ABSTRACT
A gene encoding the enzyme trehalose-6-phosphate synthase (TPS), which is part of the TPS trehalose synthesis pathway, was cloned from the deep-sea psychrotolerant bacterium Microbacterium sediminis YLB-01 and expressed in Escherichia coli BL21. The exogenously expressed TPS exhibited highest similarity (80.93% identity) to Microbacterium sp. TPS. The purified recombinant TPS was cold-tolerant, with low thermostability. The optimum temperature for TPS activity was 40°C, and the enzyme retained 72.6% of its maximal activity at 4°C. The optimum pH was 7.5. TPS activity was cation-dependent, with Mg2+, Co2+, or Ba2+ being essential for maximum activity. The kinetic constants of the recombinant TPS reaction rates confirmed that it was cold-tolerant. Molecular dynamics analysis showed that TPS was more flexible (0.8741Å) at 4°C than 1GZ5, its homolog in the mesophilic bacterium E. coli, and superposition of the 3D enzyme structures supported this.
Graphical Abstract
Enzymatic properties of recombinant trehalose-6-phosphate synthase (TPS).
The non-reducing disaccharide trehalose (α-D-glucopyranosyl (1,1)-α-D-glucopyranoside) is present in a wide range of organisms, including bacteria, yeast, fungi, plants, insects, and lower invertebrates [Citation1]. Trehalose has diverse roles: it can be used as a carbon source, for carbohydrate storage, as a structural component of cell walls, and in stress resistance [Citation1,Citation2], also it is of great potential in the food processing industry [Citation3].
Five distinct biosynthetic pathways for trehalose have been reported, namely the TPS/TPP, TreY/TreZ, TreS, TreP, and TreT pathways [Citation4,Citation5]. Among them, the TPS/TPP pathway, which involves trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP), is the most common. These two enzymes, which are encoded by otsA and otsB, catalyze two steps in the pathway: TPS transfers glucose from UDP-glucose to glucose-6-phosphate (G6P) to produce trehalose-6-phosphate, and TPP acts as a dephosphorylase to produce free trehalose [Citation6]. The TPS/TPP pathway was first described in yeast, then identified in various other organisms. Until now, only a few TPSs have been identified in bacteria, and most of them are expressed by mesophilic bacteria [Citation7–Citation15].
Deep-sea environments have an average temperature of 4°C and are inhabited by abundant psychrophilic and psychrotrophic microorganisms, which are major sources of cold-active enzymes [Citation16,Citation17]. In a previous study, we reported the isolation of a psychrotolerant microorganism, Microbacterium sediminis YLB-01 [Citation17], from a deep-sea sediment sample collected from the southwestern Indian Ocean at a depth of 2327 m. Trehalose was found to be abundant in YLB-01 through metabolic analysis [Citation18], and whole-genome sequencing revealed that YLB-01 contained a complete TPS/TPP pathway. In this study, the gene encoding TPS was cloned and expressed in Escherichia coli BL21, and the recombinant TPS protein was then purified and characterized.
Materials and methods
Bacterial strains, plasmid, and culture conditions
Microbacterium sediminis YLB-01T (= DSM 23767T = CCTCC AB2010363T = MCCC 1A06153T) is a psychrotolerant actinomycete that was isolated originally from deep-sea sediment from the southwestern Indian Ocean. E. coli DH5α was used for cloning and plasmid amplification. E. coli BL21 (DE3) was transfected with the recombinant plasmid harboring the pET28a(+) expression vector (Novagen, USA) and used for protein expression. M. sediminis YLB-01T was cultured on trypticase soy broth (TSB) medium at 28°C, and the E. coli strains were cultured on LB medium at 37°C.
Cloning of the otsA gene
Genomic DNA was extracted from strain YLB-01T as described earlier [Citation19]. The otsA gene, which encodes TPS (GenBank: WP_017830820), was amplified from YLB-01 genomic DNA using the primer pair TPS/F (5′-GTGCCGCGCGGCAGCCATATGGCTAGCGACCGTGCCGATCTTGTCGTCG-3′) and TPS/R (5′-CATTTGCTGTCCACCAGTCATGCTAGCTCATTCGCGCGGCT
TCTTCCTC-3′). The primers were designed to include homologous sequences to facilitate recombination with the sequences upstream and downstream of the NheI restriction site in pET28a(+). The amplified otsA gene was ligated into the NheI-digested pET28a(+) expression vector to obtain the pET28a-otsA construct, which was transformed into E. coli BL-21 to express recombinant TPS.
