ABSTRACT
Previous RNA-Seq analyses revealed that NAD(P)H steroid dehydrogenase-like (NSDHL) has a different expression during 3T3-L1 differentiation; however, its roles in adipogenesis are unknown. In the present study, using quantitative real-time PCR, we confirmed that NSDHL knockdown increased the proliferation of 3T3-L1 preadipocytes, but attenuated the differentiation of 3T3-L1 preadipocytes, as evidenced by reduced lipid accumulation and down-regulation of PPARγ gene expression. Further analyses showed that the expression peak of NSDHL was at the early stage of 3T3-L1 preadipocytes differentiation and LXR-SREBP1 signaling pathway was downregulated in NSDHL-knockdown 3T3-L1 cells. Collectively, our findings indicate that NSDHL is a novel modulator of adipogenesis. Moreover, our data provide insight into the complex relationships between sterol sensing, LXR-SREBP1 signaling pathway, and PPARγ in 3T3-L1 cells.
Graphical abstract
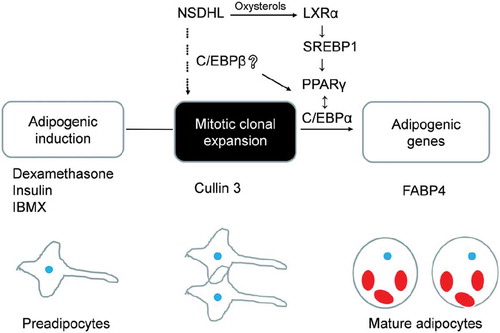
Upon adipogenic induction, NSDHL increases the relative content of oxysterols, which stimulates PPARγ expression through stimulating an increase in LXR-SREBP1 pathway.

Cholesterol and its oxysterol congeners are important components of cell membranes and function as intermediates in several crucial biosynthetic pathways. As adipose tissue is the major cholesterol storage organ and most of the intracellular cholesterol is distributed to lipid droplets (LDs), cholesterol homeostasis may have a role in the regulation of adipocyte development. NAD(P)H steroid dehydrogenase-like (NSDHL) belongs to the two C-4 methyl groups in post-squalene cholesterol biosynthesis characterized by encodes a 3β-hydroxysterol dehydrogenase or decarboxylase [Citation1]. Heterozygous mutations in NSDHL gene are associated with human CHILD syndrome (congenital hemidysplasia with ichthyosiform nevus and limb defects), responsible for the X-linked dominant, as well as the male lethal disorder mouse mutations bare patches and striated [Citation2].
C-4 methylsterols are the group of cholesterol biosynthesis known as meiosis-activating sterols (MASs). They are identified in high concentration in testis and ovary and play important roles in oocyte maturation and meiosis activation. However, the effect of MASs on cell proliferation and mitosis is not well studied [Citation3]. In addition, MASs are potent ligands for liver X receptors α and β (LXRα and LXRβ), which are oxysterol-activated nuclear receptor superfamily of transcription factors with important roles in lipid transport and glucose management. LXRβ is expressed ubiquitously, while LXRα is predominantly expressed in lipid homeostasis tissues, such as the liver and adipose tissue. LXRs modulate many metabolic and inflammatory pathways through the regulation of sterol regulatory element-binding protein-1c (SREBP-1c), ATP-binding cassette transporter A1 (Abca1) and other target genes [Citation4–Citation6]. Of note, the potential influence of LXR signaling on diabetes, insulin resistance, and metabolic syndrome remains still unclear.
