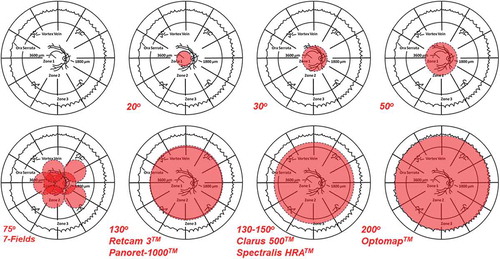
Ultra-wide-field (UWF) imaging has been used clinically in retina practices for nearly two decades to achieve rapid, single-frame photographic access to the mid- and far-retinal periphery.Citation1–Citation5 These systems, particularly the non-contact versions used most often in adults, require less time and light exposure and, consequently, allow for improved patient comfort and excellent overall image quality. Artifacts due to small pupils, lid, lash and intraocular lens projections, and reflections are not uncommon, but can be minimized with adequate dilation, patient cooperation, and operator experience. In addition, as with all UWF imaging devices, there is a peripheral non-linearity, or distortion, produced by the Mercator projection of the spherical fundus onto a flat monitor. The wider the field of view, the more this distortion becomes evident and clinically relevant, since such effects are most pronounced in the retinal periphery. The OptomapTM by Optos, which has the widest field of view at 200° (), has developed and validated a mathematical correction for Mercator projection distortions to allow accurate measurements to be made across the entire field of view.Citation6 Perhaps the greatest limitation of UWF imaging, however, is resolution, which can be half or less of that achieved with more traditional fundus photographic systems.Citation5 Thus, UWF imaging may not be the modality of choice when documentation of fine fundus detail is desired. Such limitations notwithstanding, it has become clear that UWF imaging allows for improved detection and monitoring of many retinal conditions, specifically those that involve the mid- and far-retinal periphery – Zones 2 and 3, respectively (). Five original articles and two letters in this issue of Ocular Immunology & Inflammation (OII) address the use of UWF imaging to evaluate and manage patients with uveitis.Citation7–Citation13
Figure 1. An overview of the extent of imaging capable, shown in red, with traditional photography (30–50°, and slightly beyond), 7-field montage photography of the sort used in many clinical trials (approximately 75°), and various contact (Retcam 3TM and Panoret-1000TM) and non-contact (ClarusTM, Spectralis HRATM and OptosTM) ultra-wide-field photographic imaging systems. A schematic underlay of the proportionate topographic areas of retinal Zones 1,2, and 3 used in describing retinitis is used for orientation, and to show major anatomic landmarks in relation to each of the three zones. After Cunningham ET Jr, Hubbard LD, Danis RP, Holland GN. Proportionate topographic areas of retinal Zones 1, 2, and 3 for use in describing infectious retinitis. Arch Ophthalmol. 2011 Nov; 129(11):1507–1508.
Thomas et al.Citation7 used Optos P200Tx ultra-widefield (UWF) fluorescein angiography (FA) to retrospectively study peripheral vascular leakage (PVL) in 73 eyes of 42 subjects with uveitis seen between November 2012 and January 2014, at a single uveitis referral center in Portland, Oregon. All subjects had at least three UWF images: baseline; intermediate – 3 to 11 months from baseline; and final – one year or more from baseline. The cohort included an anatomically and etiologically diverse group of uveitis patients, some of whom had clinically inactive disease and others with active disease who were either on or without treatment at the time of referral. The times from baseline to intermediate and final images ranged from 6 to 11 months (mean 7 months) and from 12 to 55 months (mean 36 months), respectively. Overall, 41 eyes (56.2%) had PVL at baseline, with just over half of these (21 eyes; 56.1%) also having diffuse vascular leakage (DVL). The authors used bivariate and multivariate linear regression analyses to interrogate whether the presence of PVL at the three tested timepoints was associated with a difference in vision (proportion of eyes with ≥20/40 vision), the proportion of eyes with cystoid macular edema (CME) on optical coherence tomography (OCT), or the proportion of patients for whom treatment was initiated or increased. Across these three timepoints, the only comparison to achieve statistical significance was a need for augmented treatment if PVL was present at baseline, which had an odds ratio of 4.39 (p = .015) after controlling for baseline severity of uveitis, including overall activity grading and the presence of either CME or DVL. The authors suggested that the presence of PVL at baseline visit was associated with a more than four-fold likelihood that treatment would be initiated or increased, but noted that treatment outcomes comparing those with or without PVL at baseline were not statistically different. They concluded that while the presence of PVL at baseline appeared to increase the likelihood of initiating or increasing treatment, they were unable to identify a benefit associated with this increased treatment tendency. Such results would appear to support the general clinical practice of ascribing limited therapeutic relevance to the presence of isolated PVL, while assigning more clinical significance to the overall severity of inflammation and the presence or absence of CME.