TPS expression and purification
The recombinant BL-21 strain harboring pET28a-otsA was grown in 200 mL LB medium supplemented with 50 μg/mL kanamycin until the culture reached an optical density of approximately 0.6–0.8 at 600 nm. Then, the cells were induced with 1 mM IPTG (isopropyl-β-D-thiogalactopyranoside) and incubated at 30°C for 4 h. The cells were harvested by centrifugation (6000 × g, 5 min, 4°C) and resuspended in binding buffer (20 mM Tris/HCl (pH 7.4), 0.5 M NaCl, 20 mM imidazole). The cells were disrupted by freezing and thawing, followed by ultrasonication. The lysed samples were centrifuged at 8000 × g for 30 min at 4°C to remove cell debris. The His-tagged recombinant TPS was affinity-purified from the soluble fraction using a Ni–NTA agarose column eluted with 200 mM imidazole. The eluate was equilibrated by dialysis with 20 mM Tris/HCl (pH 7.4) to remove the imidazole, then concentrated by ultrafiltration. The purified TPS was analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% acrylamide gel, then aliquoted and stored at −80°C. The protein concentration was determined using a BCA Protein Assay Kit (Takara Biomedical Technology Co. Ltd, China).
TPS activity assay
TPS activity was assessed by measuring UDP release from UDP-glucose in the presence of G6P, as described previously [Citation6]. Briefly, purified TPS (100 ng) was added to a final volume of 100 μL 20 mM Tris-HCl (pH 7.4) containing 10 mM G6P, 5 mM UDP-glucose, and 2 mM MgCl2, and incubated at 40°C for 30 min. Then, the sample was boiled for 5 min, and the denatured proteins were removed by centrifugation. The amount of UDP in the supernatant was detected by measuring NADH oxidation in a linked assay with pyruvate kinase and lactic acid dehydrogenase. The assay was carried out in a multiwell plate containing 2.5 mM phosphoenol pyruvate, 0.5 mM NADH, 2 mM MgCl2, 50 mM Tris-HCl (pH 7.4), 3 U pyruvate kinase mL−1, and 3 U lactic acid dehydrogenase mL−1. The decrease in absorbance at 340 nm was measured continuously in a microtiter plate reader over a 20-min period.
Enzymatic characterization of the recombinant TPS
The temperature profile of the recombinant TPS was determined in 50 mM Tris-HCl (pH 7.4) between 4°C and 60°C. To evaluate its thermostability, the recombinant TPS was preincubated in the assay buffer at 50–70°C for 0, 2, 4, or 6 min, then immediately placed on ice and allowed to cool for 1 h. The residual activity was measured at the optimum temperature. The effect of pH on recombinant TPS activity was determined in 50 mM morpholine ethanesulfonic acid (pH 5.0–7.5) or 50 mM Tris-HCl (pH 7.0–9.0). The enzyme activity was measured as described in section 2.4. The effect of metal ions was determined in 50 mM Tris/HCl (pH 7.4) with or without 2 mM Mg2+, Co2+, Mn2+, Ba2+, Zn2+, K+, Ca2+, Hg2+, or Mg2+/Ca2+. To determine the kinetic parameters of TPS, UDP formation was measured spectrophotometrically in two separate assays. In the first assay, the G6P concentration was varied from 1.0 to 5.0 mM and the uridine diphosphoglucose (UDPG) concentration was maintained constant at 5.0 mM to assess the dependence of the recombinant TPS reaction rate on G6P. In the second assay, 1.0–5.0 mM UDPG plus 5.0 mM G6P were used to determine the dependence of the recombinant TPS reaction rate on UDPG.
