ABSTRACT
Gamma-aminobutyric acid (GABA) is produced by Lactobacillus brevis using date residue fermentation. In this study, the GABA production method was improved, for which L. brevis strain JCM 1059T was the most efficient among the four L. brevis strains examined. This was presumably due to a difference in the expression level of the gene encoding glutamate decarboxylase that catalyzes GABA synthesis.
Abbreviation
GABA: gamma-aminobutyric acid
Dates are the fruit of the palm tree Phoenix dactylifera, which mainly grows in the arid and semi-arid regions of desert countries [Citation1]. Dates are rich in nutrition: the semi-dried and dried forms contain 50–80% (w/w) carbohydrate, predominantly glucose and fructose, and ranges of minerals and vitamins [Citation2,Citation3]. Date puree has been fermented with autochthonous lactic acid bacteria including Lactobacillus plantarum [Citation4] for the production of gamma-aminobutyric acid (GABA), which is known as a promising functional dietary supplement, and a conventional assay method for it was recently reported [Citation5]. Subsequently, GABA production was performed with Lactobacillus brevis type strain, JCM 1059T with date residue, which is often discarded by food industry manufacturers after puree production. Although the date residue still appeared to be enriched in carbohydrate, thus enabling bacterial growth, a substrate L-glutamate should be added for GABA production [Citation6]. In this study, aiming at future application, we improved the GABA production method using L. brevis JCM 1059T with date residue extract. As some L. brevis strains are known to be efficient cellular tools for producing GABA [Citation7–Citation9], here we also examined whether GABA production occurred with L. brevis strains other than JCM 1059T, which was then validated at the molecular levels.
First, instead of date residue, its extract was examined for GABA production using L. brevis JCM 1059T for easier handling. A mixture of date residue (derived from P. dactylifera L. cultivar Sayer) and tap water in a ratio of 1:1 (w/w) in a bag was heated at 70°C for 20 minutes, and then centrifuged (3,000 rpm, 5 min). The resulting supernatant was regarded as date residue extract. Our preliminary study revealed that the time- and cost-consuming enzyme-treatment of date residue, which had been indispensable for our previous method [Citation6], could be omitted with the addition of yeast extract. With the present method, 10 mL date residue extract, 1.5% (w/v) mono sodium L-glutamate (Ajinomoto, Japan), 0.28% (v/v) acetate (Otafuku Vinegar Brewery, Japan), and 0.1% (w/v) yeast extract (Oriental Yeast, Japan), all of which were food-grade, were mixed, followed by heating at 70°C for 20 minutes. The present method resulted in an improvement of the previous method in terms of operation time; approximately 70% compared with that for our previous method, and the pH of this material was ~4.7, the best one for GABA production with the previous method using date residue [Citation6].
The heated date residue extract (10 mL) with the necessary ingredients above was then inoculated with the L. brevis JCM 1059T cell suspension to give a final cell concentration of 108 cfu/mL, followed by fermentation at 25°C for six days, as described previously [Citation6]. With the six-day fermentation period, the GABA content steadily increased as observed with our previous method [Citation6], where experimental conditions such as the L. brevis strain used and fermentation temperature were the same as the present ones. With the present method, L. brevis strain JCM 1059T could produce GABA (~38 mM, ) to a similar extent to that with the previous one [Citation6], for which the GABA content had been measured with an amino acid analyzer (JEOL JLC-500/V, Japan), as described previously [Citation6]. Yeast extract significantly enhanced the GABA production by JCM 1059T in the presence of date residue extract (2nd and 4th bars in ). Presumably, yeast extract functioned as nitrogen source affecting GABA production, as discussed previously [Citation9]. The GABA production occurred to the same extent when the amount of date residue extract was increased to 20 L (data not shown), indicating its industrial application.
Figure 1. Effects of date residue and yeast extracts on GABA production by L. brevis JCM 1059T and other strains.
