ABSTRACT
The thermophilic hydrogenotrophic methanogen Methanothermobacter sp. CaT2 aggregates by itself. CaT2 is known to have a surface sugar layer and extracellular proteins that may be related to its aggregation. Aggregation-enhanced mutants, CHA001 and CHA002, were isolated after repeated cultivation for more than two years. When treated with proteinase K, CHA001 and CaT2 similarly exhibited a very low degree of aggregation and CHA002 exhibited less aggregation but still retained aggregation, suggesting protein-based aggregation via extracellular proteins in both CHA001 and CHA002, presumably via a putative membrane-bound and extracellularly protruding protein, MTCT_1020, identified previously. Genomic analysis revealed that CHA001 and CHA002 shared a missense mutation of MTCT_1348 and had distinct mutations. These results suggested that the MTCT_1348 mutation provides subsidiary support to the adhesive function of extracellular proteins and that there is an additional mutation(s) in CHA002 for the non-proteinous aggregation capability.
GraphicAbstract
The genomic mutations in CHA001 and CHA002 derived from an aggregating methanogen, Methanothermobacter sp. CaT2 resulted in aggregation enhancement.
Methane is produced under either mesophilic or thermophilic conditions in bioreactors, generally in upflow anaerobic sludge blanket (UASB) reactors [Citation1,Citation2]. The fermentation efficiency of a UASB reactor depends on the activity of granules that have been formed [Citation3,Citation4]. The granules are composed of many kinds of microorganisms including primary fermenting bacteria, secondary fermenting bacteria, acetoclastic methanogens and hydrogenotrophic methanogens [Citation3,Citation5–Citation7]. Of these, methanogens appear to be crucial species for granule formation [Citation1,Citation8,Citation9]. The granule environment in thermophilic reactors allows methanogens to produce a 2-3-times larger amount of methane than that in mesophilic reactors [Citation10,Citation11]. However, granule formation is generally more difficult in thermophilic reactors than in mesophilic reactors [Citation11]. The difficulty of granule formation in thermophilic reactors may be due to the difference in aggregation mechanisms including responsible molecules under mesophilic and thermophilic conditions. Therefore, elucidation of the mechanisms by which thermophilic methanogens aggregate is an attractive issue for the stabilization of fermentation in UASB reactors.
Aggregation formation in many microorganisms is known to be established via various substances including extracellular DNA (eDNA), proteins, lipids and polysaccharides [Citation4,Citation10,Citation12–Citation14]. The substances and mechanisms for the aggregation of several mesophilic methanogens have been reported. Methanosarcina mazei forms polysaccharide-based extracellular polymers that attach to the outer surfaces of cells [Citation15]. Methanobacterium formicicum and Methanobrevibacter sp. produce extracellular polymers (ECPs) to spacially connected between aggregates formed by other species [Citation3,Citation14]. Methanococcus maripaludis adheres to the surface of other microbes via flagella and pili [Citation16]. However, studies on the aggregation of thermophilic methanogens have been limited. In the case of the thermophilic aggregating hydrogenotrophic methanogen Methanothermobacter sp. CaT2, its cell-surface sugar layer has been assumed to be responsible for its aggregation properties on the basis of physiological analysis [Citation17]. Comparative genomic analysis with other thermophilic hydrogenotrophic methanogens revealed that CaT2 has specific aggregation-related adhesion genes but pili (fimbriae) cause no strong cell-cell interaction of CaT2 [Citation17,Citation18]. Recently, the analysis of an aggregation-defective mutant has strongly suggested that MTCT_1020 is involved in the aggregation of CaT2 [Citation19]. However, it is not clear whether the cell-surface sugar or specific aggregation-related adhesion proteins are involved in the aggregation formation in addition to MTCT_1020.
In order to clarify the detailed molecular mechanisms underlying cell-cell interaction of CaT2, we isolated two mutants, CHA001 and CHA002, that exhibited significantly enhanced aggregation. Both mutants were characterized by morphological analyses, proteinase K and DNase I treatments, and methane production experiments. These phenotypic characterizations and genomic analysis of CHA001 and CHA002 revealed new aspects of the aggregation mechanism of CaT2.