Previous work has demonstrated that ligand activation of LXRs promotes the upregulation of the glucose transporter in the setting of diet-induced or genetic obesity [Citation7]. More recently, the deletion of LXRs in mouse would affect adipose tissue mass and peroxisome proliferator-activated receptor γ (PPARγ) signaling pathway [Citation8]. Transcription factor PPARγ and CCAAT/enhancer-binding protein α (C/EBPα) are known as master regulators of adipogenesis. The entire process of adipogenesis consists of several stages. During mitotic clonal expansion (MCE), C/EBPβ gains the capacity to bind DNA, which leads to the upregulation of PPARγ and CEBPα [Citation9]. Surprisingly, our previous RNA-Seq analyses revealed that NSDHL has different expression during 3T3-L1 differentiation; however, its roles in adipogenesis are unknown [Citation10]. Therefore, we confirmed that NSDHL knockdown increased the proliferation of 3T3-L1 preadipocytes, but attenuated the differentiation of 3T3-L1 preadipocytes, as evidenced by reduced lipid accumulation and down-regulation of PPARγ gene expression. Further analyses showed that the expression peak of NSDHL was at the early stage of 3T3-L1 preadipocytes differentiation and LXR-SREBP1 signaling pathway was downregulated in NSDHL-knockdown 3T3-L1 cells. Our data suggest that sterol sensing by LXR-SREBP1 signaling pathway is critically linked with the regulation of proliferation and differentiation in 3T3-L1 cells.
Materials and methods
Cell culture and adipocyte differentiation
The 3T3-L1 cells were cultured in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% newborn calf serum (Gibco, Oklahoma, USA). Two days after 3T3-L1 preadipocytes reaching 80% confluence, cells were induced to differentiation (designated as day 0, D0) by adding 100 ng/μL dexamethasone, 100 ng/μL insulin, and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich, St. Louis, MO, USA). After 48 h induction, the medium was changed with a differentiation medium supplemented with 20 nM insulin. Cells were maintained after induction in DMEM containing 10% fetal calf serum (Gibco, Oklahoma, USA) with media changes every day.
Oil Red O staining
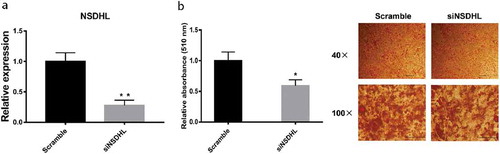
Adipocyte differentiation was monitored by measurement of lipid accumulation using an Oil Red O (Sigma-Aldrich, St. Louis, MO, USA) assay on samples, according to the manufacturer’s protocol. The analysis of lipid droplets was performed using the OLYMPUS DP Controller program and measured the absorbance at 510-nm wavelength with the Microplate Reader (Biotek, Winooski, VT, USA) for a quantitative assay.
RNA isolation and cDNA synthesis
Total RNA of each sample was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The RNA was quantified and qualified by NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) and 1% agarose gel. 1 μg total RNA with 28S/18S above 1.8 was used for the following preparation. RNA was reversely transcribed to cDNA using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) following the manufacturer’s instructions. First-strand cDNA was synthesized using PrimeScript™ Reverse Transcriptase and the second-strand cDNA was synthesized using Second Strand Synthesis Enzyme Mix.
Real-time quantitative PCR
The quantitative reverse transcription-polymerase chain reaction contained 10 μL of Luna universal qPCR Mix (NEB, Beverly, MA, USA), 25 ng of diluted cDNA, and 5 μM of each primer (Table S1) contributing a total volume of 20 μL. The thermal cycle program was 1 min at 95°C, followed by 35 cycles of 95°C for 15 s, 56 °C for 30 s and 72°C for 30 s, then 95°C for 1 min, 55°C for 1 min. A melt curve analysis was performed. After completion of the reaction, the data analysis was based on the obtained sample Cycle threshold (Ct) value. The results were calculated by the 2−ΔΔCt method and normalized by β-actin to establish relative quantification. Real-time quantitative PCR validation was performed in triplicates and repeated at least three times.
