ABSTRACT
Acute stress induces tissue damage through excessive cellular apoptosis. In our study, the effects of sesamin on apoptosis and wound healing were investigated. The angiogenesis effect of sesamin was evaluated by the abilities of adherence, migration and tube formation in human umbilical vein endothelial cells (HUVECs). Our data demonstrated that treatment with sesamin dose-dependently promoted the proliferation, adherence, migration and enhanced their angiogenic ability in vitro. Moreover, the increased apoptosis in HUVECs, which stimulated by tert-butyl hydroperoxide (TBHP) was significantly attenuated by the sesamin treatment. Furthermore, we revealed that neogenesis of granulation tissue and deposition and remodeling of the collagen matrix were accelerated by the administration of sesamin in our in vivo study. These results confirm that sesamin accelerates wound healing at least partly through its antiapoptotic effects on endothelial cells at the injury site. Thus, sesamin represents a potential therapeutic medicine for vessel injury-related wounds.
Graphic abstract

Sesamin accelerated wound healing in rats via increasing granulation thickness and collagen deposition.
Skin wounds occur for a number of reasons, such as burns, trauma, and chronic diseases, and these wounds frequently cause pain and lead to infections and even amputation, thus resulting in decreased quality of life and increased socio-economic burden; therefore, wounds represent a major public health problem worldwide [Citation1,Citation2]. The most difficult problem associated with wound healing is determining how to shorten the wound closure time while reducing scarring. In the wound bed, newly formed blood capillaries and/or angiogenesis are essential for tissue repair because they are responsible for the transport of nutrients and oxygen to the cells, which supports survival and proliferation. Endothelial cells (ECs) are among the major cell types that constitute vascular beds. Damage to ECs results in vascular dysfunction, which impacts many diseases [Citation3]. ECs are susceptible to injury, and the reaction of ECs to oxidative stress has been recognized as a key mechanism in EC injury [Citation4]. Oxidative stress arises from the excessive accumulation of reactive oxygen species (ROS), which include the superoxide anion radical (O2-), hydrogen peroxide (H2O2), and highly reactive hydroxyl radical (OH) [Citation5]. ROS are continuously generated in cells through biophylaxis and as products of cellular oxidation–reduction processes [Citation6]. Moreover, it has been shown that high levels of ROS are among the main contributors to EC injury [Citation7]. As an inducer of apoptosis, oxidative stress can cause vascular dysfunction [Citation8]. In addition, apoptosis is involved in the occurrence and development of a variety of vascular pathological injuries [Citation9]. Thus, whether apoptosis participates in the pathology of vascular injury induced by oxidative stress should be further studied. Among the various ROS, H2O2 is known as the most potent ROS involved in intracellular signaling, which transduces stimuli into downstream signaling cascades. Accordingly, in vitro models have been extensively used to study oxidative stress-related cell injury [Citation10].
The treatment effect of traditional Chinese medicine have been reported by numerous studies, especially in vascular dysfunctional diseases [Citation11]. Sesamin, which is a major lignan extract from sesame oil, has been reported to have antioxidant, antiapoptotic, anti-inflammatory, atherosclerosis-lowering, and antihypertension functions [Citation12–Citation14]. Neuronal or microglia cells injured by hypoxia or oxidative stress could be restored by the antioxidant function of sesamin [Citation15,Citation16]. However, the effects of sesamin have not been investigated in endothelial cells (ECs), which are an indispensable component of angiogenesis and wound healing.
In our study, the antiapoptotic effects of sesamin on human umbilical vein endothelial cells (HUVECs) in which apoptosis was induced by tert-butyl hydroperoxide (TBHP) were evaluated in vitro. Furthermore, an in vivo study used a full-thickness cutaneous wound model in which sesamin was administered to evaluate the wound healing therapeutic potential of this extract.
