ABSTRACT
Background
Cyclosporine A (CsA) has been used as a topical treatment for various ocular surface diseases including dry eye disease (DED). Several CsA formulations are available as solutions or emulsions.
Purpose
This review describes the development and the preclinical testing of a cationic oil-in-water emulsion of CsA (CE-CsA) in terms of pharmacodynamics, pharmacokinetics, and ocular tolerance. Due to the cationic charge, CE electrostatically interacts with the negatively-charged ocular surface, improving its residence time. Compared to other CsA formulations, CE-CsA and CE itself were found to reduce the signs and symptoms of DED, by restoring tear film stability and properties, and inhibiting the expression and secretion of pro-inflammatory factors. No delay in wound healing nor ocular toxicity were observed using CE formulations.
Conclusion
these findings indicate that the type of vehicle can significantly affect the performance of eye drops and play an ancillary role in DED treatment. CE appears as a promising strategy to deliver drugs to the ocular surface while maintaining its homeostasis.
The ocular surface encompasses a variety of structures, such as the cornea, conjunctiva, lacrimal and meibomian glands, tears, connective tissues, eyelids, eyelashes and nasolacrimal duct, all of which are connected with the trigeminal pathway and the immune system, and play a part in maintaining the homeostasis of the eye.Citation1 Dry eye disease (DED), also called keratoconjunctivitis sicca, is one of the most common ocular surface disease with a prevalence ranging from 5% to 50% worldwide.Citation2 DED is a multi-factorial and often chronic disease, characterized by multiple underlying pathophysiological mechanisms including tear film instability and hyperosmolarity, neurosensory abnormalities, ocular surface inflammation and damage, leading to loss of homeostasis.Citation2,Citation3 These combined mechanisms result in a “vicious circle” of immunopathogenesis, which can be particularly challenging to treat.Citation4
Cyclosporin A (CsA) is an immunomodulatory agent discovered in the 1970s. Since then, it has been marketed for various medical applications such as psoriasis, Crohn’s disease and organ transplants.Citation5–8 In ophthalmology, CsA was initially investigated to prevent graft versus host disease after transplantation of donor corneal tissues.Citation9 Nowadays, this medication is used for the treatment of ocular surface diseases associated with inflammation such as DED,Citation10 seasonal allergic conjunctivitisCitation11 and vernal keratoconjunctivitis (VKC), a severe and chronic form of ocular pediatric allergies.Citation12,Citation13 The efficacy of CsA comes from its ability to inhibit the activity of T cells and the production of pro-inflammatory cytokines, both responsible for hyper-inflammation,Citation14 however, its large molecular weight and hydrophobicity result in low ocular bioavailability.Citation15,Citation16 Topical solutions and emulsions represent the most common commercial formulations of CsA currently available.Citation17 Papilock mini® (marketed since 2005 in Japan by Santen Pharmaceutical Co. Ltd.), Modusik-A Ofteno® (marketed since 2003 in South America by Laboratorios Sophia), and TJ Cyporin® (marketed since 2003 in South Korea by Taejoon Pharm Co., Ldt.) are the main CsA solutions available on the market. In 2018, Sun Pharma Global marketed Cequa®, a new generation of CsA solutions based on a nanomicelle technology,Citation18 in the US and in Australia. Despite a general ease of preparation, CsA solutions require the use of surfactants and co-solvents to stabilize the formulation and prevent CsA precipitation. Unfortunately, repeated instillations of high surfactants concentrations on the ocular surface can induce ocular irritation.Citation17,Citation19,Citation20 This limitation has led to the development of new drug delivery systems (DDS) to improve CsA retention on, and penetration through, the ocular surface.Citation17
Oil-in-water emulsions are well suited to solubilize lipophilic CsA as they limit the concentration of surfactants needed.Citation17 Moreover, oily excipients also tend to prevent ocular surface desiccation by helping to restore the lipid layer of the tear film.Citation21,Citation22 Restasis® (AE-CsA, Allergan) and Ikervis® (CE-CsA, Santen) are both CsA emulsions approved by the FDA in the US and by the EMA in Europe, respectively. While AE-CsA is composed of anionic components, CE-CsA is composed of cationic ones. The advantage of cationic DDS (i.e. positively charged) is their ability to interact with the negatively-charged mucin layer of the tear film, increasing the residence time of CsA on the ocular surface.Citation15,Citation23,Citation24
In this review, we will give an overview of the development of cationic emulsions (CE) as well as its main preclinical outcomes collected over the last decade. We will compare these features with the other marketed CsA formulations and hospital-compounded preparations, in term of pharmacodynamics, pharmacokinetics, ocular tolerance and toxicity, as is recommended by health authorities for the approval of new medical products.Citation25 Finally, we will discuss the translation of these preclinical outcomes to patients with DED.
Development of a cationic emulsion of cyclosporine A
The concept of CE was first introduced in 1996 for the oral administration of progesterone.Citation26 A few years later, this technology was assessed in ophthalmology for the first time, to deliver indomethacin to the ocular surface.Citation27 In this section, we will describe the three main components of the CE-CsA (CsA, an oil-in-water emulsion and a cationic agent) as well as their interaction with the ocular surface.