Small interfering RNAs (siRNAs) and transfection
All siRNAs oligo used in this experiment were purchased from (GenePharma, Shanghai, China). Sequences of these oligonucleotides were as follows: NSDHL-specific siRNA (siNSDHL), 5ʹ- GGAGAGAGCAGUACUG-GAUTT-3ʹ, and negative control siRNA (Scramble), 5ʹ- UUCUCCGAACGUGUCACGUTT-3ʹ. 3T3-L1 preadipocytes were electroporated with siNSDHL or Scramble using the GenePulser Xcell (BIO-RAD, Hercules, CA, USA) according to the manufacturer’s protocols. Transfection efficiency was assessed by Real-time quantitative PCR.
Cell proliferation assay
Cell proliferation was assessed by CCK-8 assay. 3T3-L1 preadipocytes were seeded at a density of 2500 cells/well into 96-well plates in the culture medium. After 24 h of culture, cells were transfected with siNSDHL or Scramble. At each designated time points after transfection, 10 μL CCK-8 reagent (5 mg/mL) was added to each well and incubated for 4 h at 37°C with 5% CO2. When CCK-8 formazan crystals were solubilized, the absorbance was measured at a wavelength of 450 nm with the Microplate Reader (Biotek, Winooski, VT, USA).
Bioinformatics and prediction
Based on the GeneMANIA database (http://genemania.org/), we analyzed the interaction networks for NSDHL, and further investigated the interaction between NSDHL and eligible molecules in a given network by random chance to identify the extent of gene connectivity. Cytoscape was used to visualize the interaction networks for NSDHL and find the key genes.
Statistical analysis
Data are presented as the mean ± SEM. Statistically significant differences were determined using a two-tailed Student’s t-test by GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). For all statistical analyses, P values <0.05 were considered as significant difference. Experiments were repeated at least three times.
Results
Knockdown of NSDHL decreased lipid accumulation
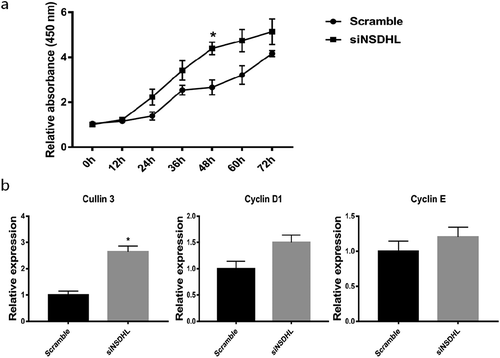
RNA-Seq analysis revealed that NSDHL has a different expression during 3T3-L1 adipogenesis, suggesting NSDHL may play a role in this process [Citation10]. To explore whether NSDHL participates in adipogenesis, we knockdown NSDHL by transfecting siNSDHL into 3T3-L1 preadipocytes during the differentiation induction. Quantitative real-time PCR analysis showed that transfection with siNSDHL significantly reduced NSDHL mRNA expression in 3T3-L1 preadipocytes at 24 h after transfection, on average, by around 70% compared with the Scramble ()), confirming the utility of this approach. Moreover, NSDHL knockdown resulted in a marked decrease of LDs by Oil Red O staining and quantification ()).
Suppression of NSDHL reduced the adipogenic genes expression
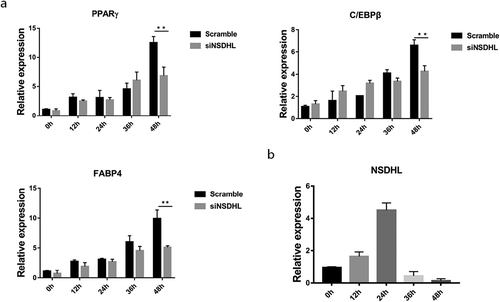
We explored the impact of NSDHL knockdown on 3T3-L1 cells adipogenesis. As shown in , consistent with oil red O-staining results ()), at day 12 of preadipocytes differentiation, the suppression of NSDHL significantly reduced the expression of adipogenic marker genes, including FABP4, and PPARγ. Moreover, we observed that NSDHL affects adipogenesis by influencing early events in the program. The C/EBPβ, C/EBPα, PPARγ, and FABP4 gene expression all significantly decreased in siNSDHL-transfected 3T3-L1 preadipocytes than scramble controls at D2 (). Interestingly, expression of NSDHL was also regulated during adipogenesis, being higher expression during preadipocytes and then declined dramatically after D2, as has been shown before [Citation10]. During MCE, growth-arrested 3T3-L1 preadipocytes reenter the cell cycle and undergo several rounds of proliferation that occurs within 48 h after adipogenic stimulation [Citation11]. The transcription factor C/EBPβ, which leads to the upregulation of PPARγ and C/EBPα, accompanied by the initiation of a transcriptional cascade that results in terminal adipogenesis [Citation12,Citation13].