Materials and methods
Cells treatment protocols
HUVECs were obtained from the American Type Culture Collection (Manassas, VA), and 5 × 105 HUVECs were cultured on 6-well plates with 10% FBS, 1% penicillin and streptomycin in 1640 medium (Sigma-Aldrich, St Louis, MO USA) until 80–90% confluence. Sesamin (C20H18O6, CAS#:607-80-7, HPLC>98%) was purchased from Shanghai PureOne biotechnology (Shanghai, China). For the in vitro studies, sesamin was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mM and diluted appropriately with cell culture medium (final DMSO concentration ≤ 1%). HUVECs were incubated with different doses of sesamin (6.25, 12.5, 25, 50, 100 μM) for 48 h or TBHP for different lengths of time (0.5, 1, 2, 4 h), or they were treated with sesamin (50 μM) for 48 h followed by TBHP stimulation for 1 h in the dark at 37°C under 5% CO2.
Assessment of cellular viability and proliferation
Cell viability was evaluated by Cell Counting Kit-8 (CCK-8, Kumamoto, Japan) assays according to the manufacturer’s instructions. In brief, HUVECs were seeded on 96-well plates for 24 h. After sesamin or TBHP treatment, 10 µL tetrazolium substrate was added to each well, which were cultured in the dark at 37°C under 5% CO2 for 1 h. The absorbance was measured using a microplate reader (Thermo Scientific, Multiskan GO) at 450 nm.
Cell adhesion assay
Cell-matrix adhesion assay
The cell-matrix adhesion assay was performed as previously described [Citation17]. Briefly, HUVECs (1 × 104cells per well), pretreated with sesamin (50 μM), were seeded into culture plates pre-coated with human fibronectin (1 μg/mL, F0895, Sigma) and incubated at 37°C for 30 min followed by washing with PBS three times. Adherent cells were then fixed with 4% paraformaldehyde for 30 min and stained with Hoechst 33258 (Beyotime, Shanghai, China) for 30 min. Samples were then washed with PBS three times. Five random fields were visualized and counted under a fluorescence microscope (Nikon).
Cell–cell adhesion assay
The effect of sesamin on the intercellular adhesion was studied by a cell–cell adhesion assay [Citation17]. Briefly, completely confluent HUVECs were first stained with Hoechst 33258. Another group of HUVECs was pretreated with sesamin (50 μM) for 48 h, followed by treatment with calcein-AM, a cyto-membrane tracker, for 30 min at 37°C, and then seeded onto the Hoechst 33258-labeled cell monolayer (acceptor cells). After 30 min, the attachment and spread of seeded cells that remained attached after three gentle washes with PBS were monitored and recorded with a fluorescence microscope (Nikon).
Cell migration assay
Transwell (Corning, 8 μm, US) assay was used to evaluate the migration ability of HUVECs. Briefly, 1 × 105cells were seeded on the upper chamber, while the lower chamber contained a culture medium with 1% FBS and with or without sesamin (50 μM). After 12 h of incubation, the membrane of the upper chamber was carefully removed with a cotton swab, fixed in 4% paraformaldehyde for 30 min at room temperature and then stained with crystal violet to quantify the migrated cells on them. The migrated cells were examined using an inverted microscope (Nikon, ECLIPSE Ti, Japan). All experiments were performed three times, and five random fields of HUVECs were counted for statistical analysis.
In vitro tube formation assay
A tube formation assay on Matrigel (BD Biosciences, US) was performed to evaluate the effects of sesamin on HUVECs’ morphogenesis and tube formation capacity. Briefly, Matrigel solution was thawed at 4°C overnight and then placed into a μ-Slide (10 μL per well, IBIDI, Germany) in a cell incubator for 1 h to solidify. A total of 5000 cells pre-treated with sesamin (50 μM) were seeded in the Matrigel precoated μ-Slide. Tube formation was observed and quantified, and five independent fields were counted for an average number under an inverted light microscope (Nikon).
