [Citation68], and the seed storage protein biosynthesis is continuing [Citation69]. At these stages, temporarily stored starch in developing seeds is breaking down [Citation70], and the seed starch content reaches <1% after seed maturation [Citation71].
One unsolved question is whether the carbon generated from starch is consumed in protein or oil biosynthesis [Citation72]. Chen et al. [Citation73] reported an association between starch breakdown and the development of protein bodies in shriveled soybean seeds. Hexose phosphate, a degradation product of starch, would be processed in glycolysis to generate PEP [Citation74]. Gmppc2 might act on the provision of carbon that is consumed in the conversion from ureides to amino acids for the biosynthesis of seed storage proteins.
The number of PEPC isogenes in soybean was shown herein to be quite larger than those in other plants; four in both Arabidopsis and Medicago, and six in rice (Suppl. Material 6), because four of the isogenes in soybean have evolutionarily close paralogs. The biological meaning of the larger gene family in soybean remains unclear, but the higher gene dosage could lead to high PEPC expression/enzymatic activity in seeds. Further studies are required to obtain a better understanding of this issue.
A gene expression divergence among the PEPC isogenes was also observed in rice [Citation3]. Four plant-type isogenes are predominantly expressed in developing rice seeds, and their developmental expression characteristics in seeds are different. Two of them, Osppc1 and Osppc3, seemed to be involved in nitrogen accumulation and fatty acid biosynthesis, respectively [Citation16]. In soybean, we observed that six isogenes are predominantly expressed in developing seeds (), and four of these isogenes showed significant expression levels in the cotyledon (). Interestingly, the four isogenes include a bacterial-type isogene (Gmppc17), and its expression level was quite lower than that of Gmppc2. The protein expressions of the bacterial-type isoforms were decreased at the late-maturation stage (). In castor oil seeds, a bacterial-type PEPC localized at the mitochondrial outer envelope has a potential role in the re-fixation of respiratory CO2 with plant-type PEPC [Citation75]. Our present data imply that Gmppc2 works under a lesser amount of bacterial-type PEPC. The seed metabolism related to PEPC in soybean and castor oil seeds might be different.
A unique observation in soybean was obtained from purified PEPC from mature seeds, which showed low sensitivity to malate (the inhibitor constant of 6.8 mM) compared to those of PEPC from other C3 plants [Citation2,Citation28]. The biochemical regulation of PEPC under a high level-malate condition such as the late stage of seed maturation could be crucial for seed development [Citation26,Citation76].
Multiple PEPC proteins have been observed in various plants by western blot analyses [Citation7,Citation9,Citation10,Citation77–Citation80]. Sugimoto et al. [Citation26] also reported that three polypeptides were seen in purified PEPC from mature soybean seeds. The presence of these polypeptides was interpreted as being due to a post-translational modification of PEPC such as protein degradation, phosphorylation, or monoubiqitination. In fact, we observed that the mono-ubiquitination site was also conserved among all of the PEPC isoforms in soybean (data not shown). Otherwise, it might be due to an N-terminal truncation of PEPC in vitro during the preparation of PEPC protein [Citation80,Citation81]. The results of our analyses suggest that the degree of protein degradation is less.
Author contribution
NY designed and conducted experiments, analyzed data, and wrote the manuscript. TS cultivated soybean plants and improved the manuscript. TT conducted computational analyses. AS assisted the experiments technically. SM improved the manuscript. KY designed computations. TM organized this study and improved the manuscript.
Supplementary_Material_6.pdf
Download PDF (100.3 KB)Supplementary_Material_5.pdf
Download PDF (322.1 KB)Supplementary_Material_4.pdf
Download PDF (80.6 KB)Supplementary_Material_3.pdf
Download PDF (134.4 KB)Supplementary_Material_2.pdf
Download PDF (5.8 KB)Supplementary_Material_1.pdf
Download PDF (26.1 KB)Acknowledgments
We thank Ms. Yoko Morimoto for her assistance with the soybean sample storage. Computations were performed in part on the NIG supercomputer at the ROIS National Institute of Genetics.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemenatry material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- O’Leary B, Park J, Plaxton WC. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J. 2011;436:15–34.
- Sánchez R, Cejudo FJ. Identification and expression analysis of a gene encoding a bacterial-type phosphoenolpyruvate carboxylase from Arabidopsis and rice. Plant Physiol. 2003;132:949–957.
- Masumoto C, Miyazawa S, Ohkawa H, et al. Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proc Natl Acad Sci. 2010;107:5226–5231.
- Plaxton WC, Podestá FE. The functional organization and control of plant respiration. Crit Rev Plant Sci. 2006;25:159–198.