Cyclosporine A
The immunosuppressive activity of CsA was first reported in 1976 by Borel et al.Citation28 Since then, mechanistic studies have shown that CsA inhibits phosphatase calcineurin, preventing nuclear factor of activated T-cell (NFAT) activation and subsequent gene expression of interleukin-2 (IL-2) in activated T cells.Citation29,Citation30 In addition to the calcineurin/NAFT pathway, other mechanisms of action of CsA have been hypothesized over the last few decades.Citation29,Citation31 It has been shown that CsA also may inhibit T cell activation by blocking JNK and p38 signaling pathway.Citation29 More recently, Liddicoat et al. described that CsA may also impact innate immune dendritic cells (DC) using the similar calcineurin/NAFT pathway, which can influence adaptive immune responses via regulatory T cells induction, T helper (CD4+) cell polarization and humoral responses.Citation31 CsA may also impact other types of innate immune cells, such as macrophages and neutrophils,Citation31,Citation32 however, more studies are needed to better understand the underlying mechanisms of action. In ophthalmology, CsA has been assessed to prevent corneal graft rejectionCitation9 and rapidly gained interest for ocular surface diseases. In addition, CsA was shown to significantly reduce the presence of CD4+ T cells on the ocular surface of patients with Sjögren’s syndrome,Citation33 while in patients with VKC, CsA also decreased the number of antigen-presenting cells (APC) cells, CD4+ T cells and B cells.Citation34 Another study showed that CsA increased the number conjunctival goblet cells and decreased the epithelial turnover, which can be beneficial for the maintenance of ocular surface homeostasis.Citation35
Oil-in-water emulsion
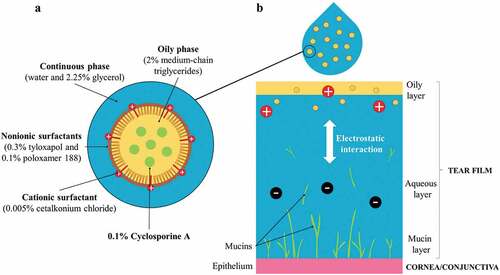
Oil-in-water emulsions are biphasic formulations containing oily droplets dispersed in a continuous water phase. They have been used as delivery systems for hydrophobic drugs due to their ability to incorporate water insoluble molecules. To create these emulsions, various oils and emulsifiers (i.e., surfactants) have been used as ocular DDS.Citation36 The choice of these components as well as their concentrations (i.e. oil/surfactant ratio) represent an important factor in the safety profile of emulsions on the ocular surface, as is described in the section 5. In CE-CsA, medium-chain triglycerides are used as the oily phase to solubilize CsA. To stabilize the oil nano-droplets (<200 nm) of CsA in the continuous phase, two nonionic surfactants are used: tyloxapol (hydrophobic) and poloxamer 188 (hydrophilic) (). These surfactants are already used in eye drop formulations, tyloxapol can exhibit anti-inflammatory effects in some instances,Citation37,Citation38 and both are known to be well tolerated in the human eye.Citation39 Glycerol can also be used in the continuous phase in order to protect the ocular surface from the hyperosmolarity induced by DED.Citation40 The tear film of the eye comprises 3 layers: the outer oily layer secreted by Meibomian glands, the intermediate aqueous layer produced by the lachrymal glands, and the inner mucin layer (). Clinically, DED is characterized by a loss of volume in all tear film layers.Citation3 Because of the similarities in composition between emulsions and the tear film (both oily and aqueous phases), emulsions can act as a tear film substitute, effectively reducing symptoms of DED.Citation41
Cationic and polar agents
The mucin layer of the tear film plays a significant role in protecting the ocular surface. Mucins are high-molecular weight glycoproteins composed of carboxyl and sulfate groups that are negatively charged.Citation42 Thus, the use of cationic agents in emulsions can induce electrostatic interaction with the anionic mucin layer and improve drug bioavailability. Moreover, cationic agents cause electrostatic repulsions between nano-droplets which help to stabilize the emulsions.Citation43 CE have demonstrated additional benefits over nonionic or anionic emulsions in drug delivery for various biomedical applications.Citation44 CE-CsA contains a cationic agent called cetalkonium chloride (CKC) a homolog of benzalkonium chloride (BAK). BAK is a quaternary ammonium compound currently used as a preservative at concentrations up to 0.02% in many eye drops formulations due to its bactericidal and microbicidal properties at high concentrations. Nevertheless, several side effects have been observed in patients using formulations containing BAK, such as dry eye exacerbationCitation45 and corneal cell damage.Citation46,Citation47 While BAK contains 12 or 14 carbons in its alkyl chains, CKC contains 16 carbons resulting in a much higher hydrophobicity and increased polarity of the molecule. As opposed to the shorter C12 and C14 alkyl chains, all the CKC is incorporated at the oil interface increasing the stability of oil nano-droplets and most importantly, reducing the amount of free molecules in the water phase that are responsible for ocular side effects.Citation48 In addition, in vitro and in vivo studies revealed that CE increased tear film elasticity and thicknessCitation49 and can potentially compensate for Meibum gland dysfunction, a common clinical sign of DED.
The originality of CE-CsA comes from the combination of CsA with emulsion and the non-conventional use CKC as cationic agent and polar lipid. Concentrations of the nonpolar and polar components have been selected in order to mimic the composition of the tear fluid lipid layer () in terms of both quantities and ratios. In particular, the limited concentration of CKC used in the CE allows to bring cationic charges as well as surfactant effects without inducing any toxic effect as it is described in section 5. Therefore, each of these components provides specific properties to the final emulsion and tear-film supplementation, which helps to break the vicious circle and restore homeostasis of a multifactorial disease such as DED. Moreover, the oils contained in CE can diffuse in the tear film lipid layer which can then act as a drug reservoir for the active molecules (eg., CsA) solubilized in the oils.Citation53
Table 1. Similarities between the composition of the tear film and a cationic emulsion.
