Brolucizumab (Novartis, Basel, Switzerland) met the primary endpoint of functional non-inferiority compared to Aflibercept (Bayer, Leverkusen, Germany) and demonstrated superiority regarding anatomic end points indicating a faster and more durable treatment effect in exudative AMD in the two pivotal clinical HARRIER and HAWK trials.Citation1 This resulted in its approval for this indication in late 2019 in the USA and in early 2020 in Europe. What followed the hope and enthusiasm about this new treatment option, were the first reports of intraocular inflammation (IOI), retinal vasculitis (RV) and retinal vascular occlusion (RO),Citation2 resulting in a post-hoc analysis of all HARRIER and HAWK IOI reports by an independent Safety Review Committee (SRC). An incidence of 4.6% for probable or definite drug-related IOI was reported, resulting in at least 0.74% of severe vision loss (≥15 ETDRS letters), the vast majority developing within the first six months.Citation3 While a vision loss of 15 or more letters has been reported in up to 1% in AMD under all anti-VEGF agents, its combination with RO seems specific to Brolucizumab.
Different pathogenic hypotheses exist, explaining why Brolucizumab causes a higher rate of intraocular inflammation and retinal vascular occlusion.Citation4 All of these advocate the generous use of systemic, i.e. intravenous, corticosteroids in this disease. The small molecular size and weight enable a higher molar concentration and increased tissue penetration. More patients develop treatment emergent anti-drug-antibodies to Brolucizumab than to other anti-VEGF agents resulting from a specific humoral and cellular immune response, leading to the formation of immune complexes at the vessel wall and consequently vascular occlusionCitation5,Citation6 strongly arguing against further use of Brolucizumab after IOI.
Since recognition of this problem, substantial efforts have been undertaken, primarily driven by Novartis, but also by independent experts to understand this phenomenonCitation4,Citation7–9 and to provide expert guidelines for its treatment.Citation2,Citation10,Citation11 Real-world evidence gathered from two large us registries (IRIS and Komodo), together covering 21394 injections, indicates that a history of intraocular inflammation or vascular occlusion in the affected eye results in a tenfold increase in the risk of IOI after Brolucizumab from 0.32–0.41% to 3.97–4.68%.Citation12 Multiple independent real world case series have meanwhile demonstrated that close monitoring, early diagnosis, aggressive and timely anti-inflammatory treatment as well as discontinuation of Brolucizumab can help to prevent progression to RV/RO and severe vision loss.Citation2,Citation10,Citation11,Citation13 Once endophthalmitis and other infectious sources of IOI have been excluded, corticosteroid treatment is to be given according to the severity of disease in a standard of care attitude.Citation9,Citation11 Fundamental for this guidance is the correct interpretation of clinical findings. These, however, are prone to misinterpretation, as outlined below:
The presentation of IOI upon treatment with Brolucizumab is modified by its strong anti-VEGF effect onto the vascular barrier resulting in less leakage than that reported for IOI/RV/VO of other origin. A seemingly less severe presentation of IOI explains an inherent risk of the underestimation of disease severity and consequently the under-treatment of IOI.
Half of the patients present a more or less mild and widely anterior uveitis which may well be controlled by topical corticosteroids. Its linkage to Brolucizumab as drug-induced inflammation may easily escape awareness. Even in mild uveitis, however, re-treatment with Brolucizumab is not recommendable.Citation2,Citation4
In instances with a mild primary presentation, timely follow-up is a prerequisite to identifying disease progression towards the posterior segment, whereas pre-and intra-retinal cells visible in OCT already indicate a more severe affection of the eye ().
As outlined above, the clinical presentation tends to underrepresent the severity of disease (): Vascular occlusion may be missed in biomicroscopy, whereas its presence has a strong impact on the aggressiveness of the treatment strategy.Citation2,Citation3,Citation9,Citation10 Therefore, wide-field fluorescein angiography is justified in any IOI possibly involving the posterior segment in order to determine the severity of vascular affection ( and 2C).Citation10
The impact of VEGF onto the uveovascular barrier in retinal vasculitis is considerable.Citation14 Based on the neutralization of this effect by Brolucizumab, fluorescein angiography at diagnosis may reveal delayed and irregular arterial filling and vascular non-perfusion, whereas classical features of retinal vasculitis, including dye leakage and vascular sheathing, may be discrete or absent ().
Since IOI/RV/RO in response to Brolucizumab represent an inflammatory disease,Citation2,Citation3 vascular occlusions without vasculitis may represent a new aspect in the spectrum of vasculitis escaping detection.
Retinal vasculitis generally results from the local precipitation of a systemic immune response.Citation15–17 Brolucizumab-associated posterior segment inflammation has been linked to the systemic presence of neutralizing anti-drug antibodies. This justifies a systemic anti-inflammatory treatment and definitive discontinuation of Brolucizumab treatment.