Materials and methods
Strains and growth conditions
Methanothermobacter sp. CaT2 (DSM 24414 and NBRC 107770) and its mutant derivatives were used in this study. Strains were grown in W medium at 55°C as previously described [Citation17], or in W-R medium (W medium without resazurin solution) under a controlled atmosphere: 160 kPa H2/CO2 (80/20, v/v) (Sumitomoseiki, Osaka, Japan).
The aggregation-enhanced mutants CHA001 and CHA002 were isolated as follows. CaT2 was repeatedly cultivated in W medium at 55°C for over two years and then the culture was diluted and spread on W medium gellan gum plates [Citation20] to form colonies. About 200 colonies were individually cultivated in W medium under H2/CO2 (80/20, v/v) to observe aggregation under a microscope. After conducting the aggregation experiments several times, cells that showed strong aggregation were selected as an aggregation-enhanced mutant. The phenotype was confirmed under a phase-contrast microscope (Eclipse E600; Nikon, Tokyo, Japan). Aggregation size in phase contrast images was measured using the polygon selection and measure function in ImageJ software [Citation21].
Observation under a phase-contrast, scanning electron microscope (SEM) and a transmission electron microscope (TEM)
Sample preparation for observation by an SEM and a TEM was carried out as described previously [Citation19]. A fluorescence microscope (Eclipse E600; Nikon, Tokyo, Japan) was used to take phase-contrast images. SEM images were taken using the following procedure. Cell pellets were fixed for 3 h with 1.25% glutaraldehyde in 0.1 M cacodylate buffer (pH 6.5) at 4°C, washed with 0.1 M cacodylate buffer, and then suspended with a small amount of the same buffer. The suspension was dropped on a 0.2 μm cellulose membrane filter and dehydrated with a graded ethanol series and t-butylalchol. The dehydrated filter was dried using vacuum freeze-drying equipment (VFD-21S; Vacuum Device, Ibaraki, Japan). The dried membrane filter was then coated with platinum (Auto Fine Coater, JFC-1600; JEOL, Tokyo, Japan). Images of sections were obtained by observation using an SEM (10 kV, 30 μs; JSM-6360LA; JEOL, Tokyo, Japan). Observation by a TEM with ruthenium red was performed as previously described [Citation17], with slight modifications. Cell pellets were fixed for 5 h with 1.25% glutaraldehyde and 0.5 mg/mL ruthenium red in 0.1 M cacodylate buffer (pH 6.5) at 4°C, washed in 0.1 M cacodylate buffer, and post-fixed for 2 h in 1% osmium tetroxide containing 0.5 mg/mL ruthenium red in 0.1 M cacodylate buffer at 4°C. After being rinsed with 0.1 M cacodylate buffer, the pellets were embedded in a 3% agarose gel and dehydrated with a graded ethanol and acetone series. The dehydrated blocks were embedded in Epok 812 (Kohken, Tokyo, Japan). Ultra-thin sections were cut using a diamond knife mounted in an ultramicrotome (EM-UC6; Leica, Wetzlar, Germany) and then placed on copper grids and stained with uranyl acetate and lead citrate. TEM examination was performed using a Tecnai G2 Spirit electron microscope (Thermo Fisher Scientific, Massachusetts, USA) at an acceleration voltage of 80 kV.
Gas-phase analysis
Methane and hydrogen were analyzed using a gas chromatography system (GC-8A and C-R6A; Shimadzu, Kyoto, Japan) with a thermal conductivity detector and a column (2 m × 3 mm stainless steel) packed with Unibeads C 60–80 (GL Science, Tokyo, Japan). The analysis conditions were as follows: injection at 150°C, column at 145°C, detector at 150°C, current for the detector at 50 mA, and flow rate of carrier Ar gas at 30 mL/min.