Construction of molecular phylogenetic
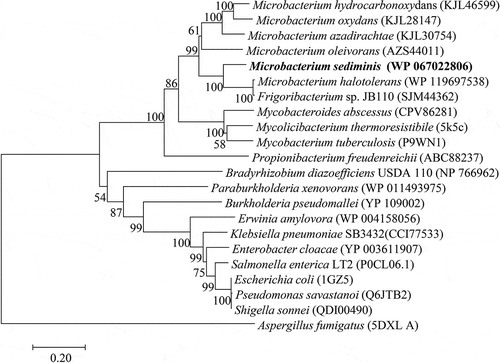
Eighteen reference otsA amino acid sequences, obtained from GenBank and PDB, were processed for Molecular Phylogenetic analysiss with MEGA version 7.0 software [Citation20], and clustering with the Maximum Likelihood method [Citation21] based on the JTT matrix-based model [Citation22]. The topologies of the trees were evaluated by performing a bootstrap analysis using 1000 replications.
Molecular dynamics analysis
Model preparation
The starting models for the molecular dynamics analysis were the 3D X-ray structures of E. coli (PDB ID: 1GZ5) TPS and Mycobacterium thermoresistibile (PDB ID: 5K5C) TPS. The initial TPS structure was the tertiary structure predicted using the Phyre2 server [Citation23]. First, water molecules and ligands were removed; then, heavy side-chain atoms missing from the structure were built in using the Phyre2 protein preparation package. All models were energy-minimized. Solvation was carried out using the simulation module of Discovery Studio 2019 (Biovia, San Diego, CA) with the default parameters.
Analysis of non-bond and hydrogen bond interactions
The numbers of non-bond intramolecular interactions (e.g. electrostatic and van der Waals forces) in the initial TPS, 1GZ5, and 5K5C structures were calculated using the “Receptor-Ligand Interactions” module in Discovery Studio 2019 (Biovia). Only salt bridges and conventional hydrogen bonds were considered in the molecular dynamics simulations.
Molecular dynamics simulations
Molecular dynamics simulations were performed using the “Standard Dynamics Cascade” protocol in Discovery Studio 2019 (Biovia) with the CHARMm force field. The system was gradually heated from 50 K to 277 K over 50 ps and equilibrated at this temperature. Further dynamics simulations (2 ns) were performed according to the OpenMM of dynamics (production) protocol. The coordinates of each atom were saved every 4 ps, thus producing a trajectory size of 500 snapshots. The trajectories were analyzed using the analysis trajectory protocol of Discovery Studio 2019. Structures were visualized and rendered using Discovery Studio 2019.
Results
Cloning of otsA, and expression and purification of recombinant TPS
The otsA gene was cloned from M. sediminis YLB-01 genomic DNA by PCR using primers TPS/F and TPS/R, which were designed to amplify the entire coding sequence for TPS. The protein encoded by otsA was 490 amino acids in length and had a calculated molecular weight of 55,145 Da and theoretical pI of 5.89. Homology analysis showed that YLB-01 TPS shared the highest similarity (80.93% identity) to Microbacterium sp. JB110 (GenBank: WP_105566529.1) TPS. YLB-01 TPS contained a conserved GT1 domain, indicating that it belonged to the glycosyltransferase GTB-type superfamily. These results indicate that the TPS expressed by YLB-01 is a novel enzyme that catalyzes trehalose biosynthesis in M. sediminis.
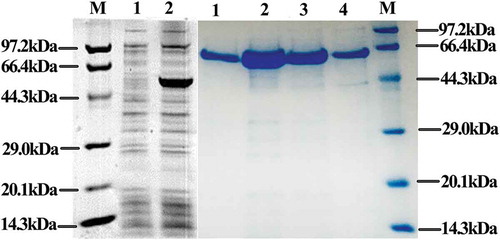
The PCR-amplified otsA sequence was introduced to pET28a to construct a recombinant plasmid. The recombinant TPS was expressed and purified, and SDS-PAGE analysis showed strong expression bands compared with the bands obtained for the control cell extracts (). The recombinant TPS had a molecular mass of approximately 56 kDa, which is consistent with the predicted molecular weight of YLB-01 TPS.