Pharmacodynamics profile
In the last decade, many preclinical studies have been published about the pharmacodynamics of CE-CsA and its vehicle using in vitro and in vivo models (). Several outcomes have been emphasized in these studies including anti-inflammatory potency, effect on DED symptoms, effect on wound healing, as well as the modulation of the gene expression profile.
Table 2. Overview of the pharmacodynamics studies realized on the CE-CsA.
Anti-inflammatory potency
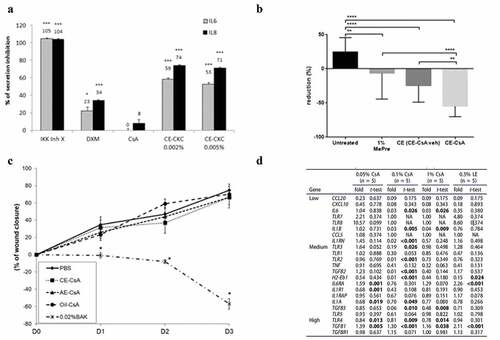
Ocular immunological diseases, such as DED, are usually associated with self-perpetuating inflammation, resulting in a chronic disease state.Citation4 In vitro studies assessed the anti-inflammatory effect of both CE-CsA and CE alone. One such study compared different formulations of CE containing two different concentrations of CKC (0.002% and 0.005%) or with tyloxapol surfactant alone.Citation55 Both CKC concentrations decreased the in vitro secretion of several proinflammatory factors in several cell types with several types of induced stress (). The study also revealed that emulsions containing only tyloxapol reduced the secretion of IL-6 and IL-8 from human corneal epithelial cells (HCECs) stressed using lipopolysaccharide.Citation55 Interestingly, a recent study has shown the anti-inflammatory activity of cationic lipids through the Protein Kinase C (PKC) pathwayCitation66 and similarly, CKC has been shown to act as an inhibitor of the PKC pathway, which downregulates proinflammatory factors,Citation55 and parallels the observation by Chen and collaborators with PKC alpha knock-out mice. Therefore, CE has higher anti-inflammatory effects compared to AE, independently of the CsA action, suggesting that CE alone has anti-inflammatory potency. These results are confirmed by another study assessing the effect of CE-CsA and AE-CsA with the same CsA concentration on an in vitro desiccation stress model of HCECs.Citation54 Results showed that levels of the proinflammatory factors (phospho Nuclear Factor kappa B (p-NF-κB p65) and phospho Inhibitor of kappa B alpha (p-IκBα)) and a proinflammatory cytokine (Tumor Necrosis Factor (TNF-α)) were found to be lower when HCECs were treated with CE-CsA compared to AE-CsA.Citation54
Figure 2. Main outcomes regarding pharmacodynamics of CsA cationic emulsions. (a) Effects of the cationic emulsions of cetalkonium chloride (CE-CKC) emulsions, IKK Inhibitor X (IKK Inh X), dexamethasone (DXM), and cyclosporine A (CsA) on IL-6 and IL-8 release by HCE-2 cells following lipopolysaccharide (LPS) stimulation. *p < .05; **p < .01; ***p < .001.Citation55 (Adapted from reference Citation55). (b) Percentage of CFS score reduction after 10 days-treatment using 1% methylprednisolone (MePre), CE (CE-CsA veh) and CE-CsA on an in vivo mouse dry eye model. **p < .01; ***p < .001; ****p < .0001.Citation62 (Adapted from reference Citation62). (c) Wound closure from day 1 to day 3 after the different treatments. * p < .0001–0.02 compared to the other four groups at the corresponding times.Citation56 (Adapted from reference Citation56). (d) Fold changes and t-test values (vs. DED untreated) for the 23 genes detected among the 34 genes followed in this study in the DED (± treatment) mice corneas. (LE, loteprednol etabonate).Citation60 (Adapted from reference Citation60).
Effect on the DED signs
Effects of CE-CsA and CE alone on signs of DED have also been tested using in vivo mice models.Citation59–64 DED was induced in mice using a controlled environment room with low humidity, as previously described in the literature.Citation67 Clinically, two common signs of DED are a loss of tear fluid volume and keratitis.Citation3 A phenol red thread (PRT) lacrimation test and corneal fluorescein staining (CFS) were performed to assess the tear fluid volume and corneal abrasion, respectively. Results showed that the CFS scores of mice with induced DED were reduced by 36% for the group treated with CE alone (CE-CsA vehicle) and by 59% for the group treated with CE-CsA when compared to the untreated groupCitation62 (), however, only CE-CsA was found to significantly increase tear fluid volume of mice.Citation64 CE-CsA also exhibited a greater reduction of CFS scores compared to other corticosteroids including 1% methylprednisoloneCitation62 and 0.5% loteprednol etabonate (LE, Lotemax®, Baush+Lomb)Citation60 as well as 5% lifitegrast solution (Xiidra®, Shire), a lymphocyte function-associated antigen-1 (LFA-1) antagonist.Citation61 Interestingly, CE alone was found to be more efficient at reducing CFS scores compared to 5% lifitegrast solution.Citation61
Compared to AE-CsA, treatment of DED with CE-CsA induced lower CFS scores, but a significant difference was not achieved in all studies.Citation59,Citation60,Citation64 However, CE-CsA treatment did reduce the occurrence of ocular lesions due to DED, compared to AE-CsA. These results highlight that CE itself can reduce keratitis induced by DED, which can be explained by its anti-inflammatory activity as well as its improved electrostatic interactions with the ocular surface and tear film lipid layer, previously described. A recent study tested different CsA formulations on mice with spontaneous development of Sjögren’s syndrome.Citation58 The results revealed that one instillation a day (QD) of CE-CsA induced a higher tear fluid volume than two instillations a day (BID) of AE-CsA. More interestingly, when comparing CE-CsA (QD) and nanomicelle-CsA (BID),Citation58 no significant difference in tear fluid volume (except at day 60) was found. Overall, these studies proved that the synergic effects of CE and CsA significantly reduced signs of DED in in vivo models using a single instillation a day.
