ABSTRACT
Objective
To evaluate the long-term efficacy of methotrexate (MTX) monotherapy in patients with juvenile idiopathic arthritis-associated uveitis (JIA-U).
Methods
We analyzed a cohort of patients with JIA-U treated with MTX monotherapy, divided into two groups depending on whether MTX was started before (on-MTX group) or after uveitis diagnosis (MTX-naïve group). The primary endpoint was the time between uveitis inactivity and first relapse.
Results
84 patients entered the study. The median duration of remission on MTX monotherapy resulted 8.2 months. The on-MTX group showed a significant longer time interval between arthritis and uveitis onset and higher need for biologic agents (bDMARD). During follow-up, 40 patients (47.6%) needed bDMARD due to poor control of uveitis. Clinical remission off medication was achieved in 11.9% of patients, all belonging to the MTX-naïve group.
Conclusions
MTX monotherapy, although effective in early stages of JIA-U, showed poor disease control in the long term.
Juvenile Idiopathic Arthritis associated uveitis (JIA-U) represents a severe sight-threatening condition due to its high risk of serious long-term complications, potentially leading to blindness.Citation1–3 Treatment strategy for JIA-U is based on a step-up approach including topical or systemic corticosteroids (CS) and Methotrexate (MTX) which is the first choice conventional synthetic disease modifying antirheumatic drug (csDMARD).Citation4 Despite its wide use and known efficacy for early disease control,Citation5 limited data are available on MTX effects on long-term JIA-U disease activity.
Aim of the present study was to evaluate the efficacy of MTX monotherapy in long-term disease control in a cohort of JIA-U patients.
Materials and methods
Study population and data collection
The present study was conducted following the principles of the Declaration of Helsinki.
We retrospectively analyzed an inception cohort of children with JIA–U, consecutively presenting at our Center between 1994 and 2017. All patients were diagnosed as having JIA according to the ILAR criteria,Citation6 and chronic anterior uveitis according to the SUN Working Group criteria.Citation7 To be included in the study, patients needed a diagnosis of JIA-U before the age of 18 years, treatment with MTX as first-line csDMARD and a regular follow-up of at least 12 months after MTX treatment start for uveitis.
Based on the timing of MTX initiation, we identified two cohorts of patients: subjects who received MTX treatment after the diagnosis of uveitis (MTX-naïve group) and patients who received MTX before the diagnosis JIA-U (on-MTX group) for the management of arthritis. During the active phase of uveitis, patients were evaluated at least monthly; once remission was achieved, at least every three months.
Clinical data were collected into an anonymized database, starting from the time of JIA-U diagnosis, and included demographic information, JIA onset, uveitis laterality, presence of ocular complications and type and dose of medications. Routine ophthalmological evaluation included measurement of best-corrected visual acuity, intraocular pressure (IOP) assessment, slit lamp examination, and ophthalmoscopy.
A “stepladder” algorithm was used for the treatment of JIA-U. New-onset uveitis was initially treated only with mydriatics and/or topical corticosteroids. In case of recurrent or persistent AU activity for >1 month, systemic corticosteroids (prednisone 0.5–1mg/kg/day) for a short period (1–2 months) were added. If intraocular inflammation was still refractory or steroid-dependent, immunosuppressive therapy with MTX was administered at a dose of approximately 15 mg/m2/week orally or subcutaneously, depending on patient/parent’s preferences. When uveitis relapse was detected despite MTX treatment, topical/systemic CS were added as described above, and MTX dosage was adjusted, if needed. Indications for a biologic DMARD (bDMARD) were intolerance or non-responsiveness to ≥6 months MTX at the maximum tolerated dose, and/or oral prednisone dependency. When uveitis inactivity was achieved, CS were tapered at the lowest possible dose and eventually stopped.
Outcome measures
The main outcome measure used to assess the effect of MTX was the time to first uveitis relapse once inactivity was achieved. According to the SUN criteria,Citation7 uveitis was graded based on the number of cells in the anterior chamber and inactive uveitis was defined as AC cell grade <0.5 + . Uveitis relapse was defined as at least a 2 grade increase in the AC. Uveitis clinical inactivity on medication (CIM) was defined as inactive disease and absence of relapses for ≥3 months on systemic treatment with or without minimal topical treatment (CS and/or mydriatic-cycloplegic eye drops, used less than once per day). Clinical remission off medication (CR) was defined as the absence of relapses for more than 3 months after stopping systemic and local treatment for eye disease.