- Andre C, Froehlich JE, Moll MR, et al. A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. Plant Cell. 2007;19:2006–2022.
- Junker BH, Lonien J, Heady LE, et al. Parallel determination of enzyme activities and in vivo fluxes in Brassica napus embryos grown on organic or inorganic nitrogen source. Phytochemistry. 2007;68:2232–2242.
- Yamamoto N, Kubota T, Masumura T, et al. Molecular cloning, gene expression and functional expression of a phosphoenolpyruvate carboxylase Osppc1 in developing rice seeds: implication of involvement in nitrogen accumulation. Seed Sci Res. 2014;24:23–36.
- Sangwan RS, Singh N, Plaxton WC. Phosphoenolpyruvate carboxylase activity and concentration in the endosperm of developing and germinating castor oil seeds. Plant Physiol. 1992;99:445–449.
- González MC, Osuna L, Echevarrı́a C, et al. Expression and localization of phosphoenolpyruvate carboxylase in developing and germinating wheat grains. Plant Physiol. 1998;116:1249–1258.
- Yamamoto N, Shimada M, Sugimoto T, et al. Dynamic protein expression of phosphoenolpyruvate carboxylase in developing rice seeds. J Cereal Sci. 2014;60:457–460.
- Yamamoto N, Sugimoto T, Masumura T. Concomitant increases of the developing seed phosphoenolpyruvate carboxylase activity and the seed protein content of field-grown wheat with nitrogen supply. Agric Sci. 2014;14:1558–1565.
- Smith AJ, Rinne RW, Seif RD. Phosphoenolpyruvate carboxylase and pyruvate kinase involvement in protein and oil biosynthesis during soybean seed development. Crop Sci. 1989;29:349–353.
- Sugimoto T, Tanaka K, Monma M, et al. Phosphoenolpyruvate carboxylase level in soybean seed highly correlates to its contents of protein and lipid. Agric Biol Chem. 1989;53:885–887.
- Sebei K, Ouerghi Z, Kallel H, et al. Evolution of phosphoenolpyruvate carboxylase activity and lipid content during seed maturation of two spring rapeseed cultivars (Brassica napus L.). C R Biol. 2006;329:719–725.
- Radchuk R, Radchuk V, Götz KP, et al. Ectopic expression of phosphoenolpyruvate carboxylase in Vicia narbonensis seeds: effects of improved nutrient status on seed maturation and transcriptional regulatory networks. Plant J. 2007;51:819–839.
- Yamamoto N, Sasou A, Saito Y, et al. Protein and gene expression characteristics of a rice phosphoenolpyruvate carboxylase Osppc3; its unique role for seed cell maturation. J Cereal Sci. 2015;64:100–108.
- Gennidakis S, Rao S, Greenham K, et al. Bacterial- and plant-type phosphoenolpyruvate carboxylase polypeptides interact in the hetero-oligomeric class-2 PEPC complex of developing castor oil seeds. Plant J. 2007;52:839–849.
- Dong LY, Masuda T, Kawamura T, et al. Cloning, expression, and characterization of a root-form phosphoenolpyruvate carboxylase from Zea mays: comparison with the C4-form enzyme. Plant Cell Physiol. 1998;39:865–873.
- Muramatsu M, Suzuki R, Yamazaki T, et al. Comparison of plant-type phosphoenolpyruvate carboxylases from rice: identification of two plant-specific regulatory regions of the allosteric enzyme. Plant Cell Physiol. 2014;56:468–480.
- Zhang YH, Zhou SL, Huang Q, et al. Effects of sucrose and ammonium nitrate on phosphoenolpyruvate carboxylase and ribulose-1, 5-bisphosphate carboxylase activities in wheat ears. Aust J Crop Sci. 2012;6:822–827.
- Sugimoto T, Sueyoshi K, Oji Y Increase of PEPC activity in developing rice seeds with nitrogen application at flowering stage. In: Ando T, Fujita K, Mae T, et al., editors. Plant Nutrition for Sustainable Food Production and Environment Proceedings of the XIII International Plant Nutrition Colloquium; September 13–19. Tokyo, Japan: Springer; 1997. p. 811–812.
- Koga N, Ikeda M. Methionine sulfoximine suppressed the stimulation of dark carbon fixation by ammonium nutrition in wheat roots. Soil Sci Plant Nutr. 2000;46:393–400.
- Pasqualini S, Ederli L, Piccioni C, et al. Metabolic regulation and gene expression of root phosphoenolpyruvate carboxylase by different nitrogen sources. Plant Cell Environ. 2001;24:439–447.