Immunofluorescence
The immunofluorescence assays were conducted using 3 × 105 HUVECs cultured on 6-well plates slides with 10% FBS, 1% penicillin and streptomycin in 1640 medium, with the cells allowed to attach for 24 h. Then, the cells were treated with sesamin (25 and 50 μM) for 48 h followed by TBHP-induced apoptosis for 1 h. For cleaved caspase-3 (Cell Signaling Technologies, USA) staining, the slides were fixed with 4% paraformaldehyde for 15 min followed by 0.5% Triton X-100 permeation for 10 min. Then, the slides were blocked with 10% bovine serum albumin (BSA) in an incubator for 1 h followed by incubation with anti-cleaved caspase-3 (1:200) at 4°C overnight. Finally, the slides were washed with PBS 3 times and incubated with Alexa Fluor 488 donkey anti-rabbit secondary antibody (Abcam, USA) for 1 h in an incubator followed by washing with PBS 3 times and labeling with 4ʹ,6-diamidino-2-phenylindole (DAPI, Beyotime, China) for 1 min at room temperature. All the images were captured using a fluorescence microscope (Nikon, Japan).
Animal experiment
Twenty-four Sprague Dawley (SD) rats were purchased from the SLAC laboratory animal company (Shanghai, China). All animal procedures were approved by the Wenzhou Medical University Animal Care and Use Committee, and the animals were cared for and handled according to the guidelines set forth by the Chinese National Institutes of Health. Before wound surgery, all rats were anesthetized with 1% pentobarbital sodium (400 µL/100 g). After anesthesia, all rats were shaved and sterilized, and then two symmetrical 20 mm diameter full-thickness wounds were made on the rat’s back. The rats in the treatment group were immediately intravenously injected with 30 mg/kg/day sesamin, which was dissolved in saline, which continued daily until the rats were sacrificed [Citation18]. The control group was injected an equivalent amount of normal saline. Wound images of rats were taken after 14 days, and the wound margins were traced using Image-Pro Plus 6.0 software.
Histology
All rats were sacrificed with 1% pentobarbital sodium (600 µL/100 g) at specific time points after surgery. First, skin wounds with 2 mm margins were dissected, treated with 4% paraformaldehyde for 24 h to fix the tissue and then embedded in paraffin. Longitudinal sections (5 µm thick) were mounted on slides. Hematoxylin and eosin (H&E) staining and Masson’s trichrome staining were performed according to the manufacturers’ instructions. Images were taken using a light microscope (Olympus, Japan).
Real-time PCR analysis
Total RNA was extracted from the cells in 12-well plates using TRIzol reagent (Invitrogen, USA). For reverse transcription to cDNA, 1 μg of total RNA was used. Amplification of the cDNA samples and quantitation were performed using SYBR Green (Bio-Rad, USA) on a RT-PCR system (Bio-Rad, USA) as follows: preheating for 3 min at 95°C; followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. The cycle threshold (Ct) values were collected and normalized to the level of the housekeeping gene β-actin. The 2−ΔΔCT method was used to calculate the relative mRNA levels of each target gene. The primers for Bcl-2, Bax, cleaved caspase-3 and β-actin are listed in .
Table 1. Primers used for real-time PCR analysis.
Western blotting
HUVECs were seeded in a 6-well cell plate at a density of 8 × 104 cells per well and the cells were treated with sesamin (25 and 50 μM) for 48 h followed by TBHP-induced apoptosis for 1 h. After treatment, the cell supernatant was discarded and washed with cold PBS twice. Then, 500 μL of RIPA lysis Kit (Beyotime, Shanghai, China) containing 1 mM phenylmethanesulfonyl fluoride (PMSF, Beyotime) were added to lysing HUVECs. After lysis for 5 min, the cells were collected by cell scraping and placed in a centrifuge tube. After centrifugation 12,000 rpm 30 min at 4°C, the supernatant protein solution was collected, and protein quantification was performed according to the Bradford method. The protein was electrophoresed on a polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Merck, Darmstadt, Germany). Membranes was blocked by 5% skim milk powder for 2h and then incubated with primary antibody at 4°C overnight. Primary antibody including anti-Bcl-2 (1:1000, Cell Signaling Technology), anti-Bax (1:1000, Cell Signaling Technology), anti-cleaved caspase3 (1:1000, Abcam), and anti-GAPDH (1:1000, Cell Signaling Technology). Following incubation with primary antibody, membranes were washed three times using TBST, then HRP-conjugated secondary antibody (1:2000, Cell Signaling Technology) were added and incubated for 2 h at room temperature. The protein bands were visualized with an ECL kit (Thermo Fisher Scientific, Waltham, MA, USA), and the images were captured on a Chemi DocXRS+ Imaging System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All experiments were repeated at least three times. Data were processed in GraphPad Prism 7.0 (GraphPad Software Inc.). All values are presented as the means ± SD. Statistical significance was analyzed using a one-way ANOVA for more than two groups. A one-way ANOVA plus Tukey’s post hoc test was used to test the differences between the groups at specific times. In all the analyses, p < 0.05 was considered statistically significant (expressed as *p < 0.05).