As secondary outcome measure, we evaluated the length of duration of MTX monotherapy, in order to evaluate how many patients could maintain stable remission with only MTX treatment. MTX monotherapy was defined as the time between the start of MTX for uveitis and the last available visit, or the time of switching to a bDMARD, where applicable.
The presence of ocular complications at baseline and after the MTX treatment start was also evaluated.
Statistical analysis
Quantitative variables were expressed as median (IQR) and qualitative variables as number (%). Comparisons in baseline demographics and disease characteristics, clinical parameters, duration of MTX treatment and duration of uveitis remission were analyzed using Pearson chi-squared test or Fisher’s exact test for categorical variables, and the Student t-test or Mann–Whitney U-test for continuous variables, where appropriate. Kaplan – Meier curves were constructed to summarize duration of remission in the study population. Statistical significance was considered for p values < .05. All analyses were performed with the IBM SPSS package (version 26.0)
Our study followed the principles of WMA Declaration of Helsinki – ethical principles for medical research involving human subjects.
Results
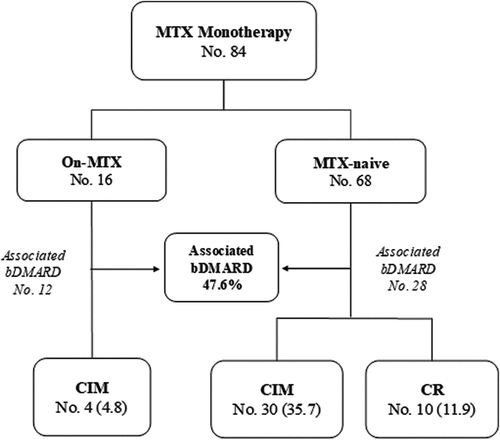
We included in the study 84 JIA-U patients, oligoarticular HLA-B27 negative subtype. The median follow-up after the diagnosis of uveitis was 7.6 years (IQR 4.1–9.7). The MTX-naïve group included 68 patients who started MTX treatment after a median of 9 months from the diagnosis of JIA-U. The on-MTX group included 16 patients treated with MTX for a median of 16.7 months before developing JIA-U (). These two groups of patients were comparable for gender distribution (p > .999), ANA positive frequency (75.0 vs 67.7%, p = .76), ESR (18.8 vs 22.1%, p > .999) and C-reactive protein (CRP) elevation (31.3 vs 27.9%, p= .76) at disease onset.
Table 1. Demographic and clinical characteristics of the patients.
Of note, patients in MTX-naïve group presented lower age at uveitis diagnosis (p = .009) as well as significantly shorter time between JIA diagnosis and uveitis onset (p < .001) and older age at MTX starting (p = .043).
Twenty patients (23.8%) had at least one ocular complication already present at the time of MTX starting. In 22 patients, new onset complications were observed during MTX treatment (). The most common were cataract and increased IOP, both presenting in 10 patients (11.9%).
At the end of the available follow-up, 81/84 patients achieved their first uveitis inactivity under MTX, after a median time of 1.9 months (IQR 1–3.1). Three patients did not achieve inactivity under MTX monotherapy, but only after bDMARD adjunctive therapy. Seventeen patients had not experienced any uveitis relapse after the first remission.
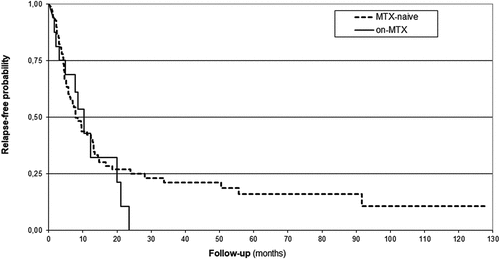
Median time between remission and first uveitis relapse was 8.2 months (IQR 4.4–19.9). 50 patients (59.5%) experienced their first uveitis relapse on MTX therapy within the first year since remission. When comparing the two study groups, there was no difference in the probability of maintaining remission (Log Rank test p = .61) ().
Figure 1. Kaplan-Meier survival estimates of the time to the first Anterior Uveitis relapse in MTX-naive and on-MTX groups (p 0.61, log rank test).
Median duration of MTX monotherapy was 34.3 months (IQR 18.2–54.7), and resulted significantly longer for MTX-naïve patients (p = .013) (). During follow-up, 40 patients (47.6%) needed adjunctive therapy with a bDMARD due to poor control of uveitis, most belonged to the on-MTX group (75 vs. 41%, p = .015).
