ABSTRACT
Purpose
To compare the prostaglandin E2 receptor subtype 3 (EP3) distribution in the lacrimal glands of normals, non-specific dacryoadenitis, and chronic Stevens-Johnson syndrome (SJS) patients.
Methods
Biopsies from lacrimal glands of four chronic SJS patients with severe dry eye disease, four dacryoadenitis patients, and five fresh body donors were assessed for EP3 expression using immunohistochemistry.
Results
In normal main and accessory lacrimal glands, EP3 is expressed strongly in nuclei and cytoplasm of majority (>75%) of acini with no ductular expression. In dacryoadenitis, EP3 expression was similar to normal glands. However, lacrimal glands from SJS patients (5–20/HPF mononuclear cells) showed a weak and reduced (<10% acini) EP3 expression within acinar cells. The reduction in intensity was more in glands with higher mononuclear cell infiltration (>10/HPF).
Conclusion
There is downregulation of EP3 expression in the lacrimal glands of SJS patients, whereas EP3 expression is preserved in non-specific lacrimal gland inflammations.
Prostaglandins include PGD2, PGI2, PGE2, PGF2a mediate tissue effects act via different G protein-linked extracellular receptors. PGE2 acts via EP1 to EP4 receptors.Citation1 Prostaglandin E2 receptor 3 (EP3), initially considered an inhibitory type of prostanoid receptor, can inhibit or activate adenylate cyclase.Citation2 EP3 is expressed in kidneys, stomach (vascular smooth muscle and mucosal cells), thalamus, intestinal mucosal epithelia (at the apex of crypts), uterine myometrium, placenta, mouth gingival fibroblasts, and eyes.Citation1 In eyes, EP3 receptors have been reported in corneal endothelium, keratocytes, trabecular cells, ciliary epithelium, conjunctival and iris stroma cells, and retinal Müller cells.Citation1,Citation2 A link has been established between EP3 and ocular surface inflammatory conditions. The proposed role of EP3 in the ocular surface is to down-regulate the ocular surface inflammation. The expression of EP3 in the conjunctival epithelium of patients with chemical burns and Mooren’s ulcer is similar to the normal human conjunctival epithelium. However, there is a downregulation of the EP3 protein in the conjunctival epithelium of Stevens-Johnson syndrome (SJS)/ocular cicatricial pemphigoid (OCP) patients, thereby contributing to the chronic ocular inflammation seen in these patients.Citation3–5 While performing EP3 staining of the exenterated specimen for normal lid margin study, we observed an intense expression of EP3 in the accessory lacrimal glands. Its expression in the main and accessory lacrimal glands has never been described before. This study explored the immunohistological expression of EP3 in normal human main and accessory lacrimal glands, and changes observed in the lacrimal glands of SJS patients. Also, the differences between non-specific dacryoadenitis and glands of SJS patients were evaluated.
Methods
Study design and participants
The study adhered to the tenets of the declaration of Helsinki, and was approved by the institutional ethics committee (LV Prasad Ethics Committee, LEC 09–18-137). All patients gave informed written consent for the lacrimal gland biopsy procedure. Included study subjects were patients of chronic SJS sequelae with severe dry eye disease (Schirmer score of 0 mm of wetting after 5 minutes and without topical anaesthesia) undergoing lid-margin mucous membrane grafting (MMG). In all cases, the direct slit lamp examination of the palpebral lobe area of the lacrimal gland revealed conjunctival scarring without any actively secreting openings onto the ocular surface. Normal lacrimal glands from five body donors with no history of recent trauma or infections that could have affected the lacrimal gland constituted the control group. Accessory lacrimal glands were examined from control eyelid tissues only as ALG biopsies from SJS patients were ethically impossible.