Results
Effect of sesamin on HUVEC viability
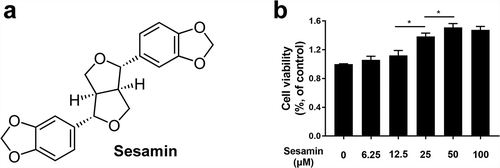
The chemical structure of sesamin is shown in ). Our group used the CCK-8 kit to evaluate the effect of sesamin on the viability of HUVECs. Cells were treated with a concentration gradient of sesamin (6.25, 12.5, 25, 50, and 100 μM) for 48 h. The cell cytotoxicity was evaluated by absorbance and calculated as a percentage of the control group. As shown in ), sesamin promoted the proliferation capability of HUVECs at doses of 25, 50 and 100 µM, and there was no significant difference in proliferation between the 50 and 100 µM treatments. Therefore, we chose sesamin (50 µM) in the subsequent experiments.
Sesamin enhanced cell–matrix adhesion and reduced cell–cell adhesion
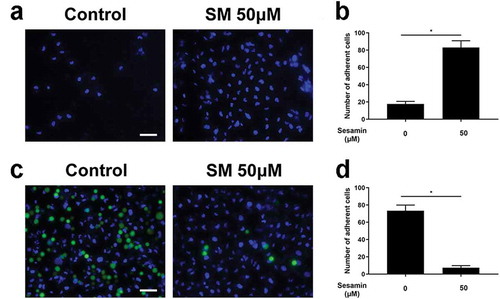
Here, human fibronectin was used as an ECM substitute to study the effect of sesamin on cell–matrix adhesion. It can be seen that sesamin-treated cells attached to the ECM gel more easily than control cells (). For cell–cell adhesion, sesamin treatment significantly reduced the adhesive ability of cells to the HUVEC monolayer at 20 μM (). Thus, sesamin augmented cell adhesion to the ECM but reduced cell–cell adhesion in this study.
Sesamin promoted migration and tube formation of HUVECs
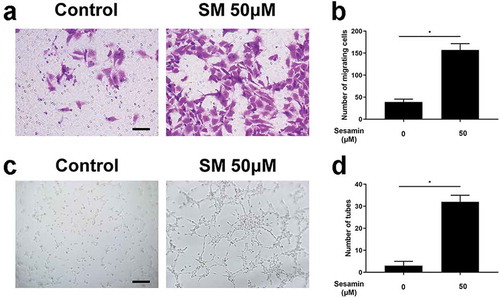
The directed migration of endothelial cells plays an important role in the beginning of angiogenesis. Here, a transwell assay was performed to investigate the sesamin-driven motility of HUVECs. Interestingly, HUVECs treated with sesamin exhibited a remarkably increased migration ability (). Moreover, an in vitro angiogenesis assay was used to investigate the capillary tube formation of HUVECs. Compared to the untreated HUVECs, the presence of sesamin () significantly increased the number of sprouting tubules in HUVECs, which indicated that sesamin strongly enhanced the ability of HUVECs to form tube-like structures.