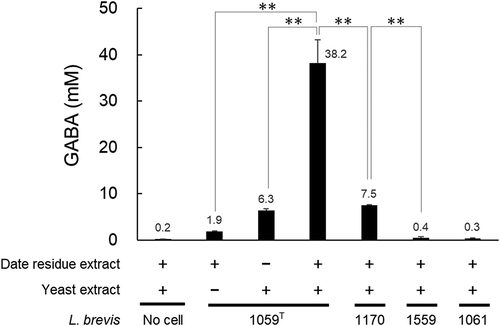
We then performed GABA production test under the same fermentation conditions using other L. brevis strains, JCM 1061, JCM 1170, and JCM 1559, which had been selected from the Japan Collection of Microorganisms, RIKEN BRC. The cell growth occurred similarly, and amount of GABA produced resulted in the order of JCM 1059T > JCM 1170 > JCM 1559 = JCM 1061 (4th to 7th bars in ). Therefore, strain JCM 1059T is the most suitable for GABA production with the present method. There is presently no explanation for the difference in the GABA production at the cellular level, but difference in the sources of isolation is of interest in the future study; JCM 1059T was isolated from human feces and other three were from plants.
We next examined the reasons for the differences in GABA production among the four L. brevis strains at the molecular level. It is known that L. brevis has two isoforms of glutamate decarboxylase (denoted as GadA and GadB, EC 4.1.1.15) [Citation10–Citation12], both catalyzing GABA synthesis from L-glutamate irreversibly. Both the gadA and gadB genes could be amplified from chromosomal DNA samples of the four strains by using specific primers (Table S1) that had been designed from the deposited L. brevis ATCC467 genome sequence (Accession number; NC_008497.1). The deduced amino acid sequences revealed that they exhibited some heterogeneity, but catalytic amino acid residues related to pyridoxal phosphate binding [Citation13,Citation14] were completely conserved (Fig. S1). Therefore, the differences in GABA production among the four strains may not be due to enzymatic catalysis by GadA and GadB, assuming that these enzymes are equally expressed in all strains.
To determine the relationship between the GABA production described above and gad gene expression in the four L. brevis strains, a reverse-transcription quantitative PCR (qRT-PCR) assay was further carried out. L. brevis cells were harvested in triplicate after six-day fermentation, which had been examined above (), and also after three-day fermentation in order to examine gene expression at an early phase during the GABA production. Total RNA was isolated using a PureLinkTM RNA Mini kit (Invitrogen, USA), followed by genomic DNA removal with a ReverTra Ace qPCR-RT Master Mix with a gDNA Remover kit (TOYOBO, Japan). For qRT-PCR, extracted mRNAs were reverse transcribed to complementary DNA with random primers using Superscript III (Invitrogen, USA), and then quantified with a MyGo Mini Real Time PCR system (IT-IS Life Science, Ireland) using specific primers for the gadA, gadB and 16S rRNA genes (Table S2) based on the L. brevis strain ATCC367 genome sequence. Relative expression of the target genes was calculated by a comparative 2−ΔΔCt method [Citation15] with the 16S rRNA gene expression levels as the endogenous reference. For the statistical analysis, a t-test was applied to reveal significant differences in the expression levels of the target genes in triplicate.
The relative expression levels of the gadA and gadB genes were compared with each other as well as between the two different growth phases (three- and six-day fermentation), and also among the four L. brevis strains. The gadA genes in the JCM 1059T and JCM 1061 strains at both growth phases exhibited significantly similar expression levels (). As the former strain produced GABA while the latter did not (), the GABA production observed here is not due to gadA expression. In the JCM 1059T strain after three-day fermentation, the relative expression level of gadB significantly differed by 3.04-fold from that of gadA, which had been regarded as the control (). These results indicated that the GABA production was conferred by GadB rather than GadA, consistent with the previous results under acidic conditions [Citation12]. Expression of the gene encoding GadB is co-regulated with L-glutamate/GABA antiporter gene, gadC in the same operon [Citation12], indicating that the increased expression level of gadB observed in this study may result in enhancement of GABA synthesis and export.
Figure 2. Relative expression levels of the gadA and gadB genes in L. brevis JCM 1059T, JCM 1170, JCM 1559, and JCM 1061 after three- and six-day fermentation.