Laovirojjanakul et al.Citation8 used Optos C200 MA UWF color and FA imaging to study 41 eyes of 24 patients with a diagnosis of non-infectious anterior and intermediate uveitis (22 eyes; 53.7%), intermediate uveitis (three eyes; 7.3%) or pars planitis (16 eyes; 39.0%) seen on a 12-month period at a single uveitis referral center in San Francisco, California. Three of these subjects (12.5%) were diagnosed with sarcoidosis. Subjects were categorized as having PVL (12 eyes; 29.3%), DVL (26 eyes; 63.4%), or no vascular leakage (three eyes; 7.3%). Peripheral vascular leakage was defined as leakage occurring anterior to the ampullae of the vortex veins. Twenty-five eyes (61.0%) had clinically active inflammation and 16 eyes (39.0%) had CME. The angiographically identified pattern of vascular leakage was then tested using both linear and logistic regression techniques for an association with best-corrected visual acuity and/or the presence of CME on OCT. The authors also tested for an association between the presence of clinically active inflammation (>0.5+ anterior chamber cell or >0.5+ vitreous haze) and leakage patterns using both regression and non-parametric techniques. Overall the authors found the presence of DVL to be associated with both 0.2 logMAR worse vision (~2 lines of Snellen visual acuity; p = 0.02) and a trend toward having clinically active inflammation (p = 0.07), but they observed no association between the presence of DVL and CME (p = 0.16). Of note, of 16 eyes (39.0%) found clinically to be inactive, 13 (81.3%) had some degree of vascular leakage, including 5 (31.3%) with PVL and 8 (50.0%) with DVL. Conversely, 20 of 26 eyes (76.9%) found on UWF FA to have DVL had no clinical evidence of vasculitis. This led the authors to suggest that UWF imaging, including FA, be considered as part of a complete baseline examination in all patients with uveitis. Babu and KumaradasCitation9 submitted a letter in response to the article by Laovirojjanakul et alCitation8, in which they described a 12-year-old Srilankan boy with idiopathic intermediate uveitis with DVL on UWF FA whose vision remained stable at approximately 6/9 in each eye following treatment with systemic corticosteroids followed by both systemic cyclosporine and mycophenolate mofetile, but in whom angiographic DVL persisted. The case emphasized the point that using UWF FA alone to initiate or assess the completeness of treatment can be problematic.
Sheemar et al.Citation10 used UWF color and FA imaging in 200 consecutive subjects with retinal vasculitis on clinical examination seen between June 2015 and September 2016, at a single uveitis referral center in New Delhi, India. Eighty-five percent were male and the retinal vasculitis was bilateral in 72.5%. Most subjects (184; 92.0%) had idiopathic uveitis, whereas nine subjects (4.5%) had non-infectious systemic disease associations, and seven (3.5%) had infectious uveitis. Duration and pattern of disease, treatment history, and follow up varied considerably across the cohort. The authors codified vascular leakage on UWF FA as occurring in retinal zones 1, 2, or 3 – with zone 3 correlating to PVL as described above. Most eyes showed leakage in all three zones (56.9%), with a minority (12.3%) having revealing leakage limited to zone 3. The vast majority of eyes (96.0%) showed exclusively or predominantly venous involvement, with only 0.5% having isolated arterial involvement. A majority of eyes (58.8%) had some degree on capillary non-perfusion (CNP), with roughly two-thirds (65.1%) of these having greater than three clock hours of ischemia. Neovascularization of the disc or elsewhere was identified much more frequently using UWF FA than clinically (47.4% vs 18.1%; p = .001), and occurred much more often when the CNP involved more than three clock hours (odds ratio 2.84; 95% CI 1.82–4.44; p = .01) or when zone 1 or zone 2 were involved (odds ratio 45.03; 95% CI 6.10–332.30; p < .001). The authors concluded that UWF imaging, including FA, provided improved detection and characterization of both retinal vasculitis and its complications, including CNP and neovascularization of the disc and elsewhere. No mention was made of adjusting these p-values for multiple comparisons.