- Kurai T, Wakayama M, Abiko T, et al. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotech J. 2011;9:826–837.
- Sentoku N, Taniguchi M, Sugiyama T, et al. Analysis of the transgenic tobacco plants expressing Panicum miliaceum aspartate aminotransferase genes. Plant Cell Rep. 2000;19:598–603.
- Sugimoto T, Tanaka K, Monma M, et al. Physiological and enzymological properties of phosphoenolpyruvate carboxylase in soybean (Glycine max L. cv Enrei) seeds. Mem Grad Sch Sci Technol Kobe Univ. 1998;16-A:77–87.
- Dornbos DL, McDonald MB. Mass and composition of developing soybean seeds at five reproductive growth stages. Crop Sci. 1986;26:624–630.
- Sugimoto T, Nomura K, Masuda R, et al. Effect of nitrogen application at the flowering stage on the quality of soybean seeds. J Plant Nutr. 1998;21:2065–2075.
- Yamamoto N, Masumura T, Yano K, et al. Pattern analysis suggests that phosphoenolpyruvate carboxylase in maturing soybean seeds promotes the accumulation of protein. Biosci Biotechnol Biochem. published online 2019;83:2238–2243.
- Sugimoto T, Kawasaki T, Kato T, et al. cDNA sequence and expression of a phosphoenolpyruvate carboxylase gene from soybean. Plant Mol Biol. 1992;20:743–747.
- Vazquez-Tello A, Whittier RF, Kawasaki T, et al. Sequence of a soybean (Glycine max L.) phosphoenolpyruvate carboxylase cDNA. Plant Physiol. 1993;103:1025–1026.
- Hata S, Izui K, Kouchi H. Expression of a soybean nodule-enhanced phosphoenolpyruvate carboxylase gene that shows striking similarity to another gene for a house-keeping isoform. Plant J. 1998;13:267–273.
- Nakagawa T, Takane K, Sugimoto T, et al. Regulatory regions and nuclear factors involved in nodule-enhanced expression of a soybean phosphoenolpyruvate carboxylase gene: implications for molecular evolution. Mol Gen Genet. 2003;269:163–172.
- Grant D, Nelson RT, Cannon SB, et al. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucl Acids Res. 2010;38:D843–D846.
- Schmutz J, Cannon SB, Schlueter J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183.
- Wang N, Zhong X, Cong Y, et al. Genome-wide analysis of phosphoenolpyruvate carboxylase gene family and their response to abiotic stresses in soybean. Sci Rep. 2016;6:38448.
- Moriya Y, Itoh M, Okuda S, et al. KAAS: an automatic genome annotation and pathway reconstruction server. Nucl Acids Res. 2007;35:W182–W185.
- Goodstein DM, Shu S, Howson R, et al. Phytozome: A comparative platform for green plant genomics. Nucl Acids Res. 2012;40:D1178–D1186.
- Kawahara Y, de la Bastide M, Hamilton JP, et al. Improvement of the Oryza sativa nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4.
- Thompson JD, Gibson T, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinf. Chapter 2: Unit 2.3. 2002. DOI:10.1002/0471250953.bi0203s00
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12.
- Yamamoto N, Takano T, Tanaka K, et al. Comprehensive analysis of transcriptome response to salinity stress in the halophytic turf grass Sporobolus virginicus. Front Plant Sci. 2015;6:1–14.
- Kim D, Pertea G, Trapnell C, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
- Trapnell C, Hendrickson DG, Sauvageau M, et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature Biotech. 2013;31:46–53.
- Howe EA, Sinha R, Schlauch D, et al. RNA-Seq analysis in MeV. Bioinformatics. 2011;27:3209–3210.
- Yano K, Imai K, Shimizu A, et al. A new method for gene discovery in large-scale microarray data. Nucl Acids Res. 2006;34:1532–1539.
- Hamada K, Hongo K, Suwabe K, et al. OryzaExpress: an integrated database of gene expression networks and omics annotations in rice. Plant Cell Physiol. 2011;52:220–229.
- Yamamoto N, Kinoshita Y, Sugimoto T, et al. Role of nitrogen-responsive plant-type phosphoenolpyruvate carboxylase in the accumulation of seed storage protein in ancient wheat (Spelt and kamut). Soil Sci Plant Nutr. 2017;63:23–28.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to image J: 25 years of image analysis. Nat Methods. 2012;9:671–675.
- Echevarria C, Pacquit V, Bakrim N, et al. The effect of pH on the covalent and metabolic control of C4 phosphoenolpyruvate carboxylase from sorghum leaf. Arch Biochem Biophys. 1994;315:425–430.