Sedimentation index assay with various treatments
Cell growth and sample treatments for a sedimentation index assay were carried out as described previously [Citation19]. Cells were grown in W-R medium at 55°C for 4 days under H2/CO2 (80/20, v/v), recovered by centrifugation, washed twice, and suspended in 10 mM Tris-HCl buffer (pH 7). The suspensions were subjected to optical density measurement or to the following treatments: proteinase K treatment, DNase I treatment and addition of CaCl2, which were carried out as described previously [Citation19]. For proteinase K treatment, proteinase K (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was added to the cell suspension at a final concentration of 10 μg/mL and the solution was incubated for 1 h at 37°C. A control examination was performed by the addition of 10 mM Tris-HCl buffer instead of proteinase K. After incubation, cells were washed once with 1 mL of 10 mM Tris-HCl buffer and subjected to a sedimentation index assay. For DNase I treatment and addition of CaCl2, DNase I, CaCl2, and Tris-HCl buffer were added to cell suspensions at final concentrations of 5 μg/mL, 10 mM, and 10 mM, respectively, and the solutions were incubated for 1 h at 37°C. A control examination was performed by the addition of 10 mM Tris-HCl buffer instead of DNase I or CaCl2. After incubation, the cells were washed once and suspended in 10 mM Tris-HCl buffer. To compare the speeds of sedimentation of the cells, the cell suspensions were mixed briefly and their values of turbidity were measured at OD600 every 10 mins for 50 min using a photometer (Libra S12; Berthold Technologies, Bad Wildbad, Germany). The sedimentation index was calculated using the following equation: Sedimentation index = (value of turbidity at each measured time)/(value of turbidity at 0 min) × 100.
Extraction of genomic DNA
Cell growth and genomic DNA preparation were carried out as described previously [Citation19]. Cells were grown in 500 mL of W medium at 55°C for 4 days under H2/CO2 (80/20, v/v), recovered by centrifugation (12,000 rpm) at 4°C for 15 min, and washed twice with dH2O. Cell pellets were suspended in TE buffer. The suspension was then subjected to a 5-times freeze-thaw treatment with freezing at −80°C for 30 min and thawing at 55°C for 10 min. The treated solutions were incubated overnight at 55°C following the addition of SDS, proteinase K, NaCl, and RNase A at final concentrations of 0.5% (w/v), 100 μg/mL, 10 mM, and 10 μg/mL, respectively. After incubation, each solution was gently mixed by inversion with double the volume of TE-saturated phenol. A half-volume of chloroform was added, and the solution was kept on ice for 5 min before being centrifuged at 14,000 rpm for 15 min at 4°C. Sodium acetate (pH 8) was then added to the transferred upper aqueous layer to a final concentration of 0.3 M. The solution was gently mixed with an equal volume of isopropanol and centrifuged at 14,000 rpm for 15 min at 4°C. The precipitate was washed with an equal volume of 70% (v/v) ethanol and centrifuged at 14,000 rpm for 15 min at 4°C. The resultant precipitate was dried and resuspended in 100 μL of TE buffer. The sample of genomic DNA was further purified using a Genomic-tip 20 kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Genome sequencing and mapping analysis of CHA001 and CHA002 strains against CaT2 genome sequences
Five micrograms of genomic DNA of CHA001 and CHA002 dissolved in Tris-EDTA buffer was digested by a Covaris S-2 sonicator (Covaris, Woburn, MA, USA) with an average size of 500 bases. Construction of a DNA library and sequencing with 300 base by a massively parallel sequencer (MiSeq; Illumina KK, Tokyo, Japan) was followed as the previous study [Citation22]. The sequenced reads were screened by a quality score higher than the Phred score 30 and were trimmed 12 bases from the 5ʹ end and 20 bases from the 3ʹ end. The truncated reads less than 150 base or with ambiguous nucleotides was removed from further analysis. We previously reported the complete genome sequence for Methanothermobacter sp. CaT2, which is available at DDBJ/EMBL/GenBank, accession numbers AP011952.1 and AP011953.1 [Citation18]. Mutation sites were searched by read mapping using CLC Genomics Workbench version 7.5 (Qiagen, Venlo, Netherland) with following parameters; match score: 1; mismatch cost: 2; Insertion/deletion cost: 3; length fraction: 0.7; similarity fraction: 0.9. The filter settings for SNP and In-del calling was followed as previous study [Citation23].