Aggarwal et al.Citation11 used UWF imaging to assess both progression and paradoxical worsening (PW) in 44 eyes of 29 patients with tubercular (TB) multifocal serpiginoid choroiditis (MSC)Citation14 seen at a single uveitis referral center in Chandigarh, India. Fundus involvement was described as either central – within 75° of the fundus center, peripheral – or both central and peripheral. Paradoxical worsening was defined as worsening of the TB lesions that occurred within six to eight weeks of initiating TB therapy. Lesions outside of the central 75°conventional imaging zone were revealed in 39 of 44 eyes (88.6%) using UWF imaging. Sixteen of 44 eyes (36.4%) showed PW and in three of these eyes (18.8%), the PW occurred outside the 75°conventional imaging zone. Overall, management was altered in 11 patients based on findings revealed with UWF imaging. The authors concluded that UWF imaging appeared to improve the diagnosis and management of PW in eyes with TB MSC.
Yang et al.Citation12 retrospectively studied the clinical profile, UWF FA findings, and long-term prognosis of 32 patients with tubuloInterstitial nephritis with uveitis (TINU) seen at a single uveitis referral center in Beijing, China, between 2000 and 2016. Ultra-wide-field imaging was performed on 13 patients using the Heidelberg Spectralis HRA 150° non-contact system. Overall, TINU constituted 1.26% of the authors' entire referral cohort seen over the period of the study. The male:female ratio was 13:19 (0.68), including eight adolescent subjects age 10 to 18. The mean age of onset differed for men vs women, at 29.4 years vs 49.0 years, respectively (p = .037). Two patients (6.3%) developed uveitis prior to nephritis, 10 (31.3%) developed uveitis concurrent with nephritis, and the majority (62.5%) developed uveitis after nephritis – with a mean time from nephritis to onset of uveitis of 7.81 ± 3.75 months. The inflammation was or became bilateral in all but two patients (93.8%). Most subjects had the non-specific systemic symptoms of fatigue (93.8%), low fever (84.4%), anorexia (68.8%), and arthralgia (31.3%). All patients had anterior uveitis and 28 (87.5%) had eye pain and blurred vision. Keratic precipitates were present in 19 patients (59.4%), and two (9.1%) had iris stromal (Busacca) nodules. Vitreous opacities were noted in nine patients (28.1%) and six (18.8%) had retinal vascular sheathing. Of the 13 patients who underwent UWF imaging, only two had overt vitreous inflammation and yet 11 (84.6%) had peripheral retinal vascular leakage and 5 (38.5%) had peripheral retinal vascular tortuosity. The mean duration of systemic prednisone therapy was 13.2 ± 10.0 months, with 18 patients receiving systemic corticosteroids for ≥12 months. In addition, 18 patients received non-corticosteroid immunosuppressive therapy. Follow up ranged from six months to seven years (mean 3.2 ± 2.2 years). Among the 30 patients followed for at least one year, 15 (50.0%) had at least one recurrence and three (10.0%) developed chronic inflammation. Vision remained 20/25 or better in all affected eyes at last visit. The authors tended to treat initial or late-onset PVL on UWF FA with increased systemic corticosteroids and noted fewer recurrences following such therapy, leading them to suggest that UWV FA may be important in the initial and long-term management of patients with TINU.
Sen et al.Citation13 used UWF imaging in the diagnosis of a case of intravitreal gnathostomiasis seen in a uveitis referral clinic in Chennai, India. The patient, a 26-year-old Asian Indian woman, had uveitis with decreased vision in her right eye to 6/24 for six months. While the worm was initially seen clinically using indirect ophthalmoscopy, OptomapTM UWF color photography allowed the authors to see anatomical details of the worm suggestive of Gnathostoma – a diagnosis that was confirmed when pars plana vitrectomy was used to remove the worm, which was identified morphologically as Gnathostoma spinigerum.
Together, these reports both confirm and advance findings from earlier studies that UWF imaging improves diagnosis and management of a patient with retinal pathology involving the mid- and far-retinal periphery. Although not reported in the studies in the current issue of OII, widefield fundus autofluorescence (FAF) appears to provide similar clinical benefits, particularly for inflammatory disorders involving the outer retina and retinal pigment epithelium – such as multifocal choroiditis (MFC), primary vitreoretinal lymphoma (PVRL), syphilis, birdshot chorioretinopathy (BSCR), acute posterior multifocal placoid pigment epitheliopathy (APMPPE), serpiginous choroidopathy, and multiple evanescent white dot syndrome (MEWDS) – among others.Citation15,Citation16 The full extent to which such increased identification of otherwise unrecognized peripheral retinal changes will influence treatment decisions and impact patient prognosis will have to await larger and longer-term, clinical trials.