Enzymatic properties of recombinant TPS
Effects of temperature on TPS activity and stability
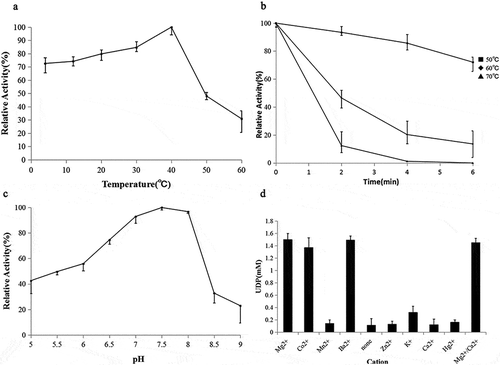
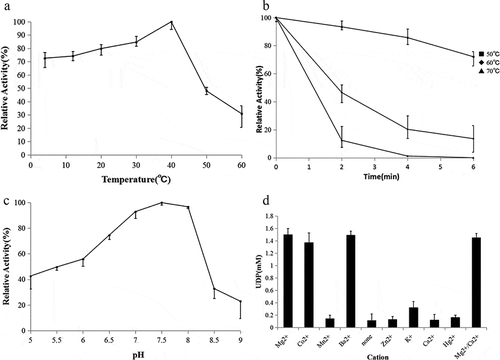
To determine the effect of temperature on the activity of the purified recombinant TPS, the temperature was varied from 4°C to 60°C. TPS activity increased with increasing temperature from 4°C and reached a peak at 40°C and then rapidly declined ()). Recombinant TPS had high activity at temperatures lower than 40°C and retained 84.7% of its maximal activity at 30°C, 79.8% at 20°C, 74.2% at 12°C, and 72.6% at 4°C. TPS activity decreased sharply above 40°C, so the heat stability of TPS was measured at 50–70°C, as shown in ). Recombinant TPS lost 50% of its maximum activity after 2 min at 60°C and was completely inactive after 4 min at 70°C, indicating that recombinant TPS was thermo-liable.
Effects of pH on TPS activity
To determine the effect of pH on the activity of the purified recombinant TPS, the pH was varied from 5.0 to 9.0 using the buffers described previously TPS activity increased with increasing pH from 5.0 and reached a peak at 7.5, but >90.0% of its optimum activity was retained at pH values from 7.0 to 8.0 ()). Furthermore, TPS was sensitive to both acid and alkaline environments, retaining only 42.8% and 23.0% of its optimum activity at pH 5.0 and pH 9.0, respectively.
Effects of metal ions on TPS activity
Recombinant TPS was cation-dependent and almost inactive in the absence of cations ()). In particular, Mg2+, Co2+, or Ba2+ was an absolute requirement for TPS activity. The presence of Ca2+ had no influence on enzyme activity, the presence of K+ resulted in much lower activity than the presence of Mg2+, Co2+, or Ba2+; and Mn2+, Zn2+, Ca2+, or Hg2+ did not stimulate recombinant TPS activity.
Kinetic constants for TPS reaction rates
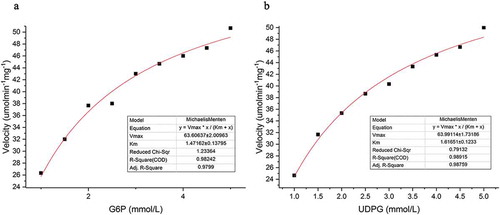
The kinetic constants for TPS reaction rates were determined at the optimum temperature (40°C) and pH (pH 7.0) by changing the concentrations of the substrates as follows: (i) increasing the G6P concentrations in the presence of saturated levels of UDPG (5 mM) or (ii) increasing the UDPG concentrations in the presence of saturated levels of G6P (5 mM). The results showed that the TPS reaction rates obeyed Michaelis–Menten kinetics (). The non-linear plots fit these data indicated Km and kcat values for G6P and UDPG of 1.47 mM/58.46s−1 and 1.62 mM/58.81s−1, respectively.