Effects of TBHP on the expression of Bcl-2, Bax and cleaved caspase-3 in HUVECs
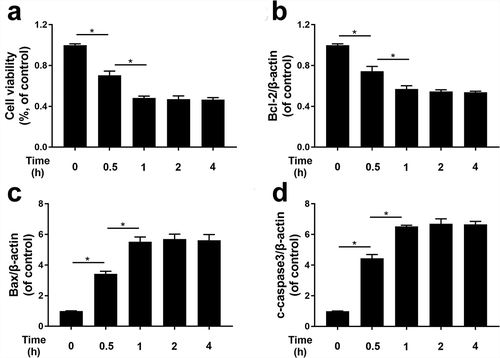
Cell survival was significantly decreased with TBHP (100 µM) in a time-dependent manner, suggesting that ROS accumulation can induce death ()). We used qRT-PCR to confirm whether TBHP increases cell death through apoptosis. As shown in the mRNA expression of Bcl-2 was decreased and the mRNA expression of Bax and cleaved caspase-3 were increased by TBHP stimulation in a time-dependent manner (0.5, 1, 2, and 4 h); however, no significant difference was found between the groups treated with TBHP for 2 and 4 h. It may be explained by ROS stress, which caused by THBP and reached the maximum effect after 1 h.
Sesamin inhibits TBHP-induced HUVEC apoptosis at gene level
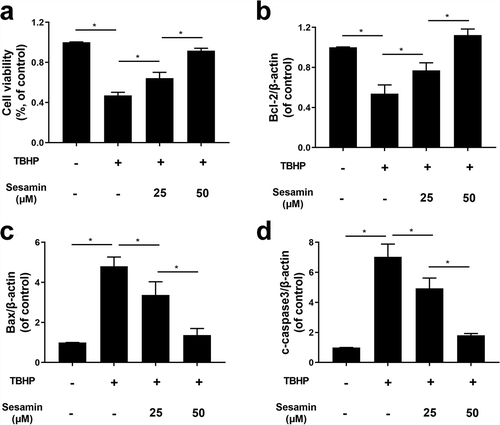
To investigate whether sesamin could upregulate cell survival in response to TBHP-induced cell apoptosis, we first evaluated cell proliferation with the CCK-8 assay. As shown in ), TBHP could induce HUVEC death, and this effect was reversed by treatment with sesamin for 48 h. Moreover, we used qRT-PCR to confirm the difference in gene expression in the experimental groups. As shown in ), Bcl-2 mRNA expression was downregulated in TBHP-treated cells while sesamin pretreatment reversed the Bcl-2 expression induced by TBHP in a dose dependent manner. Conversely, the expression of Bax and cleaved caspase-3 were significantly increased by TBHP treatment, and this effect was reversed by the sesamin pretreatment (). Taken together, the early stage apoptosis in HUVECs could be suppressed by sesamin via regulation of Bcl-2, Bax and caspase-3 gene.
Sesamin inhibits TBHP-induced HUVEC apoptosis at protein level
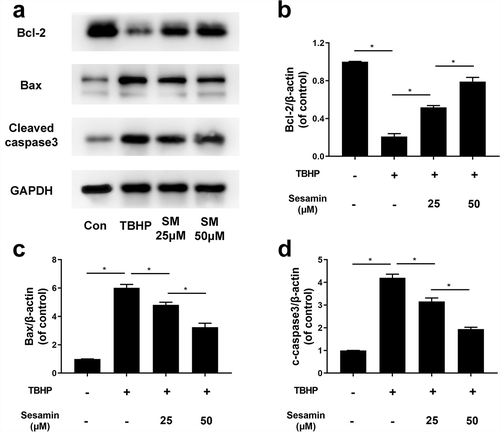
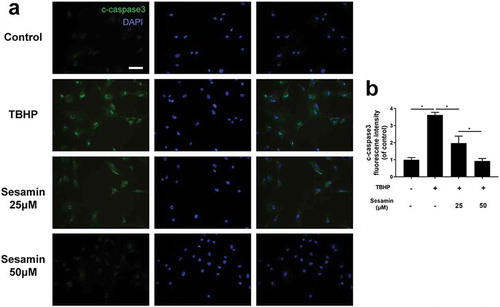
Additionally, the western blotting was performed, it was demonstrated that Bcl-2 protein expression was downregulated in TBHP-treated cells while sesamin pretreatment reversed the Bcl-2 expression induced by TBHP. Conversely, the protein expression of Bax and cleaved caspase-3 were remarkably increased by TBHP treatment, and this effect was reversed by the sesamin pretreatment in a dose dependent manner (). Furthermore, the immunofluorescence staining of active cleaved caspase-3 proteins was significantly higher in the TBHP-induced group than the control group, suggesting that TBHP could induce early stage apoptosis; however, the sesamin pretreatment inhibited the cleaved caspase-3 expression induced by TBHP ().