Effect on wound healing
Some topical anti-inflammatory medications, such as prednisolone or dexamethasone, are known to delay corneal wound healing.Citation68,Citation69 In vitro and in vivo studies have been performed with CE-CsA to assess its effect on wound healing. HCECs were used to assess the cytotoxicity of different CsA formulations, as well as their effects on cell migration and proliferation.Citation56,Citation57 Results showed that all CsA formulations, including CE-CsA, maintained an in vitro wound healing rate similar to phosphate buffered saline (PBS) ().Citation56,Citation57 Effect on wound healing has also been evaluated in vivo using a rat scrapping assay.Citation65 Rat corneas treated with CE-CsA or CE were found to heal in 5 days, a similar timeframe to corneas treated with the control (NaCl). Moreover, the number of inflammatory cells after 5-days healing was found to be lower for corneas treated with CE-CsA, compared to control group.Citation65 It is worth noting that, contrary to BAK, CKC contained in CE-CsA showed no sign of cytotoxicity nor delay of cell proliferation.
Modulation of the gene expression profile
Several studies revealed that the use of CE-CsA or CE itself can modulate the expression profile of inflammatory genes. It has been shown that the presence of CE on stress-induced cells in vitro decreased the expression of several inflammatory genes, such as interferon (IFN)-γ, IL-17A, C-X-C motif ligand (CXCL)-9 and TNF-α, Thrombospondin (THBS)-1 and C-C motif ligand (CCL)-2.Citation55 This modulation of gene expression profile has also been observed in vivo in mice models.Citation60 While both CE-CsA and AE-CsA reduced expression of IL-1α and toll-like receptor (TLR)-4, CE-CsA was the only one able to reduce the expression of numerous other inflammatory genes including 2-Eb1, IL-1β, IL-1RN, IL-6, transforming growth factor (TGF)-β2, TGF-β3, TLR-2 and TLR-3 ().Citation60
Several compelling pharmacodynamics outcomes have been highlighted in the different in vitro and in vivo studies performed during the preclinical development of CE-CsA. The anti-inflammatory properties, provided by both CsA and the vehicle, CE, indicate that CE-CsA is very effective at treating the symptoms of DED, especially as CE-CsA demonstrates significant reductions in ocular lesions while also facilitating the restoration of tear fluid volume. Moreover, in comparison to other topical treatments, no delay in wound healing was observed using CE-CsA, while the differences achieved in corneal gene expression show how the formulation can modulate ocular inflammation. Although these in vitro and in vivo findings present strong data for the efficacy of CE-CsA alone, they have additionally been confirmed clinically, as patients with severe DED have reported reductions in the signs and symptoms of their DED after using CE-CsA.Citation70,Citation71
Pharmacokinetics outcomes
Several studies assessing the pharmacokinetics profile of CE-CsA have been published over the last decade ().
Table 3. Overview of the pharmacokinetics studies realized on the CE-CsA.
It has been shown that after a single dose of either 0.05% or 0.1% CE-CsA, the CE formulation was found to deliver a higher CsA concentration (Cmax) to the rabbit corneas as well a better exposition (area under the curve (AUC)) compared to a single dose of 0.05% AE-CsA ().Citation74 CsA concentration was measured in ocular and non-ocular tissue after 10 days of treatment using 0.05% CE-CsA (QD), 0.1% CE-CsA (QD) or 0.05% AE-CsA (BID). Results showed no significant difference in CsA concentration between the three groups.Citation74 Therefore, we hypothesize that CE-CsA acts as a drug reservoir for the sustained release of CsA, resulting in a lower dose regimen requirement (QD vs. BID for AE-CsA). CE-CsA has also been compared to CsA compounded formulations prepared in hospital pharmacies. It was found that AUC(0.5–24h) was 5.4 and 3.9 times higher for CE-CsA compared to CsA hospital-compounded preparations (CsA-HP), in the cornea and the conjunctiva, respectively ().
Figure 3. Main outcomes regarding pharmacokinetics of CE-CsA. (a) Changes in CsA concentration with time after a single unilateral topical administration in the cornea of pigmented rabbits.Citation74 (Adapted from reference Citation74). (b) Pharmacokinetics profile of CsA delivery after a single unilateral topical administration of CE-CsA or CsA hospital-compounded preparations (CsA-HP) in the cornea and conjunctiva of pigmented rabbits.
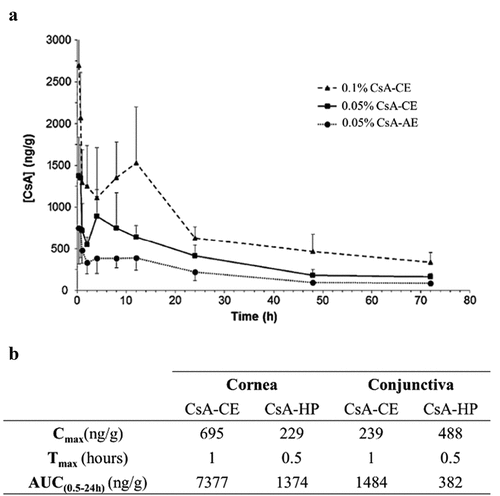
These results suggest the superiority of CE-CsA in term of ocular bioavailability compared to other formulations (AE-CsA or CsA-HP). This higher performance can be explained by the unique electrostatic interaction of CE with the tear film, as reported in section 2.2. More recently, two ex vivo studies have been published about the pharmacokinetics profile of new CsA DDS in academic research development. Results obtained to date demonstrate a slight increase of CsA in ex vivo corneas using these DDS compared to CE-CsA, but otherwise are largely comparable.Citation72,Citation73 However, significant investigations regarding the in vivo pharmacokinetics and pharmacodynamics of these new formulations still need to be performed, to evaluate the real benefit of these technologies for the treatment of ocular surface diseases.