Figure 1. Optical coherence tomography of a patient with IOI involving the posterior segment of the eye. Typical changes strongly suggestive of IOI include hyperreflective spots in the pre-retinal vitreous, an irregular inner retinal surface (arrows) and hyperreflective spots in all retinal layers without significant structural damage to the neuroretina as long as the macula is not affected by vascular occlusion. Typically, the anatomic effect of the drug with reduction of retinal fluid is also visible compared to the pre-injection OCT.
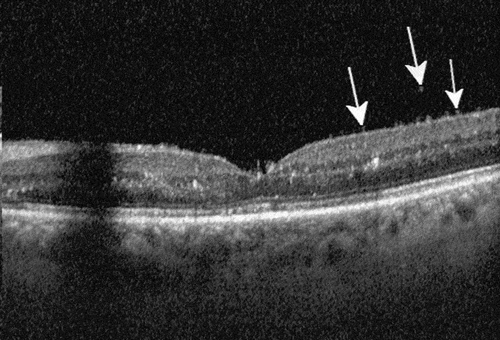
Figure 2. A. Mild anterior uveitis with discrete vision loss and mutton fat precipitates on the corneal endothelium six weeks after the second Brolucizumab injection which was well controlled under topical corticosteroids.B. Same patient, one week later. The posterior segment looks rather quiet; bleedings, vasculitic changes or vascular occlusion are not obvious despite a diffuse cellular vitreal infiltration. The dark arrow points to a small retinal vessel without obvious incoherence. C. Same patient, same visit as B.
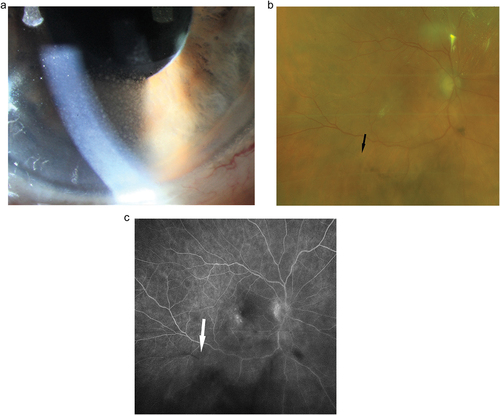
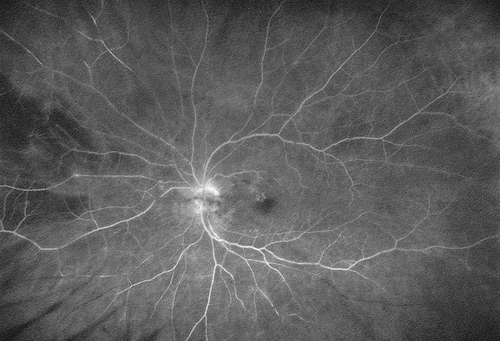
Figure 3. Eighty-four years old male presenting with foggy vision four weeks after his third Brolucizumab injection. Fluorescein angiography reveals a clinically not visible mild vasculitis and multiple vascular occlusions in the nasal and temporal inferior vessel arcades. In the absence of macular involvement, visual acuity fully recovered under intravenous corticosteroids.
The aforementioned points indicate that the disease severity is underestimated, leading to under-treatment and the risk of preventable vision loss. The risk-benefit estimation for a short-term intravenous corticosteroid treatment is clearly in favour of its use, as reported in optic neuritisCitation18,Citation19 and retinal vasculitis treatment trials.Citation20 In our and others experience,Citation21 an intravenous dose of between 0.5 and 1 mg methylprednisolone per kg of body weight given daily over up to three days depending on the clinical response may be sufficient in the majority of instances to prevent the progression of RV and RO before switching to oral prednisolone.Citation22
In conclusion, the severity of intraocular inflammation and presence of occlusive retinal vasculitis under Brolucizumab treatment may readily be underestimated owed to reduced vascular permeability resulting from anti-VEGF treatment. This advocates to base treatment decisions not only on clinically visible vasculitis severity whereas the use of wide field angiography may aid to visualize the degree of vascular damage and trigger treatment intensity.
Declaration of interest
CH none. JGG acts as an advisor to several pharmaceutical companies (AbbVie, Bayer, Novartis, Roche) and contributes to several clinical studies. None of the authors received any support for this study or have conflicting interests with the data that are presented in this report.
References
- Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021 Jan;128(1):89–99. Epub 2020 Jun 20. PMID: 32574761. doi:10.1016/j.ophtha.2020.06.028.
- Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020 Oct;127(10):1345–1359. Epub 2020 Apr 25. PMID: 32344075. doi:10.1016/j.ophtha.2020.04.017.
- Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2020 Nov 15;S0161-6420(20):31075–31077. Epub ahead of print. PMID: 33207259. doi:10.1016/j.ophtha.2020.11.011.
- Sharma A, Kumar N, Parachuri N, et al. Understanding retinal vasculitis associated with brolucizumab: complex pathophysiology or Occam’s razor? Ocul Immunol Inflamm. 2021 May 20;1-3. ( Epub ahead of print. PMID: 34014141). doi:10.1080/09273948.2021.1897628.