Analysis of TPS conformational flexibility
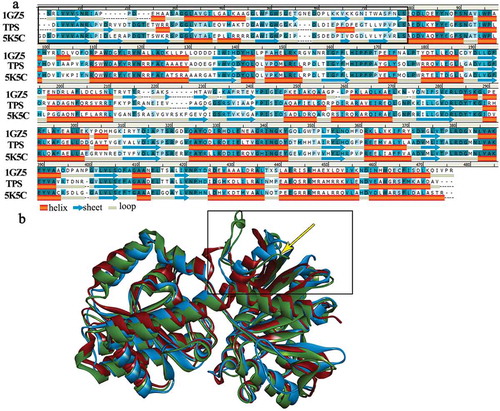
The TPS structural model and 3D X-ray structures of 1GZ5 and 5K5C showed 9, 24, and 12 salt bridges and 510, 448, and 564 hydrogen bonds, respectively. While non-bond interactions have previously been found to be important for TPS stability and rigidity [Citation24,Citation25], our results indicate that salt bridges also play a key role. – Secondary structural alignment and tertiary structure superposition analysis suggested that TPS had higher structural flexibility than 1GZ5 and 5K5C, despite having a large number of hydrogen bonds.
Molecular dynamics simulations were performed to further examine TPS flexibility. The root-mean-square fluctuations (RMSFs) of the active residues in the three structures were calculated as 0.8741Å for the TPS model, 0.799Å for 1GZ5, and 0.7955Å for 5K5C. The RMSF values for the three simulations are plotted in Figure S1. The highest RMSF value was for the TPS model (3.7Å), and the RMSF values for 1GZ5 and 5K5C were approximately 2.5Å, suggesting that TPS has the greatest flexibility out of these three enzymes.
As indicated in ), the TPS N-terminal appears to contain more loop structure than 1GZ5 and 5K5C, and 3D structure superposition analysis ()) supported this. Loop structures increase the local flexibility of a structure [Citation25], thus affecting the flexibility of protein molecules.
Analysis of phylogenetic tree and multiple amino acid sequence alignment
The phylogenetic analysis showed that TPS (WP067022806) formed a clade within the genus Microbacterium, and TPS performed a closer relative with 5K5C than 1GZ5 (). Multiple amino acid sequence alignments of TPS and other OTSA showed that all otsA have similar consensus (). Some different regions were observed in hydrophobicity analysis results, plotted base on TPS and 1GZ5 (Figure s2).
Discussion
The TPS that catalyzes trehalose synthesis in M. sediminis was cloned, expressed, and characterized in this study. The purified recombinant TPS was cold-tolerant, and, notably, retained 72.6% of its maximal activity at 4°C, which indicates greater cold tolerance than recombinant TPSs from mesophilic and thermophilic bacteria such as E. coli (in which TPS retains only 10%–15% of its maximum activity at 10°C) [Citation12] and Thermus thermophilus RQ-1 (in which TPS exhibits no activity under 30°C) [Citation11] (). Cold-tolerant enzymes such as cold-active lipases and proteases have been used in janitorial products and food manufacturing [Citation26], and cold-tolerant alkane degradation enzymes have been used in bioremediation [Citation27]. Trehalose has many uses, for example, in food processing and ophthalmology [Citation28,Citation29], so the discovery of a new cold-tolerant TPS enzyme has potential applications in the trehalose industry.
Table 1. Comparison of the enzymatic properties of trehalose-6-phosphate synthases expressed by various bacteria.
The recombinant TPS was found to require cations (Mg2+, Co2+, or Ba2+) for its catalytic activity, which is similar to most TPSs (). T. thermophilus RQ-1 TPS is an exception, as it is not divalent cation–dependent [Citation11]. Mg2+ is required for almost all TPSs, and some also require Co2+ [Citation30]. Mn2+ is necessary for the activity of the Mycobacterium smegmatis and Mycobacterium tuberculosis TPSs [Citation9,Citation10], but was not required for recombinant YLB-01 TPS. Ba2+, Mg2+, Ca2+ belong to group IIA in the element periodic table which share similar properties. The concentration of barium in seawater (21 ppb) is higher than that of manganese (0.4 ppb), ferrum (3.4 ppb), copper (0.9 ppb), and zinc (5 ppb) [Citation31].Ba2+ stimulated YLB-01 TPS catalytic activity in this study,tudy, which is the first report of Ba2+ stimulating the activity of a bacterial TPS. Although Ba2+ could enhance the activity of YLB-01 TPS, no complex structrues with Ba2+ were avaliable in PDB. Crystallization of TPS with Ba2+ as a ligand in the later study may contribute to understand how does this ion affect TPS activities in a deep-sea environment.