Ocular toxicity and tolerance
Exploratory and regulatory (according to the Good Laboratory Practices) studies of ocular toxicity and tolerance have been performed to assess the safety of CE-CsA ().
Table 4. Overview of the ocular toxicity and tolerance studies realized on the CE-CsA.
The first study focused on the assessment of the safety of CE without an active molecule. For that, repeated instillations (15 times at 5-min interval) of 0.002% CKC versus 0.02% BAK in solution (CKC-sol, BAK-sol) or as a CE were tested on rabbits.Citation75 Results showed that BAK-sol induced the highest corneal epithelial damage, inflammatory infiltration as well as clinical signs of eye inflammation (hyperemia, chemosis and purulent secretions) (). In contrast, CKC-CE exhibited the lowest toxicity, similar to the control group (PBS). Both BAK-CE and CKC-sol were found to induce moderate toxicity.Citation75 Contrary to the 0.02% BAK usually used in many marketed eye drops as preservative, a concentration of CKC of 0.002% in CKC-CE is well-tolerated on the eye without inducing any side effects. The second study assessed the toxicity of three formulations of CsA including CE-CsA, AE-CsA and CsA-oil using a Draize test (eye irritation test).Citation56 CE-CsA showed a lower Draize test score, indicating less ocular irritation, 4 hours after instillation compared to both CE-CsA and CsA-oil (). Following these two successful exploratory studies, 28-day (GLP) local tolerance studies () with 0.002% and 0.005% CKC in CKC-CEs were performed before pivotal phase III studies were initiated, as recommended for new drug approval. Results of these GLP regulatory studies showed that CE-CsA containing 0.005% CKC was safe and well-tolerated following 4 to 6 daily installations over 28 days. Additionally, no delayed contact hypersensitivity was found using a local lymph node assay on mice (data on file). Finally, CE-CsA or CE did not show any phototoxic or photosensitizing (photoallergic) potential (data on file).
Figure 4. Main outcomes regarding ocular toxicity and tolerance of CE-CsA. (a) Microphotographs of typical clinical features of rabbit eyes after 15 instillations at 5-min interval of 0.002% CKC or 0.02% BAK containing in a solution (sol) or a CE.Citation75 (b) Draize test score calculated at different time points (75 min, 4h and 1 day) after 15 instillations of CE-CsA, AE-CsA, Oil-CsA or 0.02% BAK. * p < .02 compared to PBS and p < .004 compared to 0.02% BAK. # p < .01 compared to AE-CsA, Oil-CsA, and 0.02% BAK. ♦ p < .0001 compared to PBS. $ p = .0003 compared to PBS, CE-CsA, AE-CsA, and Oil-CsA groups.Citation56 (Adapted from reference Citation56).
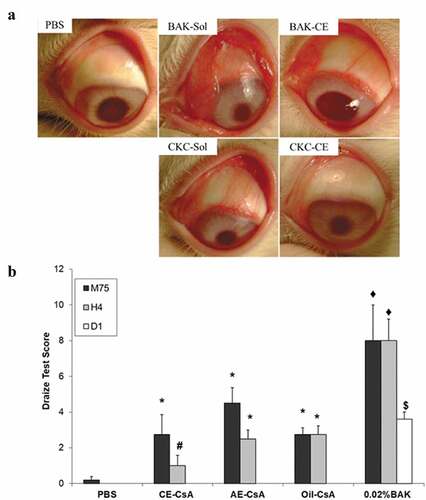
These studies indicate that the excipients (i.e., its concentration) of the vehicle and its formulation (solution vs emulsion) used to deliver CsA to the ocular surface can significantly impact the ocular tolerance of the formulation. The use of emulsion containing 0.002% or 0.005% CKC induced the lowest toxicity compared to the other CsA formulations tested, showing that CE using low CKC concentrations is well-tolerated by the eye. These findings can also be explained by the high anti-inflammatory potency of CE described in section 3.1.
Translation of preclinical outcomes to patients with DED
The previous sections described the main outcomes observed through the preclinical development of a CE-CsA. The promising results obtained during this phase allowed testing of CE-CsA on human patients via clinical trials (SANSIKA and SICCANOVE for DED and VEKTIS for VKC). Very interestingly, the clinical studies showed clinical outcomes that can be explained with the preclinical findings in term of therapeutic performance on patients with DED signs and symptoms ().
Table 5. Similarities between preclinical outcomes and results of clinical studies.