At the end of the available follow-up, 10 patients (11.9%), all from the MTX-naïve group, maintained prolonged CR (median 2.9 years, IQR 2.5–3.9) ().
Discussion
In the present study, we evaluated the long-term outcome of MTX monotherapy in an inception cohort of consecutive oligoarticular JIA-U patients, followed at our tertiary care center. The majority were females, with higher frequency than in other reported cohorts.Citation8–11
Methotrexate is widely used in clinical practice as first csDMARD for the treatment of pediatric noninfectious uveitis and is currently recommended as the immunosuppressive drug of choice both for treatment of arthritis and uveitis in children with JIA.Citation4,Citation12–15 However, while strong evidence supports the role of MTX in the initial treatment of JIA arthritis,Citation14 data on its long-term efficacy in JIA-U, as monotherapy, are limited and based mostly on small observational studies that provide a mean follow-up duration of less than two yearsCitation5 which is much less than in our study (7.6 years).
Previous studies have pointed out a potential preventive role of MTX toward uveitis in JIA patients. Papadopoulous et alCitation8 showed that uveitis occurred more in patients who received only nonsteroideal antinflammatory drugs (NSAIDs) or local treatment, as compared to those receiving MTX. Seemingly, a Russian study with similar design demonstrated that the absence of MTX treatment is a predictor for uveitis development.Citation11 In addition, data from a German registry showed how MTX treatment, both alone and in combination with tumor necrosis factor-α (TNF-α) inhibition, reduced uveitis incidence over time.Citation9 In our cohort, the difference in the two groups in the age at the time of uveitis onset seem to confirm the preventive effect of MTX: patients who were already on treatment developed uveitis almost two years later than MTX-naïve children did.
Evidence on the efficacy of MTX in the treatment of noninfectious uveitis has been summarized in a meta-analysis, which showed that 73% of children achieved significant improvement of ocular inflammation, as defined by the SUN criteria.Citation5 Indeed, efficacy of MTX in inducing uveitis inactivity was achieved after a median 3.5 months, and studies conducted only on JIA-U patients reported an overall rate of clinical inactivity on medication (CIM) ranging between 71 and 86%.Citation10,Citation16,Citation17 Our study also confirms the high efficacy of MTX in the short-term control of ocular inflammation. In fact, the initial response rate was high, with only three (3.7%) patients being refractory to MTX monotherapy, and, among MTX-responders, inactivity was achieved after 1.9 months. However, our main objective was to evaluate the long-term efficacy of MTX monotherapy, and in this regard, our results are less reassuring. In fact, we found that the median duration of uveitis inactivity on MTX was only 8 months; this is comparable to data from two previous retrospective studies, reporting a median duration of first remission of 10.3 months.Citation16,Citation17 Moreover, survival curve, in our study, showed that by five months, 25% of the patients had already experienced a relapse and only 25% were still in remission on treatment after two years.
The need for adjunctive bDMARD treatment, in our cohort, is another relevant aspect as, after a mean 2.5 years, almost half of our patients did so. Notably, patients in the on-MTX group had a shorter duration of remission on MTX monotherapy as 75% needed to switch to a bDMARD therapy.
Finally, only 10 patients (11.9%) maintained prolonged remission off-MTX while 6 out of 16 (37.5%) who stopped MTX treatment, reported a relapse after treatment discontinuation. In two previous studies reporting data on MTX discontinuation, relapses were noted in one-third of patients in a German study,Citation16 and in up to two-thirds of the patients in another cohort.Citation10 In these cohorts, a prolonged clinical remission off medication was achieved only in 16 and 18% of patients, respectively. In addition, Heiligenhaus et al. reported that no patient in their study maintained remission of uveitis after MTX discontinuation.Citation17 In the previously mentioned metanalysis of nine studies, despite the short follow up of the patients, remission off medication was globally achieved in 16% of the patients, as well.Citation5
Of note, in our cohort, none of the patients of the on-MTX group maintained stable remission off therapy (). Hence, despite the early introduction of MTX for arthritis is capable to delay uveitis onset, it seems to be unable to arrest, alone, a severe ocular disease course, as confirmed by the significantly shorter time of remission on monotherapy and the higher need for an adjunctive bDMARD.