- Smith AM, Zeeman SC, Smith SM. Starch degradation. Annu Rev Plant Biol. 2005;56:73–98.
- Katayose Y, Kanamori H, Shimomura M, et al. DaizuBase, an integrated soybean genome database including BAC-based physical maps. Breed Sci. 2012;61:661–664.
- Salvagiotti F, Cassman KG, Specht JE, et al. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008;108:1–13.
- Zapata F, Danso SKA, Hardarson G, et al. Time course of nitrogen fixation in field-grown soybean using nitrogen-15 methodology. Agron J. 1987;79:172–176.
- Ohyama T, Minagawa R, Ishikawa S, et al. Soybean seed production and nitrogen nutrition. INTECH Open Access Publisher; 2013. DOI:10.5772/52287.
- Harper JE, Gibson AH. Differential nodulation tolerance to nitrate among legume species. Crop Sci. 1984;24:797–801.
- Streeter JG, Wong PP. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci. 1988;7:1–23.
- Davidson IA, Robson MJ. Effect of contrasting patterns of nitrate application on the nitrate uptake, N2-fixation, nodulation and growth of white clover. Ann Bot. 1986;57:331–338.
- Streeter JG. Nitrate inhibition of legume nodule growth and activity I. Long term studies with a continuous supply of nitrate. Plant Physiol. 1985;77:321–324.
- Yashima H, Fujikake H, Sato T, et al. Systemic and local effects of long-term application of nitrate on nodule growth and N2 fixation in soybean (Glycine max [L.] Merr.). Soil Sci Plant Nutr. 2003;49:825–834.
- Yashima H, Fujikake H, Yamazaki A, et al. Long-term effect of nitrate application from lower part of roots on nodulation and N2 fixation in upper part of roots of soybean (Glycine max (L.) Merr.) in two-layered pot experiment. Soil Sci Plant Nutr. 2005;51:981–990.
- Melzer E, O’Leary MH. Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol. 1987;84:58–60.
- Pedersen P. 2004. Soybean growth and development. Ames, IA: Iowa State University, University Extension. Available from: https://crops.extension.iastate.edu/files/article/SoybeanGrowthandDevelopment_0.pdf
- Bender RR, Haegele JW, Below FE. Nutrient uptake, partitioning, and remobilization in modern soybean varieties. Agron J. 2015;107:563–573.
- Meinke DW, Chen J, Beachy RN. Expression of storage-protein genes during soybean seed development. Planta. 1981;153:130–139.
- Monma M, Sugimoto T, Monma M, et al. Starch breakdown in developing soybean seeds (Glycine max cv. Enrei). Agric Biol Chem. 1991;55:67–71.
- Wilson LA, Birmingham VA, Moon DP, et al. Isolation and characterization of starch from mature soybeans. Cereal Chem. 1978;55:661–670.
- Stevenson DG, Doorenbos RK, Jane JL, et al. Structures and functional properties of starch from seeds of three soybean (Glycine max (L.) Merr.) varieties. Starch. 2006;58:509–519.
- Chen Z, Ilarslan H, Palmer R, et al. Development of protein bodies and accumulation of carbohydrates in a soybean (Leguminosae) shriveled seed mutant. Am J Bot. 1998;85:492.
- Ainsworth EA, Rogers A, Vodkin LO, et al. The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 2006;142:135–147.
- Park J, Khuu N, Howard AS, et al. Bacteria- and plant-type phosphoenolpyruvate carboxylase isozymes from developing castor oil seeds interact in vivo and associate with the surface of mitochondria. Plant J. 2012;71:251–262.
- Tuin LG, Shelp BJ. In situ [14C] glutamate metabolism by developing soybean cotyledons. I. metabolic routes. J Plant Physiol. 1994;143:1–7.
- Matsuoka M, Hata S. Comparative studies of phosphoenolpyruvate carboxylase from C3 and C4 plants. Plant Physiol. 1987;85:947–951.
- Blonde JD, Plaxton WC. Structural and kinetic properties of high and low molecular mass phosphoenolpyruvate carboxylase isoforms from the endosperm of developing castor oilseeds. J Biol Chem. 2003;278:11867–11873.
- Rao S, Reiskind J, Bowes G. Light regulation of the photosynthetic phosphoenolpyruvate carboxylase (PEPC) in Hydrilla verticillata. Plant Cell Physiol. 2006;47:1206–1216.
- Uhrig RG, She YM, Leach CA, et al. Regulatory monoubiquitination of phosphoenolpyruvate carboxylase in germinating castor oil seeds. J Biol Chem. 2008;283:29650–29657.
- Chollet R, Vidal J, O’Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Biol. 1996;47:273–298.