Sequence data deposition
Illumina sequence reads of the CHA001 and CHA002 strains have been deposited in the DDBJ Sequence Read Archive. The BioSample accession numbers of CHA001 and CHA002 are SAMD00184547 and SAMD00184548, respectively.
Results
Characterization of the aggregation-enhanced mutants CHA001 and CHA002
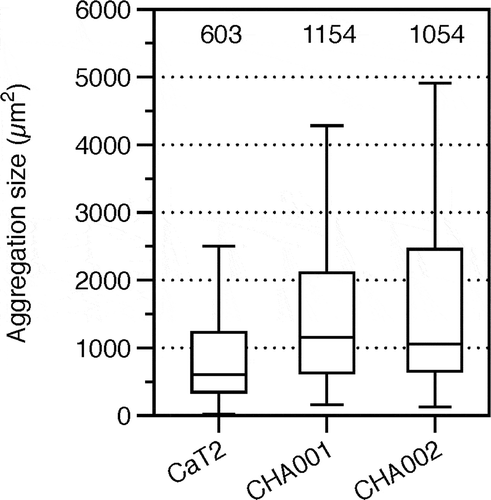
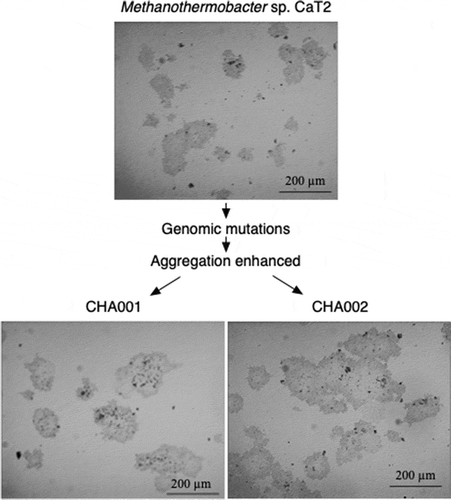
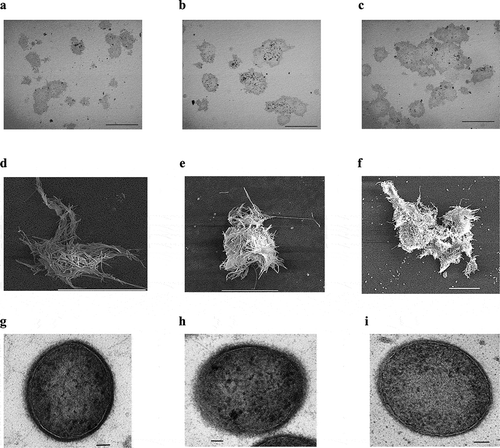
Methanothermobacter sp. CaT2 with an intrinsic aggregation ability was repeatedly cultivated for over two years, from which aggregation-enhanced mutants, CHA001 and CHA002, were obtained. Morphologies of the mutants were observed under a phase-contrast microscope, a scanning electron microscope (SEM) and a transmission electron microscope (TEM). Phase-contrast and SEM analyses revealed that CHA001 and CHA002 formed larger and denser aggregates than those formed by CaT2 () and that the size of aggregates of CHA002 were larger than that of aggregates of CHA001 (). In addition, the aggregation size of CHA001 was larger than that of CaT2 (). Moreover, SEM analysis revealed that the two mutants exhibited similar smooth cell-surface structures (). TEM analysis with sugar-staining samples revealed that the thicknesses of surface sugar layer of CHA001 and CHA002 were similar to that of CaT2 or slightly thinner, respectively, and that the thicknesses of cell wall of CHA001 and CHA002 were slightly thinner or thinner than that of CaT2, respectively ( and ). On the basis of these findings, it is likely that the order of aggregation strength is CHA002, CHA001, and CaT2 and that the mutations of both mutants have no effect on the surface sugar layer.