First, a significant decrease of HLA-DR, a cell surface inflammatory marker, was observed on patients with DED after treatment with CE-CsA or CE alone,Citation70,Citation71,Citation76 further confirming the anti-inflammatory potency observed during the preclinical testing.Citation55,Citation60 Interestingly, HLA-DR was shown to be well correlated with some inflammatory markers that are observed in both dry eye patients and dry eye mice model using transcriptomics and gene profiling.Citation60,Citation78
Tear breakup time (TBUT) was found to be higher for patients after treatment with both CE-CsA or CE.Citation76 This result corroborates the increased tear fluid elasticity and thickness observed after the same treatment in animal models.Citation49 In addition, the tear fluid volume was found to increase using CE-CsA and CE alone, in both preclinical and clinical studiesCitation76 with phenol red thread (PRT) and Schirmer tests respectively. Finally, a decrease in corneal epithelial damage, similar to that observed during preclinical development, has also been well observed in clinical studies via a significant improvement in CFS after treatment with CE-CsA and CE.Citation71,Citation76
Conclusion
The multiple benefits of using CsA to treat ocular surface diseases, such as DED, are widely accepted, and position CsA formulations among the armamentarium of anti-inflammatory drugs used for DED management. Many alternative CsA formulations are currently used in clinical settings, the most common being solutions, AE, CE and hospital-compounded preparations. In this review, we discussed the mechanisms of action and preclinical outcomes of a sophisticated CE-CsA. The use of CE as vehicle provides unique properties to the CsA formulation: (1) reduced secretion of pro-inflammatory factors promoted by the components composing the oil-in-water emulsion, (2) stabilization and restoration of tear fluid surface and (3) high precorneal residence time of CsA, due to the innovative and non-conventional use of CKC. These combined modes of action result in a improved reduction of the signs and symptoms of DED compared to the other CsA formulations. Very interestingly, CE alone was found to modulate the expression of many pro-inflammatory genes, and more studies should be performed in gene expression profile to better understand the underlying mechanisms of action of CE on the ocular surface. Additionally, although the use of quaternary ammonium in eye drops is contested due to well-known BAK ocular toxicity, no signs of cytotoxicity, ocular toxicity nor delay of wound healing was observed using CE-CsA containing CKC. The safety of CKC can likely be explained by its chemical properties (high hydrophobicity and polarity), allowing for a better incorporation of CKC at the surface of the oil nanodroplets and thus resulting in a very low concentration of free CKC molecules in the aqueous phase from where they can contribute to ocular toxicity.
The good ocular tolerance and efficiency observed during the preclinical and clinical testing of CE-CsA lead to the successful approval of this technology by Santen for the treatment of DED in Europe, Asia and Australia, and for the treatment of VKC in Europe and Canada. The higher performance of CE-CsA over AE-CsA, led to a decreased posology requirement (QD vs BID), while still significantly reducing the signs and symptoms of DED in patients. While two instillations of AE-CsA are required per day, only one instillation of CE-CsA is needed. This lower dose regimen may result in better patient compliance and treatment experience. With regards to hospital preparations, in addition to the higher in vivo efficacy, CE-CsA presents other major advantages including well-defined product information, a standardized manufacturing process and quality controls which include adherence to strict regulations and pharmacovigilance.
CE appears to be an optimal vehicle by which hydrophobic drugs, such as CsA, can be delivered. This delivery system is additionally able to protect the ocular surface, which is often altered or damaged during both acute and chronic diseases. Therefore, we believe that CE could represent a promising drug delivery solution for many other types of ocular surface diseases.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. JSG and PD are employees of Santen SAS.
Acknowledgments
Clotilde Jumelle/writing support, Sylviane Cadillon for bibliographic searches.
References
- Gipson IK. The ocular surface: the challenge to enable and protect vision. Invest Ophthalmol Vis Sci. 2007;48(10):4390–4398. doi:10.1167/iovs.07-0770.
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003.
- Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798.e1. doi:10.1016/j.ajo.2010.10.032.
- Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300–306. doi:10.1136/bjophthalmol-2015-307415.
- Cohen DJ, Loertscher R, Rubin MF, Tilney NL, Carpenter CB, Strom TB. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984;101(5):667–682. doi:10.7326/0003-4819-101-5-667.
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256(22):3110–3116. doi:10.1001/jama.1986.03380220076026.
- Brynskov J, Freund L, Rasmussen SN, et al. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic crohn’s disease. N Engl J Med. 1989;321(13):845–850. doi:10.1056/NEJM198909283211301.
- Faulds D, Goa KL, Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45(6):953–1040. doi:10.2165/00003495-199345060-00007.
- Hunter PA, Wilhelmus KR, Rice NS, Jones BR. Cyclosporin A applied topically to the recipient eye inhibits corneal graft rejection. Clin Exp Immunol. 1981;45:173–177.
- Akpek EK, Amescua G, Farid M, et al. Dry eye syndrome preferred practice pattern®. Ophthalmology. 2019;126(1):P286–P334. doi:10.1016/j.ophtha.2018.10.023.
- Wan KH-N, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. 2013;120(11):2197–2203. doi:10.1016/j.ophtha.2013.03.044.
- Fauquert J-L. Diagnosing and managing allergic conjunctivitis in childhood: the allergist’s perspective. Pediatr Allergy Immunol. 2019;30(4):405–414. doi:10.1111/pai.13035.
- Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314–322. doi:10.1111/pai.12197.
- PubChem. Cyclosporin A. https://pubchem.ncbi.nlm.nih.gov/compound/5284373. Accessed January 26, 2021.
- Jumelle C, Gholizadeh S, Annabi N, Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J Control Release. 2020;321:1–22. doi:10.1016/j.jconrel.2020.01.057.
- Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56(3):307–318. doi:10.1016/S0939-6411(03)00138-3.
- Lallemand F, Schmitt M, Bourges J-L, Gurny R, Benita S, Garrigue J-S. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi:10.1016/j.ejpb.2017.03.006.
- Weiss SL, Kramer WG. Ocular distribution of cyclosporine following topical administration of OTX-101 in New Zealand white rabbits. J Ocul Pharmacol Ther. 2019;35(7):395–402. doi:10.1089/jop.2018.0106.
- Jester JV, Li HF, Petroll WM, et al. Area and depth of surfactant-induced corneal injury correlates with cell death. Invest Ophthalmol Vis Sci. 1998;39(6):922–936.