Biopsy and fixation procedures
A total of 4 SJS patients had their orbital lobe of the lacrimal gland biopsied under general anaesthesia at the time of the MMG surgery. A small incision biopsy from the orbital lobe of the lacrimal gland was taken via eyelid crease incision of 8–10 mm length. After making skin incision, the orbital rim was reached in the pre-septal plane, and the periosteum was reflected off the rim. A small nick was made into the periorbita and lacrimal gland biopsy measuring 2–3 mm was taken using conjunctival scissors. The tissue biopsies were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Serial sections (4 µm) made with a microtome (Leica, RM2125RTS) were stained with Haematoxylin and Eosin (H&E) and immunohistochemistry (IHC). Tissue blocks of diagnosed non-specific dacryoadenitis patients (N = 4) were also processed for H&E and EP3 staining. The diagnosis of non-specific dacryoadenitis was made as per consensus laid by orbital society.Citation6
Immunohistochemistry
The expression of EP3 has never been studied before in the lacrimal glands. The brain tissue (cytoplasmic expression in neurons) served as a positive control. The tissue sections of the study, control groups, and brain were stained for immunohistochemistry using antibodies against EP3 receptor (rabbit anti-human EP3 polyclonal antibody, 1:200 (101760; Cayman Chemicals Co., Ann Arbor, MI). Deparaffinized sections were rehydrated with graded alcohol of 100%, 90%, 80% dilution followed by antigen retrieval (heating the sections with EDTA buffer (pH-9.0) for 30 minutes). Then the slides were incubated with bovine serum albumin (catalog no-A7906, Sigma, USA) for 20 minutes to avoid non-specific antibody reaction. The EP3 primary antibody (1:200) was applied for one hour at room temperature. The prediluted secondary antibodies (Dako, Denmark, Europe) were incubated at room temperature for 30 minutes. Samples were analysed under an Olympus light microscope (BX51. Images were captured using Aperio image scope software (Leica biosystems, Mumbai, India). The number of mononuclear cells were counted in 10 high power fields.
Results
Normal lacrimal glands
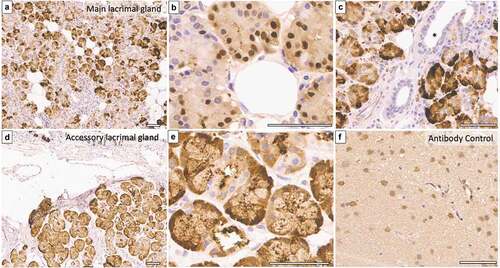
The mean age of five body donors was 57.8 years, and the mean duration between the time of death and tissue retrieval was 60 minutes. Light microscopy of all samples showed typical acinar and ductal architecture. EP3 protein was detected in the nuclei and cytoplasm of acini of normal human main and accessory lacrimal glands (). Intra- and interlobular ducts did not express EP3 in the normal glands. The expression was strong and present in 50–75% of acini observed at low and higher magnification.
Figure 1. EP3 expression in normal main and accessory lacrimal glands of a 66-year-old body donor. (a–e), Immunohistochemistry photomicrograph shows strong expression of EP3 in more than 75% of acini’s nuclei and cytoplasm of main lacrimal gland (a & b) as well as accessory lacrimal gland (d & e). (c), Interlobular ducts (marked with asterisk) are devoid of any immunostaining. (f) represents the brain tissue taken as positive control (Scale bar = 60 microns).
Stevens-Johnson syndrome
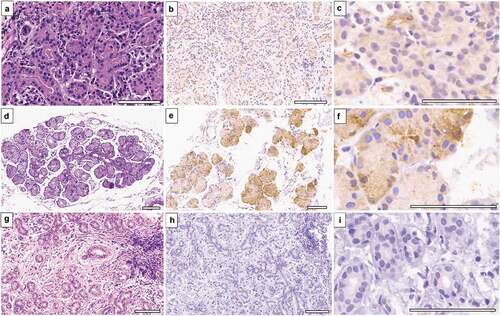
The mean age of four SJS patients was 20.7 years (6 to 36 years). The ocular surface was dry (Schirmer = 0). The acute attack was less than 6-months old in two patients, and the other two had chronic SJS of 48 months duration. All patients had chronic ocular sequelae of SJS with a mean lid complication score of 8.7 (range: 8 to 9) and a mean corneal complication score of 4.5 (range: 2 to 4). Visual acuity and ocular surface details are summarized in . Lacrimal glands from SJS patients showed a marked reduction in the expression of EP3 protein (). This reduction was present in glands with mononuclear cell infiltration (> 20/HPF). Interstitial inflammatory cells did not express EP3 protein. Two biopsies from patients with SJS duration of 48 months revealed a mild reduction in the intensity of EP3 expression but expressed in 50–75% of acini. These biopsies had less than five mononuclear cells per high power field.
Table 1. Summary of demographics, ocular surface details and histopathology findings of normal, dacryoadenitis and SJS patients.
Figure 2. EP3 expression in main lacrimal glands of Stevens-Johnson syndrome patients. (a), Photomicrograph (h&e stained) of lacrimal gland biopsy from SJS patient (disease duration = 4 months) displays acini having atrophic changes with mononuclear cell infiltration. (b & c), EP3 receptor expression is absent within acini, only endothelial cells are expressing it weakly. (d–f), A 19-year-old SJS patient’s gland biopsy (disease duration = 48 months) show normal acinar arrangement with strong expression of EP3 and minimal inflammation. (g–i), Lacrimal gland biopsy from a 36-year-old-female shows periductular fibrosis with atrophic acini and negative EP3 immunostaining. (Scale bar = 80 microns).