Sesamin accelerates wound healing in rats
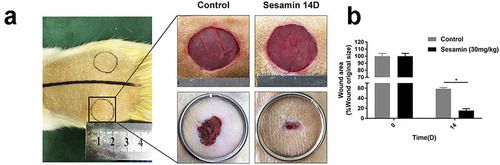
In our in vivo experiment, we used a full-thickness cutaneous wound model of rats to evaluate the time of wound closure based on the antiapoptotic effects of sesamin in vitro. As shown in , the sesamin-treated group healed more quickly than the control wounds (). On day 14 after the operation, the healing rate of the sesamin group was approximately 80%, while that of the control group was 40%, thus demonstrating that sesamin accelerated wound healing in vivo.
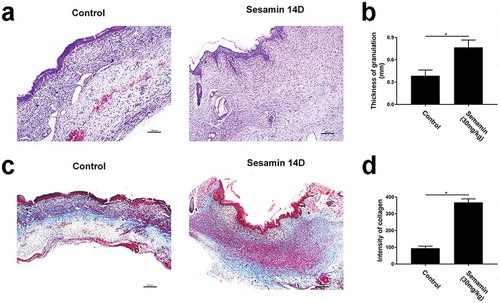
Sesamin improved granulation and accelerated collagen deposition in vivo
H&E staining of wound sections ()) at day 14 revealed that the granulation of the sesamin-treated group was thicker than that of the control groups. Moreover, the sesamin-treated group had thicker and more continuous layers of granulation across the entire wound gap compared to the control ()). These results indicated that sesamin can increase the speed of granulation formation, suggesting that sesamin treatment can significantly enhance granulation formation in vivo. Masson’s trichrome staining of wound sections at day 14 revealed strong blue staining in the wound bed in the sesamin-treated groups ()), indicating the abundant deposition of regenerated collagen ()). Additionally, the collagen fibers in the sesamin-treated wounds were densely packed and in a parallel arrangement, whereas the collagen fibers in the wounds of the control groups were irregularly organized and loosely packed.
Discussion
Difficult wound healing is a hard clinical problem [Citation19], angiogenesis is considered an indispensable step in wound healing process [Citation20]. Many previous studies have reported that growth factors applied externally can significantly expedite wound healing [Citation21]. However, the high price of growth factors, short half-life of proteins and unforeseeable adverse side effects limit the clinical application of growth factors [Citation22]. Thus, promising therapeutic strategies for wound healing that shorten the time until wound closure are urgently needed. In our study, a traditional Chinese medicine named sesamin was investigated, which has multiple therapeutic effects, was employed, and its angiogenic and wound healing abilities.
Angiogenesis is a complex process requiring multiple sequential steps, including the interplay between cells, soluble factors and ECM components. In this study, a significant proangiogenic effect of sesamin on HUVECs was observed through faster cell proliferation, as well as regulated adhesion, migration, and in vitro tube formation. Generally, vascular endothelial proliferation is the beginning of angiogenesis followed by separation from adjacent cells, migration, adherence to the ECM and differentiation [Citation23,Citation24]. Under the conditions used in this study, sesamin significantly promoted proliferation of HUVECs at doses over 25 μM, which should be an initial sign of angiogenesis. Furthermore, not only is the large number of endothelial cells enhancing revascularization at the wound site, but motility and differentiation are also critical processes in the reconstruction of blood vessel networks. In this study, enhanced cell migration was found in sesamin stimulated HUVECs, which ultimately benefits the angiogenesis process. Furthermore, the pretreatment of HUVECs with sesamin decreased cell-cell adhesion and increased the cellmatrix adhesion, which also supported migration. Furthermore, the ability of endothelial cells to separate from adjacent cells and adhere into ECM would help cells differentiate into new blood vessels, which was confirmed by the faster tube formation rate and tube numbers, which ultimately indicated the pro-angiogenesis effects of sesamin.