- Maurer JK, Li HF, Petroll WM, Parker RD, Cavanagh HD, Jester JV. Confocal microscopic characterization of initial corneal changes of surfactant-induced eye irritation in the rabbit. Toxicol Appl Pharmacol. 1997;143(2):291–300. doi:10.1006/taap.1996.8097.
- Khanal S, Tomlinson A, Pearce EI, Simmons PA. Effect of an oil-in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26(2):175–181. doi:10.1097/ICO.0b013e31802b492d.
- McCulley JP, Arciniega JC. Effects of an oil-in-water emulsion eye drop on tear film evaporation in patients with dry eye disease. Invest Ophthalmol Vis Sci. 2011;52:3822–3822.
- Quinteros DA, Ferreira LM, Schaffazick SR, Palma SD, Allemandi DA, Cruz L. Novel polymeric nanoparticles intended for ophthalmic administration of acetazolamide. J Pharm Sci. 2016;105(10):3183–3190. doi:10.1016/j.xphs.2016.06.023.
- Imperiale JC, Acosta GB, Sosnik A. Polymer-based carriers for ophthalmic drug delivery. J Control Release. 2018;285:106–141. doi:10.1016/j.jconrel.2018.06.031.
- Jordan D. An overview of the common technical document (CTD) regulatory dossier. MEW. 2014;23:101–105.
- Gershanik T, Benita S. Positively charged self-emulsifying oil formulation for improving oral bioavailability of progesterone. Pharm Dev Technol. 1996;1(2):147–157. doi:10.3109/10837459609029889.
- Klang S, Abdulrazik M, Benita S. Influence of emulsion droplet surface charge on indomethacin ocular tissue distribution. Pharm Dev Technol. 2000;5(4):521–532. doi:10.1081/pdt-100102035.
- Borel JF, Feurer C, Gubler HU, Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468–475. doi:10.1007/BF01973261.
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2):119–125. doi:10.1016/S0162-3109(00)00192-2.
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi:10.1146/annurev.immunol.15.1.707.
- Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. 2019;163:472–480. doi:10.1016/j.bcp.2019.03.022.
- Warcoin E, Baudouin C, Gard C, Brignole-Baudouin F. In Vitro Inhibition of NFAT5-mediated induction of CCL2 in hyperosmotic conditions by cyclosporine and dexamethasone on human HeLa-modified conjunctiva-derived cells. PLoS One. 2016;11(8):e0159983. doi:10.1371/journal.pone.0159983.
- Power WJ, Mullaney P, Farrell M, Collum LM. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjögren’s syndrome. Cornea. 1993;12(6):507–511. doi:10.1097/00003226-199311000-00008.
- El-asrar AM, Tabbara KF, Geboes K, Missotten L, Desmet V. An immunohistochemical study of topical cyclosporine in vernal keratoconjunctivitis. Am J Ophthalmol. 1996;121(2):156–161. doi:10.1016/s0002-9394(14)70579-3.
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi:10.1001/archopht.120.3.330.
- Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004;58(2):357–368. doi:10.1016/j.ejpb.2004.03.033.
- Thomassen MJ, Antal JM, Divis LT, Wiedemann HP. Regulation of human alveolar macrophage inflammatory cytokines by tyloxapol: a component of the synthetic surfactant Exosurf. Clin Immunol Immunopathol. 1995;77(2):201–205. doi:10.1006/clin.1995.1144.
- Thomassen MJ, Antal JM, Barna BP, Divis LT, Meeker DP, Wiedemann HP. Surfactant downregulates synthesis of DNA and inflammatory mediators in normal human lung fibroblasts. Am J Physiol. 1996;270(1 Pt 1):L159–163. doi:10.1152/ajplung.1996.270.1.L159.
- Soliman KA, Ullah K, Shah A, Jones DS, Singh TRR. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov Today. 2019;24(8):1575–1586. doi:10.1016/j.drudis.2019.05.036.
- Corrales RM, Luo L, Chang EY, Pflugfelder SC. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea. 2008;27(5):574–579. doi:10.1097/ICO.0b013e318165b19e.
- Sindt CW, Foulks GN. Efficacy of an artificial tear emulsion in patients with dry eye associated with meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1713–1722. doi:10.2147/OPTH.S35833.
- Royle L, Matthews E, Corfield A, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25(8):763–773. doi:10.1007/s10719-008-9136-6.
- Benita S. Prevention of topical and ocular oxidative stress by positively charged submicron emulsion. Biomed Pharmacother. 1999;53(4):193–206. doi:10.1016/S0753-3322(99)80088-2.
- Yang SC, Benita S. Enhanced absorption and drug targeting by positively charged submicron emulsions. Drug Dev Res. 2000;50(3–4):476–486. doi:10.1002/1098-2299(200007/08)50:3/4<476::AID-DDR31>3.0.CO;2-6.
- Kuppens EV, de Jong CA, Stolwijk TR, de Keizer RJ, Van Best JA. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br J Ophthalmol. 1995;79(4):339–342. doi:10.1136/bjo.79.4.339.
- Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980;19:308–313.
- Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001.
- Daull P, Lallemand F, Garrigue J-S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. J Pharm Pharmacol. 2014;66(4):531–541. doi:10.1111/jphp.12075.
- Georgiev GA, Yokoi N, Nencheva Y, Peev N, Daull P. Surface chemistry interactions of cationorm with films by human meibum and tear film compounds. Int J Mol Sci. 2017;18(7). doi:10.3390/ijms18071558.
- Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27. doi:10.1016/j.exer.2013.05.010.
- Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles1. J Lipid Res. 2014;55(2):289–298. doi:10.1194/jlr.M044826.