Table 1. Thicknesses of the cell wall (CW) and sugar layer (SL) in aggregation-enhanced mutants CHA001 and CHA002.
Figure 1. Images of Methanothermobacter sp. CaT2 and the aggregation-enhanced mutants, CHA001 and CHA002.
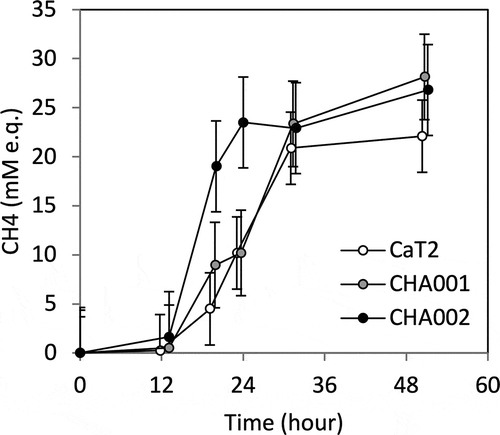
In order to examine the relationship between aggregation strength and methane productivity, the mutants and CaT2 were cultured with hydrogen as a substrate under a shaking condition (), which led to dispersal of large aggregates. The amount of methane produced by CHA001 was almost the same as that produced by CaT2. On the other hand, CHA002 produced methane at an early exponential phase compared to CaT2. It is thus assumed that CHA002 can perform efficient metabolism via its stronger cell-cell interaction that is sufficient to some extent even under a shaking condition.
Aggregation of CHA001 and CHA002 mediated by extracellular proteins
To evaluate the aggregation capability, experiments for determining the sedimentation index of cells were performed as described previously [Citation17]. The indices of CHA001 and CHA002 were similar to those of CaT2 ( and ), suggesting that the enhancement of aggregation of mutants compared with that of CaT2 were difficult to distinguish on sedimentation indices.
Figure 4. Effects of proteinase K treatment, DNase treatment and addition of CaCl2 on the sedimentation indices of CaT2 and CHA001.
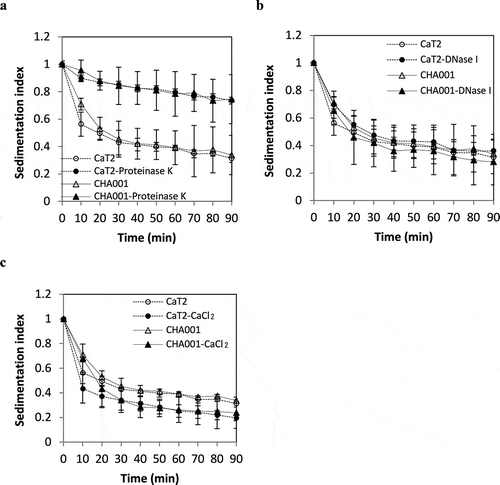
Figure 5. Effects of proteinase K treatment, DNase treatment and addition of CaCl2 on the sedimentation indices of CaT2 and CHA002.
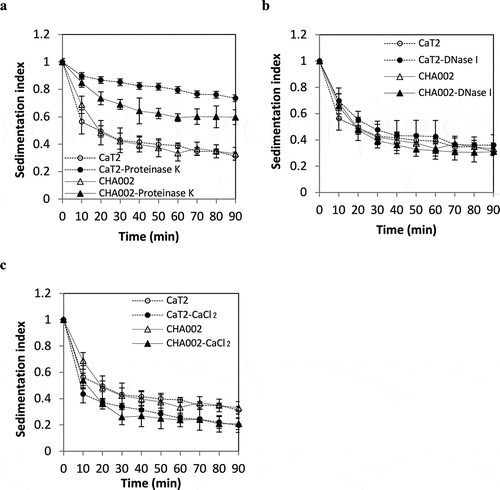
The results of proteinase K treatment in a previous study suggested that CaT2 has extracellular proteins responsible for its aggregation [Citation19]. Proteinase K treatment was thus performed for CHA001 and CHA002 to examine whether extracellular proteins are involved in their aggregations. CHA001 showed a large decrease in sedimentation as did CaT2 after the proteinase K treatment (). CHA002 also showed a decrease in sedimentation but still sedimented to some extent compared to that of CaT2 even after proteinase K treatment (). These findings suggest that CaT2 and that CHA002 has an additional aggregation factor(s) in addition to the proteinous factors.