Figure 2. Suppression of NSDHL decreased the expression of adipogenic genes in 3T3-L1 cells adipogenic program.
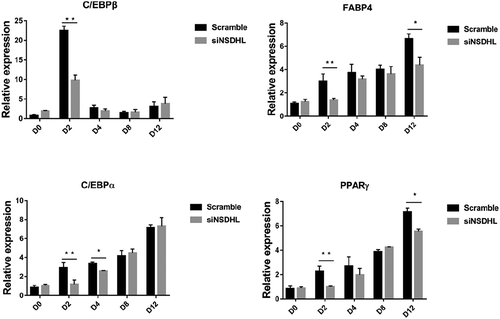
We next measured NSDHL effects early in adipogenesis, during the MCE phase. Quantitative analysis using quantitative PCR showed that knockdown of NSDHL was associated with a reduction in the mRNA levels of FABP4, PPARγ, and C/EBPβ after the 48-h induction of differentiation ()), which confirmed the previous result (). In addition, we revealed that NSDHL expression peaked at 24 h of 3T3-L1 cells differentiation ()). NSDHL expression fluctuates during events early in the 3T3-L1 cells adipogenic program, suggesting NSDHL may play a role in the MCE phase. Our results demonstrated that NSDHL is a critical regulator during the early stage of adipogenesis in 3T3-L1 cells.
Bioinformatics and prediction
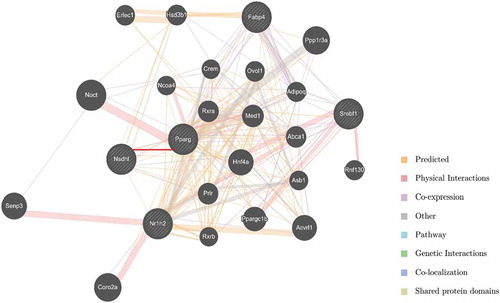
In order to identify the candidate key genes that potentially regulate adipogenesis of 3T3-L1 cells with NSDHL, we constructed the interaction networks using GeneMANIA. 282 total links were identified. The network associated with cholesterol metabolism, nuclear receptor activity, and fat cell differentiation and comprised PPARγ, LXRβ, SREBP1, and other lipid metabolism-related genes ().
LXR-SREBP1 signaling pathway was downregulated in NSDHL-knockdown 3T3-L1 cells
No previous studies have suggested a mechanism for how the NSDHL might influence adipogenesis of 3T3-L1 cells. NSDHL is a downstream gene of cholesterol biosynthesis and plays vital roles in this process. As expected, many interactions reflecting their participation in the cholesterol biosynthesis pathway as well as additional interactions with genes annotated for roles in lipid synthesis and metabolism (). Moreover, there has a predicted interaction link between NSDHL and PPARγ based on a previous study () [Citation14]. We, therefore, tested the inference that NSDHL and associated molecules may regulate adipogenesis of 3T3-L1 cells.