Furthermore, we revealed that TBHP could induce early stage apoptosis in ECs, which represent an important component of the vascular system. Moreover, sesamin treatment could attenuate apoptosis and reduce HUVEC damage. In general, oxidative stress can exacerbate endothelial dysfunction and even lead to cell death because of the associated increase in endogenous ROS production [Citation25], which could attack DNA, transcription factors, and the cytomembrane [Citation26]. Endothelial cell apoptosis is a vital initial step in many vascular diseases, such as atherosclerosis [Citation27], and the early stage of apoptosis can be induced by H2O2-mediated oxidative stress [Citation28]. Interestingly, stress-induced apoptosis is activated by decreases in Bcl-2 protein, which is an antiapoptotic protein, and increases in Bax protein, which is a proapoptotic protein. In our study, cell apoptosis was verified via qRT-PCR and Western blot. We observed that the mRNA expression of Bcl-2 decreased and the mRNA expression of Bax and cleaved caspase-3 increased in a time-dependent manner after TBHP stimulation. Additionally, the changes of mRNA and protein expression of Bcl-2, Bax and cleaved caspase-3 in HUVECs after exposure to TBHP could be reversed by sesamin treatment, suggesting that it may protect against TBHP-induced apoptosis by regulating the Bcl-2 protein family.
The apoptosis pathway is a complex cellular protein cascade reaction, and the highly conserved family of cysteine proteases containing cellular caspases and the cleaved form of cysteine protease are regulated during the apoptosis process [Citation29]. According to recent studies, oxidative stress can lead to considerable activation of the caspase pathway [Citation30]. Under proapoptotic stimuli, an apoptosis protein cascade reaction could be activated, and it involves promoting caspase-3 protein into its activated form, i.e., cleaved caspase-3 [Citation31]. A number of studies have reported that apoptosis is related to the increased expression of cleaved caspase-3 in a variety of organisms [Citation32]. In our study, the expression of the cleaved caspase-3 gene and protein were increased after TBHP exposure; however, sesamin pretreatment remarkably decreased the TBHP-induced caspase-3 activity in HUVECs. These results indicate that sesamin may protect HUVECs against TBHP-induced apoptosis.
Additionally, we performed an in vivo experiment and used a full-thickness cutaneous wound model of rats to evaluate the effects of sesamin. In the early stage of wound healing, granulation tissue, which usually forms in wound sites 2–5 days after injury and contains abundant cells and cytokines that are able to repair damage, protect against infection. In our research, we observed that granulation tissue was markedly thicker in sesamin-treated groups than the control groups, suggesting that sesamin could accelerate wound healing by strengthening granulation tissue formation. Furthermore, collagen deposition and remodeling, which promote the strength and appearance of scars formed after healing [Citation33–Citation35], were enhanced by the sesamin treatment in our present study. Collagen fibers are mainly secreted by fibroblasts in the wound bed, which are distributed to a great degree in granulation tissue and can be remodeled by the growth factors secreted by the granulation tissue. Moreover, our experiments show that the well-organized, abundant collagen in the sesamin-treated wounds could be related to the thick granulation tissue. Taken together, the histopathological results indicate that the sesamin treatment results in faster and better wound healing than the control conditions.
In summary, our findings suggest that sesamin protected HUVECs against TBHP-induced apoptosis. Moreover, in full-thickness cutaneous wounds, neogenesis of granulation tissue and the deposition and remodeling of the collagen matrix were accelerated by the administration of sesamin. These findings suggest that sesamin is a promising therapeutic strategy for wound healing that can shorten the time until wound closure.