Effects of DNase I and metal ions on aggregation of CHA001 and CHA002
The aggregation of CaT2 was not influenced by treatment with DNase I but was strengthened by the addition of Ca2+ [Citation19]. We thus examined the effects of treatment with DNase I and addition of CaCl2 on the sedimentation of CHA001 and CHA002 cells. DNase I treatment had almost no effect on the sedimentation of CHA001 or CHA002 cells ( and ), suggesting that eDNA is not engaged in the aggregation of either mutant. On the other hand, the addition of CaCl2 slightly increased the sedimentation of CHA001 and CHA002 as in the case of CaT2 ( and ). These results suggest that Ca2+ ions enhance the aggregation of both CHA001 and CHA002 as well as CaT2.
Genomic analysis of mutations in CHA001 and CHA002
CaT2 bears an extracellular protein MTCT_1020 that is responsible for its aggregation [Citation19]. CHA001 and CHA002 as derivatives of CaT2 exhibited enhanced aggregation and were thus expected to have mutations. Genomic sequencing was performed to identify the mutations in CHA001 and CHA002. Both mutants were found to have no mutation in MTCT1020. On the other hand, there were mutations in two genes and three genes in CHA001 and CHA002, respectively, in addition to a deletion mutation in the non-coding region in both mutants (). Of these, two mutations were shared by the mutants. One of the two mutations was an amino acid substitution mutation in MTCT_1348, which encodes a pyridoxamine 5ʹ-phosphate oxidase. In CHA001, there was an additional mutation causing an amino acid substitution in MTCT_0393, which encodes the ATP-binding subunit, consisting of 480 amino acid residues, of a cobalt transporter. The mutation in MTCT_0393 was a nonsense mutation, resulting in a reduction in the size of the product to 8 amino acid residues. In CHA002, there was an additional mutation causing an amino acid substitution in MTCT_0179 for acetate-CoA synthetase with 635 amino acid residues. The mutation in MTCT_0179 was a nonsense mutation, resulting in a small product with 72 amino acid residues. The other mutation in CHA002 was in MTCT_0445 for a DUF2085 domain-containing protein with 111 amino acid residues. The mutation in MTCT_0445 was a frame-shift mutation causing a small product with 50 amino acid residues. The other mutations in both mutants were found in a non-coding region.
Table 2. Summary of mutations in CHA001 and CHA002.
Discussion
Methanothermobacter sp. CaT2 possesses an extracellular protein, MTCT_1020, which is responsible for its aggregation [Citation19] in addition to a surface sugar layer, which has been proposed to be involved in cell-cell interaction [Citation17]. The former has genetically been suggested, but additional aggregating factors have not been investigated. In this study, aggregation-enhanced mutants were isolated and characterized. TEM observations suggested that the isolated mutants, CHA001 and CHA002, have a surface sugar layer similar to that of CaT2 or slightly thinner, respectively, and have a cell wall slightly thinner or thinner than that of CaT2, respectively ( and ). Such difference in these thicknesses may be due to mutations in the mutants. Treatment with proteinase K significantly reduced the sedimentation of both mutants. These findings suggest that the cell-cell interactions in aggregates of both mutants as well as in aggregates of CaT2 are mediated by extracellular proteins as core factors. However, CHA002, but not CHA001, aggregated to some extent even after proteinase K treatment ()), suggesting the presence of another factor(s) for the aggregation of CHA002.