- Brown SHJ, Kunnen CME, Duchoslav E, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013;54(12):7417–7424. doi:10.1167/iovs.13-12916.
- Garrigue J-S, Amrane M, Faure M-O, Holopainen JM, Tong L. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharmacol Ther. 2017;33(9):647–661. doi:10.1089/jop.2017.0052.
- Hwang S-B, Park JH, Kang -S-S, et al. Protective effects of cyclosporine A emulsion versus cyclosporine A cationic emulsion against desiccation stress in human corneal epithelial cells. Cornea. 2020;39(4):508–513. doi:10.1097/ICO.0000000000002244.
- Daull P, Guenin S. Hamon de Almeida V, Garrigue J-S. Anti-inflammatory activity of CKC-containing cationic emulsion eye drop vehicles. Mol Vis. 2018;24:459–470.
- Liang H, Baudouin C, Daull P, Garrigue J-S, Brignole-Baudouin F. Ocular safety of cationic emulsion of cyclosporine in an in vitro corneal wound-healing model and an acute in vivo rabbit model. Mol Vis. 2012;18:2195–2204.
- Liang H, Baudouin C, Daull P, Riancho L, Garrigue J-S, Brignole-Baudouin F. Ocular safety evaluation of newly developed cyclosporine in cationic emulsion in an in vitro corneal wound healing model and in an acute in vivo rabbit model. Invest Ophthalmol Vis Sci. 2011;52:2026–2026.
- Burade V, Zalawadia R, Patel A, Ogundele A. Preclinical efficacy comparison of cyclosporine ophthalmic solution 0.09% vs cyclosporine ophthalmic emulsion 0.05% vs ciclosporin ophthalmic emulsion 0.1% in a NOD mouse model of dry eye disease. Clin Ophthalmol. 2020;14:2747–2755. doi:10.2147/OPTH.S259331.
- Daull P, Nagano T, Okada S, Gros E, Feraille L, Garrigue J-S. Efficacy of preservative-free cyclosporine emulsion eye drops in a mouse model of dry eye. Invest Ophthalmol Vis Sci. 2019;60:292–292.
- Daull P, Barabino S, Feraille L, et al. Modulation of inflammation-related genes in the cornea of a mouse model of dry eye upon treatment with cyclosporine eye drops. Curr Eye Res. 2019;44(5):476–485. doi:10.1080/02713683.2018.1563197.
- Garrigue J-S, Nicolas C, Kessal K, et al. Comparative efficacy of preservative-free anti-inflammatory eye drops in a mouse model of dry eye. Invest Ophthalmol Vis Sci. 2017;58(8):800–800.
- Daull P, Feraille L, Barabino S, et al. Efficacy of a new topical cationic emulsion of cyclosporine A on dry eye clinical signs in an experimental mouse model of dry eye. Exp Eye Res. 2016;153:159–164. doi:10.1016/j.exer.2016.10.016.
- Daull P, Feraille L, Barabino S, Garrigue J-S. Efficacy evaluation of a cationic emulsion of cyclosporine in a mouse model of dry eye. Invest Ophthalmol Vis Sci. 2015;56:4468–4468.
- Garrigue J-S, Daull P, Feraille L, Barabino S. Comparative efficacy of cyclosporine eye drop formulations in a mouse model of dry eye. Invest Ophthalmol Vis Sci. 2016;57:421–421.
- Daull P, Feraille L, Amrane M, Elena PP, Garrigue J-S. Cationic Emulsion of Cyclosporine for the Management of Ocular Surface Inflammation: A Preclinical Evaluation. 2015.
- Filion MC, Phillips NC. Anti-inflammatory activity of cationic lipids. Br J Pharmacol. 1997;122(3):551–557. doi:10.1038/sj.bjp.0701396.
- Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46(8):2766–2771. doi:10.1167/iovs.04-1326.
- Barba KR, Samy A, Lai C, Perlman JI, Bouchard CS. Effect of topical anti-inflammatory drugs on corneal and limbal wound healing. J Cataract Refract Surg. 2000;26(6):893–897. doi:10.1016/s0886-3350(00)00364-3.
- Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y. Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol. 1982;66(11):705–708. doi:10.1136/bjo.66.11.705.
- Robert P-Y, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol. 2016;26(6):546–555. doi:10.5301/ejo.5000830.
- Baudouin C, Figueiredo FC, Messmer EM, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol. 2017;27(5):520–530. doi:10.5301/EJO.5000952.
- Agarwal P, Scherer D, Günther B, Rupenthal ID. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int J Pharm. 2018;538(1–2):119–129. doi:10.1016/j.ijpharm.2018.01.019.
- Grimaudo MA, Pescina S, Padula C, et al. Poloxamer 407/TPGS mixed micelles as promising carriers for cyclosporine ocular delivery. Mol Pharm. 2018;15(2):571–584. doi:10.1021/acs.molpharmaceut.7b00939.
- Daull P, Lallemand F, Philips B, Lambert G, Buggage R, Garrigue J-S. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits. Cornea. 2013;32(3):345–354. doi:10.1097/ICO.0b013e31825e83f4.
- Liang H, Brignole-Baudouin F, Rabinovich-Guilatt L, et al. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: an in vivo study in rabbits. Mol Vis. 2008;14:204–216.
- Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016;26(4):287–296. doi:10.5301/ejo.5000779.
- Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671–681. doi:10.1016/j.ophtha.2018.12.027.
- Kessal K, Liang H, Rabut G, et al. Conjunctival inflammatory gene expression profiling in dry eye disease: correlations with HLA-DRA and HLA-DRB1. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02271.