Non-specific dacryoadenitis
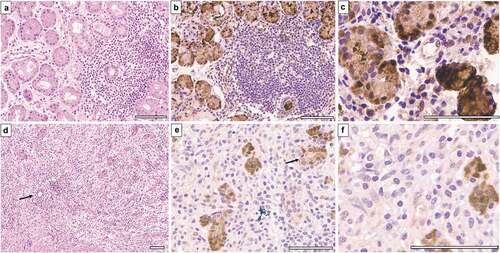
Four patients with non-specific dacryoadenitis had granulomatous inflammation with fibrosis (n = 1), and mixed lymphoplasmacytic infiltrate (n = 4) along with atrophic acini in three biopsies. The degree of mononuclear cell infiltration ranged between 10–20/HPF in all dacryoadenitis samples. The demographic and ocular examination details are available in . The visible acini had nuclear and cytoplasmic EP3 expression with no immunostaining of mononuclear cells (). In one sample where no acini could be visualized (the whole biopsy had inflammatory cell infiltration), there was a strong expression of EP3 in inflammatory cells ().
Figure 3. EP3 expression in dacryoadenitis specimens. (a) 19-year-old patient with unilateral dacryoadenitis has lymphoplasmacytic infiltrate within interstitial space surrounding normal acini. (b & c), EP3 is expressed strongly within acinar epithelium without no immunostaining in lymphocytes. (d–f), Dacryoadenitis specimen from a 17-year-old-male shows diffuse lymphoplasmacytic infiltrate with severely atrophic acini. The expression of EP3 can be observed in atrophic acini (e & f), marked with an arrow). (Scale bar = 80 microns).
Discussion
The current study provides a first-time description of EP3 receptor localization in acinar cells of main and accessory lacrimal glands. Though further studies are needed for elucidating their specific role, they could be having an anti-inflammatory effect and/or stimulating the secretion of bicarbonate and mucus from the acinar cells as observed in the duodenum or gastric antral epithelial cells. There is downregulation of EP3 expression in SJS lacrimal glands with inflammation, which is similar to the changes observed in the conjunctival epithelium of SJS patients. The EP3 expression in non-specific lacrimal gland inflammations is preserved despite mononuclear cell infiltration. The differences indicate the specific switch happening in the lacrimal glands of SJS patients as compared to dacryoadenitis.
PGE2 mediates pain and pyresis resulting from inflammation.Citation1 Its action via EP3 is protective and anti-inflammatory. The expression of EP receptors and PGE synthase has not been described for human lacrimal glands. The earlier description of pathological changes in lacrimal glands of SJS patients have demonstrated preserved acinar structure with periductular fibrosis and mild interstitial inflammatory infiltration.Citation8,Citation9 In the current study, lacrimal gland biopsies from SJS patients showed normal acinar structure in two biopsies and focal acinar atrophy with inflammatory cell infiltration in the rest two biopsies. Immunohistochemistry revealed a decreased EP3 expression in the lacrimal gland epithelial cells of biopsies with mononuclear cell infiltration. This reduction is similar to the changes observed in conjunctival epithelium EP3 expression of SJS patients. A correlation has been found between EP3 gene (PTGER3) polymorphisms and severe ocular surface complications in Japanese SJS patients.Citation3,Citation4 The downregulation of EP3 expression in the ocular surface of SJS patients was implicated for persistent inflammation observed clinically even when acute phase is over. In the current study, the decreased expression of EP3 correlated with the levels of inflammatory cells within these glands. We postulate that reduced EP3 expression in the lacrimal gland represents a continuum of ocular surface changes within lacrimal glands with an interplay between the tear-producing gland and ocular surface.