Genomic analysis revealed that CHA001 and CHA002 have three and four mutations, respectively (). In a previous study, seven genes were predicted to be responsible for the aggregation of CaT2 [Citation17], but no mutations in these genes were found in either mutant (). Furthermore, although both mutants exhibited stronger aggregation than that of CaT2, there was no mutation in MTCT_1020. CHA001 and CHA002 share an amino acid substitution in MTCT_1348 (UniProt: T2GJ51), which is annotated to be a pyridoxamine 5ʹ-phosphate oxidase. However, the protein may play a different role than the annotated enzyme. The domain analysis suggested that its function is unclear and the domain IPR011576 of the protein appears to be unrelated to the catalysis of pyridoxamine 5′-phosphate oxidase. This mutation appears to strengthen the interaction by extracellular proteins because proteinase K treatment caused CHA001 to disperse aggregates to a level similar to that of CaT2 treated with proteinase K (). Therefore, the missense mutation of MTCT_1348 may strengthen the protein function of MTCT_1020 or increase the expression of MTCT_1020. The possibility of the contribution of the nonsense mutation of MTCT_0393 to the enhanced aggregation in CHA001 should not be ignored. The MTCT_0393 gene encoding an ATP-binding subunit of the ABC cobalt transporter may form an operon with MTCT_0394 for cobaltochelatase and MTCT_0390 for magnesium chelatase. The mutation in MTCT_0393 may affect the cobalt uptake of CHA001 from the environment and might cause the enhanced aggregation phenotype of CHA001 because cobalt is important for methanogen growth, especially on Methanothermobacter species [Citation24,Citation25]. CHA002 may have an additional factor(s) responsible for the remaining aggregation capability after proteinase K treatment. It is thus assumed that the factor(s) is not a protein. The remaining aggregation capability was provided by either a nonsense mutation in MTCT_0179 or a frame-shift mutation in MTCT_0445. MTCT_0179 (UniProt: T2GIP8) is annotated as acetyl-CoA synthetase but the BLAST search suggested that MTCT_1462 and MTCT_0630 are also acetyl-CoA synthetases because the identical homologies of their amino acid sequences with MTCT_0179 were 55% and 32%, respectively. Therefore, the activity for acetyl-CoA synthesis is conceivably complemented by the two paralogous proteins in CHA002. On the other hand, MTCT_0445 (UniProt: T2GI40) is an unknown membrane protein with three times the membrane-spanning segments and the mutation in CHA002 probably disrupts the function of MTCT_0445. The disruption is assumed to cause the thinner membrane and surface sugar layer in CHA002, resulting in the enhancement of cell aggregation. However, there is no evidence to support these assumptions. At least we can conclude that there is a non-proteinous material(s) as an aggregation factor, which seems to be emphasized by a mutation(s), in addition to the main extracellular proteins for aggregation in CaT2.
Author contribution
Conceived and designed the experiments: Kana Sumikawa, Tomoyuki Kosaka, Mamoru Yamada. Performed the almost experiments: Kana Sumikawa. Performed electroscopic observation: Kana Sumikawa, Koichi Udo. Performed genomic sequencing and analysis: Yu Kanesaki, Hirofumi Yoshikawa. Wrote the paper: Kana Sumikawa, Tomoyuki Kosaka, Mamoru Yamada.
Acknowledgments
We thank Mitsuaki Kimoto for obtaining the electron microscopic images and Kazunobu Matsushita, Toshiharu Yakushi, Naoya Kataoka, and Minenosuke Matsutani for their helpful discussion. This work was partially supported by KAKENHI Grant Number 16K07666, Japan (TK) and the MEXT-supported Program for Strategic Research Foundation at Private Universities, 2013-2017 (S1311017) (YK and HY).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Uemura S, Harada H. Microbial characteristics of methanogenic sludge consortia developed in thermophilic UASB reactors. Appl Microbiol Biotechnol. 1993;39:654–660.
- Schmidt JE, Ahring BK. Granulation in thermophilic upflow anaerobic sludge blanket (UASB) reactors. Antonie Van Leeuwenhoek. 1995 Nov;68:339–344.
- Schmidt JE, Ahring BK. Granular sludge formation in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol Bioeng. 1996 Feb 5;49:229–246.