- Haug SJ, Hien DL, Uludag G, et al., Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. Mar 31, 2020;18:100680. PMID: 32258827; PMCID: PMC7125319. doi:10.1016/j.ajoc.2020.100680.
- Iyer PG, Peden MC, Suñer IJ, Patel N, Dubovy SR, Albini TA. Brolucizumab-related retinal vasculitis with exacerbation following ranibizumab retreatment: a clinicopathologic case study. Am J Ophthalmol Case Rep. Nov 10, 2020;20:100989. PMID: 33294727; PMCID: PMC7695942. doi:10.1016/j.ajoc.2020.100989.
- Anderson WJ, Da Cruz NFS, Lima LH, Emerson GG, Rodrigues EB, Melo GB. Mechanisms of sterile inflammation after intravitreal injection of anti-angiogenic drugs: a narrative review. Int J Retina Vitreous. 2021 May 7;7(1):37. PMID: 33962696; PMCID: PMC8103589. doi:10.1186/s40942-021-00307-7.
- Cox JT, Eliott D, Sobrin L. Inflammatory complications of intravitreal anti-VEGF injections. J Clin Med. 2021 Mar 2;10(5):981. PMID: 33801185; PMCID: PMC7957879. doi:10.3390/jcm10050981.
- Iyer PG, Albini TA. Drug-related adverse effects of anti-vascular endothelial growth factor agents. Curr Opin Ophthalmol. 2021 May 1;32(3):191–197. PMID: 33770015. doi:10.1097/ICU.0000000000000757.
- Holz FG, Heinz C, Wolf A, Hoerauf H, Pleyer U. Intraokulare Entzündungen bei Brolucizumab-Anwendung: patientenmanagement – diagnose – therapie. Ophthalmologe. 2021 Mar;118(3):248–256. Epub 2021 Feb 8. PMID: 33555415; PMCID: PMC7935813. doi:10.1007/s00347-021-01321-8.
- Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2020 Sep 29; S2468-6530(20):30400–0. Epub ahead of print. PMID: 33007521. doi:10.1016/j.oret.2020.09.020.
- Ip M, Albini T, Zarbin M, et al. The brolucizumab experience thus far: a health economics and outcomes research analysis. AAO Annual Meeting, 13-15 November 2020 Virtual meeting.
- Singer M, Albini TA, Seres A, et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. 2021 May 7; S2468-6530(21):00162–7. Epub ahead of print. PMID: 33971353. doi:10.1016/j.oret.2021.05.003.
- Eskandarpour M, Nunn MA, Weston-Davies W, Calder VL. Immune-mediated retinal vasculitis in posterior uveitis and experimental models: the leukotriene (LT)B4-VEGF axis. Cells. 2021 Feb 15;10(2):396. PMID: 33671954; PMCID: PMC7919050. doi:10.3390/cells10020396.
- Deuter CM, Kötter I, Wallace GR, Murray PI, Stübiger N, Zierhut M. Behçet’s disease: ocular effects and treatment. Prog Retin Eye Res. 2008 Jan;27(1):111–136. Epub 2007 Nov 26. PMID: 18035584. doi:10.1016/j.preteyeres.2007.09.002.
- Au A, O’Day J. Review of severe vaso-occlusive retinopathy in systemic lupus erythematosus and the anti-phospholipid syndrome: associations, visual outcomes, complications and treatment. Clin Exp Ophthalmol. 2004 Feb;32(1):87–100. PMID: 14746601. doi:10.1046/j.1442-9071.2004.00766.x.
- Ksiaa I, Abroug N, Kechida M, et al. Eye and Behçet’s disease. J Fr Ophtalmol. 2019 Apr;42(4):e133–e146. Epub 2019 Mar 5. PMID: 30850197. doi:10.1016/j.jfo.2019.02.002.
- De Lott LB, Burke JF, Andrews CA, et al. Association of individual-level factors with visual outcomes in optic neuritis: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020 May 1;3(5):e204339. PMID: 32379333; PMCID: PMC7206503. doi: 10.1001/jamanetworkopen.2020.4339.
- Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993 Jun;111(6):773–775. PMID: 8512477. doi:10.1001/archopht.1993.01090060061023.
- Mohammadi M, Shahram F, Shams H, Akhlaghi M, Ashofteh F, Davatchi F. High-dose intravenous steroid pulse therapy in ocular involvement of Behcet’s disease: a pilot double-blind controlled study. Int J Rheum Dis. 2017 Sep;20(9):1269–1276. Epub 2017 May 19. PMID: 28524639. doi:10.1111/1756-185X.13095.
- Kataoka K, Horiguchi E, Kawano K, et al. Three cases of brolucizumab-associated retinal vasculitis treated with systemic and local steroid therapy. Jpn J Ophthalmol. 2021 Mar;65(2):199–207. PMID: 33543352. doi:10.1007/s10384-021-00818-8.
- Talat L, Lightman S, Tomkins-Netzer O. Ischemic retinal vasculitis and its management. J Ophthalmol. 2014;2014:197675. doi:10.1155/2014/197675.