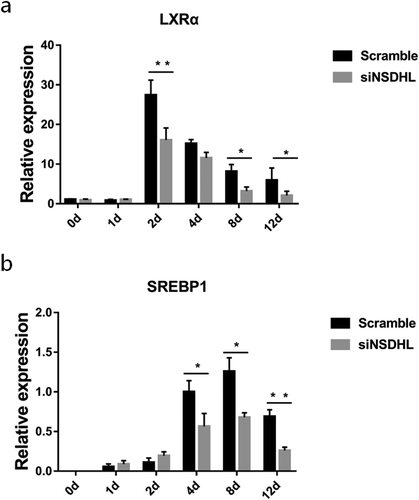
We demonstrated that suppression of NSDHL decreased the expression of LXRα ()), but had an inapparent effect on LXRβ (data not show). To assess whether the effect of NSDHL is mediated by PPARγ, we also tested the mRNA expression of SREBP1, a protein thought to be important in the regulation of fatty acid synthesis, which was significantly decreased along with knockdown of NSDHL ()). The previous study had demonstrated that an increase in the expression of LXR and SREBP1 induces the accumulation of LDs [Citation15]. In addition, administration of the PPARγ agonist in obese Zucker rats led to increased LXRs mRNA expression in adipose tissue in vivo [Citation15]. This cross-talk had also been observed in macrophages [Citation16,Citation17]. Therefore, our outcome suggests that suppression of NSDHL in preadipocytes can downregulate the pathway of LXR-SREBP1 and the expression of PPARγ during the progress of adipogenesis.
Impact of NSDHL knockdown on proliferation of 3T3-L1 preadipocytes
Adipogenesis involves cell proliferation and differentiation. We demonstrated that NSDHL may play a role in the MCE phase of adipogenesis in 3T3-L1 cells. We then addressed whether NSDHL regulates the proliferation of 3T3-L1 preadipocytes by siRNA-mediated suppression of NSDHL. The impact of NSDHL silencing on 3T3-L1 preadipocytes proliferation was assessed using the CCK-8 assay. As shown in ), suppression of NSDHL significantly increased cell proliferation of 3T3-L1 preadipocytes at 48 h after siRNA transfection compared to Scramble (P < 0.05). The fact that mutations in lamin B receptor (LBR), sterol-C4-methyl oxidase–like gene (SC4MOL), NSDHL, and emopamil binding protein (EBP) results in proliferative skin disease [Citation18]. Recently, it has been shown that alterations in sterol methyl oxidase impact activation of the cell cycle [Citation19]. To understand the underlying mechanism by which NSDHL regulates 3T3-L1 preadipocytes proliferation, we evaluated the impact of NSDHL knockdown on the expression of cyclin D1, cyclin E and cullin 3 in 3T3-L1 preadipocytes using quantitative real-time PCR. The results showed that NSDHL knockdown significantly increased the mRNA expression of cullin 3 compared to Scramble ()). We also observed a trend for increasing expression of cyclin E and cyclin D1 in siNSDHL-transfected cells compared to Scramble-transfected cells, but the differences did not reach statistical significance ()). Overall, these results demonstrate that NSDHL knockdown activates the proliferation of 3T3-L1 preadipocytes and suggest its involvement in the regulation of 3T3-L1 preadipocytes proliferation.
Taken together, our results indicate that NSDHL knockdown is associated with decreased adipogenic capacity. These effects are potentially mediated through the relative decrease in the LXR-SREBP1 pathway. This is summarized in a schematic diagram ().
Discussion
Cholesterol is an important constituent of cell membranes and lipid rafts and serves as the immediate precursor of steroids, vitamin D, and bile acids. The MASs are the first group of cholesterol biogenesis intermediates that were identified to have important extrahepatic functions in mammals. These include 4,4′-dimethyl-5α-cholesta-8,24-dien-3β-ol (testis meiosis-activating sterol [T-MAS]), 4,4′-dimethyl-5α- cholesta-8,14,24-trien-3β-ol (follicular fluid meiosis-activating sterols [FF-MASs]), and zymosterol. FF-MAS is also a ligand for LXRs [Citation20]. LXR signaling is known to regulate crosstalk between inflammatory and cholesterol metabolism.