Declaration of interest
The authors report no potential conflicts of interest.
Financial Conflicts
MRM is a consultant to Zeiss, Bayer, Novartis, Lumithera, and Gensight Biologics. SK is a consultant to Adverum, Alcon, Allergan, BioMarin, Genentech/Roche, Novartis, Optos, Regeneron, and RegenXBIo. MZ is a consultant to AbbVie, Alimera, Santen, and Gilead. ETC has no relevant financial conflicts.
Acknowledgments
Supported in part by The Pacific Vision Foundation (ETC) and The San Francisco Retina Foundation (ETC).
Additional information
Funding
REFERENCES
- Quinn N, Csincsik L, Flynn E, et al. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res. Jan 2019;68:83–109. doi:10.1016/j.preteyeres.2018.10.001.
- Nagiel A, Lalane RA, Sadda SR, Schwartz SD. Ultra-widefield fundus imaging: a review of clinical applications and future trends. Retina. 2016 Apr;36(4):660–678. doi:10.1097/IAE.0000000000000937.
- Shoughy SS, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015 Jul;63(7):575–581. doi:10.4103/0301-4738.167122.
- Patel M, Kiss S. Ultra-wide-field fluorescein angiography in retinal disease. Curr Opin Ophthalmol. 2014 May;25(3):213–220. doi:10.1097/ICU.0000000000000042.
- Witmer MT, Kiss S. Wide-field imaging of the retina. Surv Ophthalmol. 2013 Mar-Apr;58(2):143–154. doi:10.1016/j.survophthal.2012.07.003.
- Fan W, Uji A, Borrelli E, et al. Precise measurement of retinal vascular bed area and density on ultra-wide fluorescein angiography in normal subjects. Am J Ophthalmol. Apr 2018;188:155–163. doi:10.1016/j.ajo.2018.01.036.
- Thomas AS, Redd T, Campbell JP, et al. The impact and implication of peripheral vascular leakage on ultra-widefield fluorescein Angiography in Uveitis. Ocul Immunol Inflamm. 2019; 27(3):349–355.
- Laovirojjanakul W, Acharya N, Gonzales JA. Ultra-widefield fluorescein angiography in intermediate uveitis. Ocul Immunol Inflamm. 2019; 27(3):356–361.
- Babu K, Kumaradas M. In response to Laovirojjanakul W, acharya N and Gonzales JA‘s “ultra-widefield fluorescein angiography in intermediate uveitis.” Ocul Immunol Inflamm. 2019; 27(3):362–364. doi:10.1080/09273948.2017.1408844.
- Sheemar A, Temkar S, Takkar B, et al. Ultra-wide field imaging characteristics of primary retinal vasculitis: Risk factors for retinal neovascularization. Ocul Immunol Inflamm. 2019; 27(3):383–388. doi:10.1080/09273948.2018.1508729.
- Aggarwal K, Agarwal A, Deokar A, et al. Ultra-wide field imaging in paradoxical worsening of tubercular multifocal serpiginoid choroiditis after the initiation of anti-tubercular therapy. Ocul Immunol Inflamm. 2019; 27(3):365–370. doi:10.1080/09273948.2017.1373829.
- Yang M, Chi Y, Guo C, Huang J, Yang L, Yang L. Clinical profile, ultra-wide-field fluorescence angiography findings, and long-term prognosis of uveitis in tubulointerstitial nephritis and uveitis syndrome at one tertiary medical institute in China. Ocul Immunol Inflamm. 2019; 27(3):371–379. doi:10.1080/09273948.2017.1394469.
- Sen P, Dutta Majumder P, Biswas J, Rao C, Das K. Role of ultra-wide-field imaging in the diagnosis of intravitreal gnathostomiasis: a case-report. Ocul Immunol Inflamm. 2018 Aug 10:1–3. [Epub ahead of print] (OII production editor to add final citation.). doi:10.1080/09273948.2018.1498525.
- Cunningham ET Jr, Gupta A, Zierhut M. The creeping choroiditides - serpiginous and multifocal serpiginoid choroiditis. Ocul Immunol Inflamm. 2014 Oct;22(5):345–348. doi:10.3109/09273948.2014.962924.
- Pfau M, Fleckenstein M, Schmitz-Valckenberg S, Holz FG. Autoflourescence imaing. Cunha-Vaz J, Koh A, eds. Imaging Techniques. Vol. 10. Basel, Karger: ESASO Course Series. 2018; 65–87.
- Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retina Vitreous. 2016 Apr;8(2):12. doi:10.1186/s40942-016-0035-x.