Some organisms adapt to cold environments by adjusting the kinetic parameters of the proteins they express; for example, by reducing Km and/or enhancing kcat constants [Citation32]. The Km of YLB-01 TPS was lower than that of the TPSs expressed by mesophilic bacteria such as E. coli, M. tuberculosis, and Ectothiorhodospira halochloris (). Furthermore, although only a small number of kcat values for bacterial TPSs have been calculated, the kcat of YLB-01 TPS was much higher than the kcat for the TPS expressed by the mesophilic E. coli (). Cold-active enzymes tend to have flexible structures, which helps improve their catalytic efficiency at low temperatures. Structural flexibility can be local or global [Citation33]. Molecular dynamics simulations indicated that the flexibility of the YLB-01 TPS structure may be localized to a loop region. The numbers of salt bridges and hydrogen bonds that contributed to the stability of the TPSs were confirmed in this study. Introducing more bonds (such as disulfide bonds) to TPS by site-directed mutagenesis may improve thermal stability, whereas the removal of bonds may reduce thermal stability. These results imply that YLB-01 TPS may have adapted to low temperatures by optimization of its kinetic parameters.
Table 2. Comparison of the kinetics of trehalose-6-phosphate synthases expressed by various bacteria.
Trehalose accumulates in the psychrotrophic Arthrobacter strain A3 under cold conditions [Citation31,Citation33]. Moreover, in low-temperature environments, trehalose synthesis is induced and is essential for E. coli viability [Citation2]. Trehalose is a major component of the glycolipids that are present in the cell walls of mycobacteria and corynebacteria [Citation3,Citation34]. However, although trehalose was abundant in YLB-01 [Citation18], there was no significant change in otsA expression or TPS abundance after prolonged cold treatment at 4°C for 7 days, as determined by transcriptomic and proteomic analysis (Tables S1 and S2, unpublished data). This indicates that trehalose may be used to construct YLB-01 cell components rather than participating in cold adaptation.
Author contributions
Xixiang Tang conceived and designed the research. Zhiwei Yi and Xiashutong Xu wrote the manuscript. Zhiwei Yi, Rufang Xu and Ping Huang conducted experiments and analyzed data. Libo Yu isolated the M. sediminis YLB-01 strain. All authors have read and approved this manuscript.
Supplement_table_YT.docx
Download MS Word (19.5 KB)Figure_S2_.pdf
Download PDF (155.8 KB)Figure_S1_.pdf
Download PDF (167.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Argüelles JC. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol. 2000;174(4):217–224.
- Kandror O, DeLeon A, Goldberg AL. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. PNAS. 2002;99:9727–9732.
- Elbein AD, Pan YT, Pastuszak I, et al. New insights on trehalose: a multifunctional molecule. glycobiology. 2003;13(4):17R–27R.
- Avonce N, Mendoza-Vargas A, Morett E, et al. Insights on the evolution of trehalose biosynthesis. BMC Evol Biol. 2006 Dec;19(6):109.
- Paul MJ, Primavesi LF, Jhurreea D, et al. Trehalose metabolism and signaling. Annu Rev Plant Biol. 2008;59:417–441.
- De Smet KA, Weston A, Brown IN, et al. Three pathways for trehalose biosynthesis in mycobacteria. Microbiology. 2000;146:199–208.
- Lapp D, Patterson BE, Elbein AD. Properties of a trehalose phosphate synthetase from Mycobacterium smegmatis. J Biol Chem. 1971;246:4567–4579.
- Lippert K, Galinski EA, Truper HG. Biosynthesis and function of trehalose in Ectothiorhodospira halochloris. Antonie Van Leeuwenhoek. 1993;63:85–91.
- Klutts S, Pastuszak I, Edavana VK, et al. Purification, cloning, expression, and properties of mycobacterial trehalose-phosphate phosphatase. J Biol Chem. 2003 Jan 24;278(4):2093–2100.