The levels of PGE2 are elevated in the tears of dry eye patients; however, expression of EP3 protein was not evaluated in their study.Citation10, Citation11 The only description regarding PGs in the lacrimal gland is their effect on the secretory activity of rabbit lacrimal glands.Citation9 Of all PGs, only PGE1 showed a dose-dependent stimulatory effect on the lacrimal gland secretion. This effect was thought to result from stimulation of postganglionic sympathetic nerves to release noradrenaline or direct action on beta-receptors located on the secretory acinar cells.Citation12
Shim et al. quantitatively compared the values of PGE2 in tears of dry eye patients and found significantly elevated levels in DED patients compared to controls.Citation13 In the evaporative DED mice model, increased expression of cyclooxygenase 2 and PGE synthase was found in lacrimal glands, meibomian glands, corneas, and conjunctivas. Within lacrimal glands, COX2 levels were elevated in acinar and ductal cells, but PGE synthase expression was elevated in periductal infiltrating cells only. PGE synthase was not detected in acinar cells of lacrimal glands from DED mice. They found lacrimal glands to be producing more PGE2 than the ocular surface and postulated neural network between the lacrimal gland and ocular surface to induce PGE2 production. Also, EP3 receptor expression was not studied in their paper. The increased PGE2 levels but reduced anti-inflammatory EP3 receptor levels in the lacrimal glands of DED patients indicate the pro-inflammatory pathway activation and the significant role prostaglandins play in ocular tissues of SJS patients.
Conclusion
This study explores the changes in EP3 expression in the lacrimal glands of SJS patients and non-specific dacryoadenitis. The PGE2 receptor changes in the lacrimal glands of SJS patients could be involved in the lacrimal gland activity other than subconjunctival fibrosis. The long-discussed issue of persistent inflammation even after the active phase is over could be due to constitutive downregulation of anti-inflammatory prostaglandins. Further studies are needed to evaluate whether prostaglandins are the mediator of ocular surface changes or a result of lymphocytes mediated hypersensitivity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Breyer RM, Clapp L, Coleman RA, et al. Prostanoid receptors (version 2020.5) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide Pharmacol CITE. 2020;2020(5). doi:10.2218/gtopdb/F767/2020.5.
- Doucette LP, Walter MA. Prostaglandins in the eye: function, expression, and roles in glaucoma. Ophthalmic Genet. 2017;38(2):108–116. doi:10.3109/13816810.2016.1164193.
- Ueta M, Sotozono C, Yokoi N, Inatomi T, Kinoshita S. Prostaglandin E receptor subtype EP3 expression in human conjunctival epithelium and its changes in various ocular surface disorders. PLoS One. 2011;6(9):e25209. doi:10.1371/journal.pone.0025209.
- Mieno H, Ueta M, Yamada K, et al. Expression of prostaglandin E2receptor 3 in the eyelid epidermis of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Ophthalmol. 2020;104(7):1022–1027. doi:10.1136/bjophthalmol-2018-313587.
- Ueta M, Matsuoka T, Yokoi N, Prostaglandin KS. E2 suppresses polyinosine-polycytidylic acid (polyI:C)-stimulated cytokine production via prostaglandin E2 receptor (EP) 2 and 3 in human conjunctival epithelial cells. Br J Ophthalmol. 2011;95(6):859–863. doi:10.1136/bjo.2010.199679.
- Mombaerts I, Bilyk JR, Rose GE, et al., Expert Panel of the Orbital Society. Consensus on diagnostic criteria of idiopathic orbital inflammation using a modified Delphi approach. JAMA Ophthalmol. 2017;135(7):769–776. doi:10.1001/jamaophthalmol.2017.1581.
- Sotozono C, Ang LP, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114(7):1294‐1302. doi:10.1016/j.ophtha.2006.10.029.
- Singh S, Mishra DK, Shanbhag S, et al. Lacrimal gland involvement in severe dry eyes after Stevens-Johnson syndrome. Ophthalmology. 2021;128(4):621–624. doi:10.1016/j.ophtha.2020.08.016.
- Singh S, Ali MJ, Mittal V, Brabletz S, Paulsen F. Immunohistological study of palpebral lobe of the lacrimal gland in severe dry eyes secondary to Stevens-Johnson syndrome. Curr Eye Res. 2020;18:1–7.
- Lekhanont K, Sathianvichitr K, Pisitpayat P, Anothaisintawee T, Soontrapa K, Udomsubpayakul U. Association between the levels of prostaglandin E2 in tears and severity of dry eye. Int J Ophthalmol. 2019;12(7):1127–1133. doi:10.18240/ijo.2019.07.12.
- Pholpramool C. Secretory effect of prostaglandins on the rabbit lacrimal gland in vivo. Prostaglandins Med. 1979;3(3):185–192. doi:10.1016/0161-4630(79)90102-2.
- Pholpramool C, Tangkrisanavinont V. Evidence for the requirement of sympathetic activity in the PGE1-induced lacrimal secretion in rabbits. Arch Int Pharmacodyn Ther. 1983;265(1):128–137.
- Shim J, Park C, Lee HS, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012;119(11):2211–2219. doi:10.1016/j.ophtha.2012.05.038.