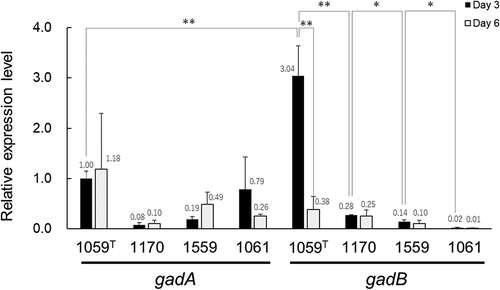
In the JCM 1059T strain, the relative expression level of gadB was significantly reduced after six-day fermentation as compared with that after three-day (), indicating that gadB expression at the early phase (three-day fermentation) resulted in the GABA accumulation at the later phase (six-day fermentation). Among the four strains after three-day fermentation, the relative expression levels of gadB decreased in the order JCM 1059T > JCM 1170 > JCM 1559 > JCM 1061. This order was the same with that of GABA production after six-day () and also after three-day fermentation (Fig. S2). Taken together, differences in GABA production among the four strains may be attributed to the expression level of gadB at the early phase.
Although efficiency for GABA production using L. brevis JCM 1059T in the present study was the same as that in our previous one [Citation6], operation time and cost before fermentation were reduced, and thus the method could be improved. With the present improved method using date residue extract and by adding yeast extract, GABA production was found to be the most efficient with the JCM 1059T strain among the four L. brevis strains examined, where the gene expression level of gadB was possibly responsible for the production. As date residue is usually discarded during industrial food processing, the present findings will contribute to efficient and economical GABA production in future food industry.
Author contributions
M. H., S. F., and Y. S. conceived and performed the experiments, and analyzed the data. All the authors wrote the paper.
200107_GABA_Supplement.docx
Download MS Word (241.5 KB)Acknowledgments
The L. brevis strains used were provided by the Japan Collection of Microorganisms, RIKEN BRC, which is participating in the National BioResource Project of the MEXT, Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Supplementary material
Supplemental data of this article can be accessed here.
Additional information
Funding
References
- Chao CT, Krueger RR. The date palm (Phoenix dactylifera L.): overview of biology, uses, and cultivation. HortScience. 2007;42:1077–1082.
- Roukas T, Kotzekidou P. Pretreatment of date syrup to increase citric acid production. Enzyme Micorb Technol. 1997;21:273–276.
- Chandrasekaran M, Bahkali A. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology – review. Saudi J Biol Sci. 2013;20:105–120.
- Di Cagno R, Filannino P, Cavoski I, et al. Bioprocessing technology to exploit organic palm date (Phoenix dactylifera L. cultivar Siwi) fruit as a functional dietary supplement. J Funct Foods. 2017;31:9–19.
- Nishiyama T, Sulistyaningdyah WT, Ueda K, et al. Gaba enzymatic assay kit. Biosci Biotechnol Biochem. 2020;84:118–125.
- Hasegawa M, Yamane D, Funato K, et al. Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. J Biosci Bioeng. 2018;125:316–319.
- Kim JY, Lee MY, Ji GE, et al. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol. 2009;130:12–19.
- Lim HS, Cha IT, Roh SW, et al. Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J Microbiol Biotechnol. 2017;27:450–459.
- Wang Q, Liu X, Fu J, et al. Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microb Cell Fact. 2018;17:80.
- Huang J, Mei LH, Sheng Q, et al. Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chin J Chem Eng. 2007;15:157e161.
- Fan EY, Huang J, Hu S, et al. Cloning, sequencing and expression of a glutamate decarboxylase gene from the GABA-producing strain Lactobacillus brevis CGMCC 1306. Ann Microbiol. 2012;62:689e698.
- Lyu C, Zhao W, Peng C, et al. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb Cell Fact. 2018;17:180.
- Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym. 2000;10:67–79.
- Huang J, Fang H, Gai Z-C, et al. Lactobacillus brevis CGMCC 1306 glutamate decarboxylase: crystal structure and functional analysis. Biochem Biophys Res Commun. 2018;503:1703–1709.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25:402–408.