LXRs are members of a large family of nuclear receptors which bind to the regulatory region of target genes and, upon ligand binding, stimulate their transcription. Both LXRα and LXRβ function as heterodimers with retinoid X receptor (RXR), a common partner for several nuclear receptors, including PPAR, vitamin D receptor (VDR), thyroid hormone receptor (TR), and farnesoid X receptor (FXR). During the last decade, oxidized cholesterol derivatives or oxysterols were identified as specific ligands of LXRs, which are also named “oxysterol receptors.” Studies performed later suggest that LXRs are “sterol sensors” which, in response to excess cholesterol, stimulate its transport to the liver and adipocyte tissue. Despite the fact that both LXRs are highly expressed in adipose tissue, few studies have focused on the role of LXRs in this tissue [Citation15,Citation21–Citation24]. Our study indicated that the distribution of NSDHL mRNA reveals an expression pattern similar to that of LXRα with strong expression in metabolic organs such as liver, kidney, and stomach (Figure S1) [Citation25]. The NSDHL mRNA is also notably present in adipose tissue and to a lesser extent in the intestine (Figure S1). The analysis of gene expression in LXR knockout mice established that LXR regulates a number of candidate target genes involved in both cholesterol and fatty acid metabolism, which implicates LXR in a broader role in metabolic conversion [Citation26,Citation27]. Furthermore, LXR agonists could markedly stimulate fatty acid synthesis in hepatocytes. This effect is partially mediated by increased expression of SREBP-1c, which subsequently binds to the sterol response element (SRE) within the promoter region of genes encoding various lipogenic enzymes [Citation4].
These findings imply a role for LXR in controlling lipid storage capacity in mature adipocytes and point to an intriguing physiological interplay between LXR and PPARγ in controlling pathways in lipid handling. The LXR-SREBP1 pathway provides a mechanism whereby cholesterol and fatty acid metabolism can be coupled. The availability that this regulatory arrangement is conserved in the control regions of other adipogenic genes is under investigation.
The work presented here describes a physiological role for NSDHL in 3T3-L1 adipocytes. The role of NSDHL in the regulation of adipogenesis was confirmed in NSDHL knockdown 3T3-L1, in which the lipid accumulation was decreased compared with scramble (). Furthermore, we show a down-regulation of PPARγ expression in mature 3T3-L1 adipocytes at D12 to further confirm the effect of NSDHL knockdown (). In addition, we also demonstrated the down-regulation of C/EBPβ, FABP4, and PPARγ expression by NSDHL knockdown during MCE ()). Based on the interaction analysis, our data support the idea that NSDHL acts by regulating the adipogenesis-related LXR-SREBP1 pathway. We show a down-regulation of LXRα and SREBP1, after treatment with siNSDHL ()). The observed transcript is probably the SREBP-1c isoform because this isoform is a direct target gene of LXRs. Furthermore, knockdown of NSDHL in 3T3-L1 preadipocytes increased cell proliferation and expression of cullin 3 (). The observation that NSDHL is fatty appears to accumulate on the surface of lipid droplets suggests that it plays a critical role in lipid homeostasis [Citation28].
Taken together, our studies provide evidence that NSDHL gene not only is important in sterol synthesis but also regulates 3T3-L1 cells proliferation and differentiation. NSDHL expression level is rather dramatically decreased within the first 2 days ()), suggesting that NSDHL might have a decisive role in the initiation of adipogenesis. The compartmentalization of genes involved in the cholesterol biosynthetic pathway may provide a mentality to help understand the regulation of adipogenesis. However, further validation studies are needed to determine authentic function at the protein level or discover other underlying mechanisms in future studies. Greater knowledge about adipose tissue has become increasingly more important in light of the increasing incidence of obesity and its associated disorders, such as type 2 diabetes. Our results rather suggest that a link between fatty acid and cholesterol metabolism and stimulated us to study the potential cross-regulation between sterol and lipid metabolism in adipocytes, which play a central role in maintaining lipid homeostasis and energy balance.