A possible limitation of our study was that it included patients followed at a single tertiary care center, specialized in the treatment of severe course uveitis, where difficult-to-treat patients from other Regions of Italy are referred. This may represent a possible referral bias.
In conclusion, MTX treatment, although effective in the early stages, was associated with poor long-term control of JIA-U and even appearance of new ocular complications in one-fourth of the patients. Based on these findings, prolonged MTX monotherapy cannot fully control the uveitis course. Therefore, we suggest that patients who develop uveitis despite ongoing MTX treatment for arthritis should benefit from an adjunctive bDMARD treatment at the time of the first uveitis onset. In MTX-naïve patients, uveitis should be carefully followed over time even in case of initial good response to MTX and bDMARD should promptly be added in case of early relapse or prolonged uveitis activity under medication. This treat-to-target strategy will probably improve the long-term prognosis of JIA-U, as recently reported.Citation18
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Clarke SL, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J. 2016;14(1). 27 Published 2016 Apr 27. doi:10.1186/s12969-016-0088-2.
- Lee JJY, Duffy CM, Guzman J, et al. Prospective determination of the incidence and risk factors of new-onset uveitis in juvenile idiopathic arthritis: the research in arthritis in Canadian children emphasizing outcomes cohort. Arthritis Care Res (Hoboken). 2019;71(11):1436–1443. doi:10.1002/acr.23783.
- Zulian F, Martini G, Falcini F, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29(11):2446–2453.
- Constantin T, Foeldvari I, Anton J, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;77(8):1107–1117. doi:10.1136/annrheumdis-2018-213131.
- Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology (Oxford). 2013;52(5):825–831. doi:10.1093/rheumatology/kes186.
- Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392.
- Jabs DA, Nussemblatt RB, Rosembaum JT, the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi:10.1016/j.ajo.2005.03.057.
- Papadopoulou C, Kostik M, Böhm M, et al. Methotrexate therapy may prevent the onset of uveitis in juvenile idiopathic arthritis. J Pediatr. 2013;163(3):879–884. doi:10.1016/j.jpeds.2013.03.047.
- Tappeiner C, Schenck S, Niewerth M, Heiligenhaus A, Minden K, Klotsche J. Impact of antiinflammatory treatment on the onset of uveitis in juvenile idiopathic arthritis: longitudinal analysis from a nationwide pediatric rheumatology database. Arthritis Care Res (Hoboken). 2016;68(1):46–54. doi:10.1002/acr.22649.
- Kalinina Ayuso V, van de Winkel EL, Rothova A, De Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. 2011;151(2):217–222. doi:10.1016/j.ajo.2010.08.021.
- Kostik MM, Gaidar EV, Hynnes AY, et al. Methotrexate treatment may prevent uveitis onset in patients with juvenile idiopathic arthritis: experiences and subgroup analysis in a cohort with frequent methotrexate use. Clin Exp Rheumatol. 2016;34(4):714–718.
- Henderson LA, Zurakowski D, Angeles-Han ST, Lasky A, Rabinovich CE, Lo MS, Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry Investigators. Medication use in juvenile uveitis patients enrolled in the childhood arthritis and rheumatology research alliance registry. Pediatr Rheumatol Online J. 2016;14(1):9. Published 2016 Feb 16. doi:10.1186/s12969-016-0069-5.
- Angeles-Han ST, Ringold S, Beukelman T, et al. 2019 American college of rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res (Hoboken). 2019;71(6):703–716. doi:10.1002/acr.23871.
- Ringold S, Angeles-Han ST, Beukelman T, et al. 2019 American college of rheumatology/arthritis foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol. 2019;71(6):846–863. doi:10.1002/art.40884.
- Ferrara G, Mastrangelo G, Barone P, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol Online J. 2018; 16(1):46. Published 2018 Jul 11. doi:10.1186/s12969-018-0255-8.
- Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32:362–365.
- Heiligenhaus A, Mingels A, Heinz C, Ganser G. Methotrexate for uveitis associated with juvenile idiopathic arthritis: value and requirement for additional anti-inflammatory medication. Eur J Ophthalmol. 2007;17(5):743–748. doi:10.1177/112067210701700509.
- Cheung CSY, Mireskandari K, Ali A, Silverman E, Tehrani N. Earlier use of systemic immunosuppression is associated with fewer ophthalmic surgeries in paediatric non-infectious uveitis. Br J Ophthalmol. 2020;104(7):938–942. doi:10.1136/bjophthalmol-2019-314875.