- Liu Y, Xu HL, Yang SF, et al. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res. 2003 Feb;37:661–673.
- Satoh H, Miura Y, Tsushima I, et al. Layered structure of bacterial and archaeal communities and their in situ activities in anaerobic granules. Appl Environ Microbiol. 2007 Nov;73:7300–7307.
- Liu WT, Chan OC, Fang HH. Characterization of microbial community in granular sludge treating brewery wastewater. Water Res. 2002 Apr;36:1767–1775.
- Sekiguchi Y, Kamagata Y, Nakamura K, et al. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol. 1999 Mar;65:1280–1288.
- Fernández N, Díaz EE, Amils R, et al. Analysis of microbial community during biofilm development in an anaerobic wastewater treatment reactor. Microb Ecol. 2008 Jul;56:121–132.
- Zinder SH, Cardwell SC, Anguish T, et al. Methanogenesis in a thermophilic (58°C) anaerobic digestor: Methanothrix sp. as an important aceticlastic methanogen. Appl Environ Microbiol. 1984 Apr;47:796–807.
- Orell A, Fröls S, Albers SV. Archaeal biofilms: the great unexplored. Annu Rev Microbiol. 2013;67:337–354.
- Harada H, Ohashi A, Imachi H. Realization of super high-rate methane fermentation bioreactor and rRNA-based molecular analysis of sludge consortium. J Environ Biotechnol. 2004;4:19–27.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010 Sep;8:623–633.
- Liu YQ, Liu Y, Tay JH. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl Microbiol Biotechnol. 2004 Aug;65:143–148.
- Veiga MC, Jain MK, Wu W, et al. Composition and role of extracellular polymers in methanogenic granules. Appl Environ Microbiol. 1997 Feb;63:403–407.
- Robinson RW, Aldrich HC, Hurst SF, et al. Role of the cell surface of Methanosarcina mazei in cell aggregation. Appl Environ Microbiol. 1985 Feb;49:321–327.
- Jarrell KF, Stark M, Nair DB, et al. Flagella and pili are both necessary for efficient attachment of Methanococcus maripaludis to surfaces. FEMS Microbiol Lett. 2011 Jun;319:44–50.
- Kosaka T, Toh H, Fujiyama A, et al. Physiological and genetic basis for self-aggregation of a thermophilic hydrogenotrophic methanogen, Methanothermobacter strain CaT2. Environ Microbiol Rep. 2014 Jun;6:268–277.
- Kosaka T, Toh H, Toyoda A. Complete genome sequence of a thermophilic hydrogenotrophic methanogen, Methanothermobacter sp. strain CaT2. Genome Announc. 2013;1:e00672–13.
- Sumikawa K, Kosaka T, Mayahara N, et al. An aggregation-defective mutant of Methanothermobacter sp. CaT2 reveals unique protein-dependent aggregation. Microbes Environ. 2019 Sep 25;34:244–251.
- Nakamura K, Tamaki H, Kang MS, et al. A six-well plate method: less laborious and effective method for cultivation of obligate anaerobic microorganisms. Microbes Environ. 2011;26:301–306.
- Schneider CA, Rasband WS, Eliceiri KW. NH image to ImageJ: 25 years of image analysis. Nat Methods. 2012 Jul;9:671–675.
- Akuzawa S, Nagaoka J, Kanekatsu M, et al. Draft genome sequence of Oceanobacillus picturae Heshi-B3, isolated from fermented rice bran in a traditional japanese seafood dish. Genome Announc. 2016 Feb 4;4:e01621–15.
- Hirokawa Y, Kanesaki Y, Arai S, et al. Mutations responsible for alcohol tolerance in the mutant of Synechococcus elongatus PCC 7942 (SY1043) obtained by single-cell screening system. J Biosci Bioeng. 2018 May;125:572–577.
- Murray WD, Van Den Berg L. Effects of nickel, cobalt, and molybdenum on performance of methanogenic fixed-film reactors. Appl Environ Microbiol. 1981;42:502–505.
- Schönheit P, Moll J, Thauer RK. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123:105–107.