Author contributions
Ye Sunzhi (first author): Devise and design all experiments, performed experiments, Wrote manuscript. Wang Wei: provided design and technical support for experiments, reviewing and editing of this draft manuscript. Chen Xiaoyan: Data processing, reviewing the manuscript’s discussion part. Deng Yingbin: Funding Acquisition, Extensively taught the design and error of all experiments.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130:333–346.
- Valencia IC, Falabella A, Kirsner RS, et al. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401–424.
- Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022.
- Yamada T, Egashira N, Imuta M, et al. Role of oxidative stress in vinorelbine-induced vascular endothelial cell injury. Free Radic Biol Med. 2010;48:120–127.
- Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci. 2005;109:217–226.
- Lau YS, Ling WC, Dharmani Murugan MRM. Boldine ameliorates vascular oxidative stress and endothelial dysfunction: therapeutic implication for hypertension and diabetes. J Cardiovasc Pharmacol. 2015;65:522.
- Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–439.
- Aoki M, Nata T, Morishita R, et al. Endothelial apoptosis induced by oxidative stress through activation of NF-κB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38:48–55.
- Han D, Haudenschild CC, Hong MK, et al. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995;147:267.
- Ryu JM, Lee HJ, Jung YH, et al. Regulation of stem cell fate by ROS-mediated alteration of metabolism. Int J Stem Cells. 2015;8:24.
- Xing -S-S, Yang X-Y, Zheng T, et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol. 2015;72:141–152.
- Hsieh PF, Hou C-W, Yao P-W, et al. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J Neuroinflammation. 2011;8:57.
- Lee -C-C, Chen P-R, Lin S, et al. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: possible implications for its antihypertensive effect. J Hypertens. 2004;22:2329–2338.
- Wu WH, Wang SH, Kuan II, et al. Sesamin attenuates intercellular cell adhesion molecule‐1 expression in vitro in TNF‐α‐treated human aortic endothelial cells and in vivo in apolipoprotein‐E‐deficient mice. Mol Nutr Food Res. 2010;54:1340–1350.
- Hou RCW, Huang HM, Tzen JT, et al. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res. 2003;74:123–133.
- Hou RC-W, Wu -C-C, Yang C-H, et al. Protective effects of sesamin and sesamolin on murine BV-2 microglia cell line under hypoxia. Neurosci Lett. 2004;367:10–13.
- Tang Y, Huang B, Sun L, et al. Ginkgolide B promotes proliferation and functional activities of bone marrow-derived endothelial progenitor cells: involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell Mater. 2011;21:459–469.
- Liu Y-L, Xu Z-M, Yang G-Y, et al. Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol Sin. 2017;38:1445.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321.
- Zeng Z, Zhu B-H. Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. J Ethnopharmacol. 2014;154:653–662.
- Morimoto N, Yoshimura K, Niimi M, et al. An exploratory clinical trial for combination wound therapy with a novel medical matrix and fibroblast growth factor in patients with chronic skin ulcers: a study protocol. Am J Transl Res. 2012;4:52.
- Epstein SE, Kornowski R, Fuchs S, et al. Angiogenesis therapy. Circulation. 2001;104:115–119.
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794.
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887.
- Urso C, Caimi G. Oxidative stress and endothelial dysfunction. Minerva Med. 2011;102:59–77.
- Simon H-U, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418.
- Yu E, Mercer J, Bennett M. Mitochondria in vascular disease. Cardiovasc Res. 2012;95:173–182.
- Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H2O2: submicromolar levels of H2O2 induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–1018.
- Fischer U, Jänicke R, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76.
- Anuradha CD, Kanno S, Hirano S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic Biol Med. 2001;31:367–373.
- Ho FM, Liu SH, Liau CS, et al. High glucose–induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624.
- Abu-Qare AW, Abou-Donia MB. Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2ʹ-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J Toxicol Env Heal B. 2001;4:313–332.
- Leivonen S-K, Häkkinen L, Liu D, et al. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-β-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124:1162–1169.
- Brem H, Kodra A, Golinko MS, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–2287.
- Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008.