Author contribution
HY Zhang, CP Li, and YZ Xin performed most of the research works: designed and carried out experiments, and wrote the manuscript. X Cui and JW Cui helped to construct vector. GL Zhou supervised the overall research and wrote the manuscript. All authors read and approved the manuscript.
ZHOU_et_al.-BBB-supFigYT.pptx
Download MS Power Point (104.9 KB)ZHOU-BBB-Supplementary_materials.pdf
Download PDF (72.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Liu XY, Dangel AW, Kelley RI, et al. The gene mutated in bare patches and striated mice encodes a novel 3β-hydroxysteroid dehydrogenase. Nat Genet. 1999;22(2):182–187.
- Herman GE. X-Linked dominant disorders of cholesterol biosynthesis in man and mouse. Biochim Biophys Acta. 2000;1529(1–3):357–373.
- Cukurcam S, Betzendahl I, Michel G, et al. Influence of follicular fluid meiosis-activating sterol on aneuploidy rate and precocious chromatid segregation in aged mouse oocytes. Hum Reprod. 2007;22(3):815–828.
- Repa JJ, Liang, G, Ou, J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830.
- Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-Mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun. 2000;274(3):794–802.
- Venkateswaran A, Repa JJ, Lobaccaro JM, et al. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J Biol Chem. 2000;275(19):14700–14707.
- Commerford SR, Vargas L, Dorfman SE, et al. Dissection of the insulin-sensitizing effect of liver X receptor ligands. Mol Endocrinol. 2007;21(12):3002–3012.
- Beaven SW, Matveyenko A, Wroblewski K, et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 2013;18(1):106–117.
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81(2):715–736.
- Xin YZ, Li CP, Guo Y, et al. RNA-Seq analysis reveals a negative role of MSMO1 with a synergized NSDHL expression during adipogenesis of 3T3-L1. Biosci Biotechnol Biochem. 2019;83(4):641–652.
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci. 2003a;100(1):44–49.
- Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci. 2003b;100(3):850–855.
- Zhang JW, Tang QQ, Vinson C. et al. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad. Sci. 2004;101(1):43–47.
- Guan Y, Myers CL, Lu R, et al. A genomewide functional network for the laboratory mouse. PLoS Comput Biol. 2008;4(9):e1000165.
- Juvet LK, Andresen SM, Schuster GU, et al. On the role of liver X receptors in lipid accumulation in adipocytes. Mol Endocrinol. 2003;17(2):172–182.
- Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7(1):161–171.
- Chinetti G, Lestavel S, Bocher V, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7(1):53–58.
- Gibson KM, Hoffmann G, Schwall A, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J Lipid Res. 1990;31(3):515–521.
- He M, Kratz LE, Michel JJ, et al. Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. J Clin Invest. 2011;121(3):976–984.
- Janowski BA, Willy PJ, Devi TR, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–731.
- Laffitte BA, Repa JJ, Joseph SB, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci. 2001;98(2):507–512.
- Dalen KT, Ulven SM, Bamberg K, et al. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J Biol Chem. 2003;278(48):48283–48291.
- Laffitte BA, Chao LC, Li J, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci. 2003;100(9):5419–5424.
- Hummasti S, Laffitte BA, Watson MA, et al. Liver X receptors are regulators of adipocyte gene expression but not differentiation. Identification of apoD as a direct target. J Lipid Res. 2004;45(4):616–625.
- Willy PJ, Umesono K, Ong ES, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045.
- Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell. 1998;93(5):693–704.
- Alberti S, Schuster G, Parini P, et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXR-deficient mice. J Clin Invest. 2001;107(5):565–573.
- Caldas H, Herman GE. NSDHL, an enzyme involved in cholesterol biosynthesis, traffics through the Golgi and accumulates on ER membranes and on the surface of lipid droplets. Hum Mol Genet. 2003;12(22):2981–2991.