- Edavana VK, Pastuszak I, Carroll JD, et al. Cloning and expression of the trehalose-phosphate phosphatase of Mycobacterium tuberculosis: comparison to the enzyme from Mycobacterium smegmatis. Arch Biochem Biophys. 2004 Jun 15;426(2):250–257.
- Silva Z, Alarico S, da Costa MS. Trehalose biosynthesis in Thermus thermophilus RQ-1: biochemical properties of the trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Extremophiles. 2005 Feb;9(1):29–36.
- Jang IC, Oh SJ, Seo JS, et al. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol. 2003 Feb;131(2):516–524.
- Cardoso FS, Castro RF, Borges N, et al. Biochemical and genetic characterization of the pathways for trehalose metabolism in Propionibacterium freudenreichii, and their role in stress response. Microbiology. 2007 Jan;153(Pt(1)):270–280.
- Nobre A, Alarico S, Fernandes C, et al. A unique combination of genetic systems for the synthesis of trehalose in Rubrobacter xylanophilus: properties of a rare actinobacterial TreT. J Bacteriol. 2008 Dec;190(24):7939–7946.
- Seo H, Koo Y, Lim J, et al. Characterization of a Bifunctional Enzyme Fusion of Trehalose-6-Phosphate Synthetase and Trehalose-6-Phosphate Phosphatase of Escherichia coli. Appl Environ Microbiol. 2000;66:2484–2490.
- Yan BQ, Chen XL, Hou XY, et al. Molecular analysis of the gene encoding a cold-adapted halophilic subtilase from deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913: cloning, expression, characterization and function analysis of the C-terminal PPC domains. Extremophiles. 2009;13:725–733.
- D’Amico S, Collins T, Marx JC, et al. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006 Apr;7(4):385–389.
- Xia JM, Hu XM, Huang CH, et al. Metabolic profiling of cold adaptation of a deep-sea psychrotolerant Microbacterium sediminis to prolonged low temperature under high hydrostatic pressure. Appl Microbiol Biotechnol. 2019;1–13.
- Yu L, Lai Q, Yi Z, et al. Microbacterium sediminis sp. nov., a psychrotolerant, thermotolerant, halotolerant and alkalitolerant actinomycete isolated from deep-sea sediment. Int J Syst Evol Microbiol. 2013 Jan;63(Pt 1):25–30.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376.
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8(3):275–282.
- Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845.
- Feller G. Molecular adaptations to cold in psychrophilic enzymes. Cell Mol Life Sci. 2003;60(4):648–662.
- Siddiqui KS, Cavicchioli R. Cold-adapted enzymes. Annu Rev Biochem. 2006;75:403–433.
- Marsha C. Cold-adapted enzymes. Annu Rev Biochem. 1997;15:359–364.
- Nichols D, Bowman J, Sanderson K, et al. Developments with Antarctic microorganisms: culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr Opin Biotech. 1999;10:240–246.
- Rose B. Trehalose, a new approach to premium dried foods. Trends Food Sci Tech. 1991;2:166–169.
- Luyckx J, Baudouin C. Trehalose: an intriguing disaccharide with potential for medical application in ophthalmology. Clin Ophthalmol. 2011;5:577–581.
- Li YT, Zhang HH, Sheng HM, et al. Cloning, expression and characterization of trehalose-6-phosphate phosphatase from a psychrotrophic bacterium, Arthrobacter strain A3. World J Microbiol Biotechnol. 2012 Aug;28(8):2713–2721.
- Turekian KK, Karl K. Turekian. Found Earth Sci Ser. 1968.
- D’Amico S, Claverie P, Collins T, et al. Molecular basis of cold adaptation. Philos Trans R Soc Lond B Biol Sci. 2002 Jul 29;357(1423):917–925.
- Chen XM, Jiang Y, Li YT, et al. Regulation of expression of trehalose-6-phosphate synthase during cold shock in Arthrobacter strain A3. Extremophiles. 2011;15(2011):499–508.
- Lapp D. Cord factor and related trehalose esters. Chem Phys Lipids. 1976;16:91–106.