ABSTRACT
Background
Periorbital necrotizing fasciitis (PNF) is a rare complication of bacterial infection, associated with irreversible inflammatory destruction of soft tissues like subcutaneous tissue and superficial fascia. PNF can cause visual loss, septic shock and death within hours to days. Since the infection progresses rapidly from a local disease to septic shock, prompt identification and decisive interventions are mandatory.
Aim
Considering pathophysiology, differential diagnosis, and treatment options, we report a case of PNF and its outcome.
Methods
A 69 years old male with febrile periorbital swelling had been diagnosed with bilateral PNF, caused by dual infection with Streptococcus pyogenes (S. pyogenes) and Staphylococcus aureus (S. aureus) based on conjunctival swabs.
Results
The superantigens produced by S. pyogenes have been identified as key to the rapid dissemination of infection and severity of systemic manifestations.
Conclusion
A combination of intravenous antibiotics and regular surgical debridements resulted in a beneficial outcome in our patient.
Necrotizing fasciitis is an uncommon, severe, primarily superficial bacterial infection that rapidly spreads into the surrounding tissue, inducing extensive necrosis of the superficial fascial layers. When associated with systemic disorders, NF can be potentially lethal. If not treated quickly with antibiotics and debridement of the infected tissue, the patient may develop septic shock within hours, which can progress to multi-organ failure (MOF).1–Citation4 NF can affect any part of the body, but the highest prevalence occurs in the extremities and perineum.Citation5,Citation6 NF is uncommon in the periorbital region because of its excellent blood supply.Citation1,Citation3 The specific anatomy of the eyelids and anterior orbita including the orbital septa generally usually prevents a rapid spread of infection and superficial necrosis of the skin in this region. The dermis adheres to the nasojugal fold and laterally to the cheek fold (malar fold) on the periost. Together with the musculus orbicularis oculi, it acts as an anatomical barrier toward the spread of infection into the lower regions. Once the anatomical barriers of the dermis and muscles are compromised, however, the infection may rapidly spread to the orbital apex and the throat. Periorbital NF (PNF) may cause disfigurement,Citation7,Citation8 loss of vision,Citation2,Citation3 MOF, disseminated intravascular coagulation, and death in about 6% to 15% of patients.Citation2,Citation9–12 A summary of literature review on clinical presentation and related pathophysiology of periocular or periorbital necrotizing fasciitis are presented in .
Table 1. Clinical presentation and related pathophysiology of periocular and periorbital necrotizing fasciitis.
Based on the infectious agents involved, NF has been categorized into four types.Citation20 Type I is characterized by polymicrobial infections with mixed anaerobic and aerobic bacteria, including Streptococcus species, Klebsiella species (Klebsiella pneumoniae), S. aureus and Escherichia coli. Apart from the live pathogens, a pathogenic role has also been reported for superantigens of S. aureus and S. pyogenes.Citation8,Citation14,Citation21 In type II a monomicrobial infection is at the source, predominantly caused by group A beta-hemolytic Streptococcus species, such as S. pyogenes and either accompanied or not by S. aureus.Citation14 Methicillin-resistant S. aureus (MRSA) has been reported in the same category.Citation14 The rare type III is caused by exposure to marine Gram-negative pathogens, like Vibrio vulnificus or Aeromonas hydrophilaCitation5,Citation14,Citation20 and Clostridium species.Citation7 Type IV is linked to fungi like Apophysomyces (Mucorales) and Aspergillus species.Citation22,Citation23 So far, types I, II and IV have been documented in periorbital necrotizing fasciitis.Citation2,Citation7,Citation11,Citation24
Due to the high potential of this infection to cause irreversible destruction in a short time, rapid diagnosis and treatment, based on the knowledge of the etiological agents involved and their influence on the host’s immune defense are mandatory. Based on a case of periorbital necrotizing fasciitis with the contribution of a superantigen-producing S. pyogenes in our clinics, we provide a review of the literature including differential diagnostic considerations and a treatment approach based on the pathophysiology of the infection.
Case description
A 69-year-old male patient with rapidly increasing eyelid edema on both sides and in critical general condition presented to the hospital’s emergency department. A history of wasp venom allergy with a pronounced local reaction, urticaria and photodermatosis were known. Initially, steroids and antihistamines were administered because of a suspected diagnosis of angioedema under treatment of an angiotensin-converting enzyme inhibitor for arterial hypertension. The patient is a smoker with a history of alcohol abuse. His medical history includes a COPD GOLD II and diabetes mellitus type II treated since about 5 years with lifestyle modification and metformin, and in addition, he suffers from chronic rhinitis and rosacea.
Antibiotic therapy was initiated a few hours after admission due to septic signs that occurred, including tachycardia, fever, confusion, a C-reactive protein (CRP) level of 157 mg/l (normal <5), a leukocytosis of 24.8 x109/l (normal <10), creatinine of 58 umol/l and a blood glucose of 11.7 mmol/l. Based on progressive inflammatory signs, the diagnosis of PFN was suspected, and microbiological swabs from the eyelid region was obtained before treatment with meropenem and clarithromycin was initiated. The antibiotics were selected according to the differential diagnosis of sepsis and pneumonia, and supplemented by intravenous acyclovir because of herpesvirus infection including encephalitis in the differential diagnosis. Computer tomography excluded any cerebral affection, orbital phlegmon, sinusitis or sinus vein thrombosis.
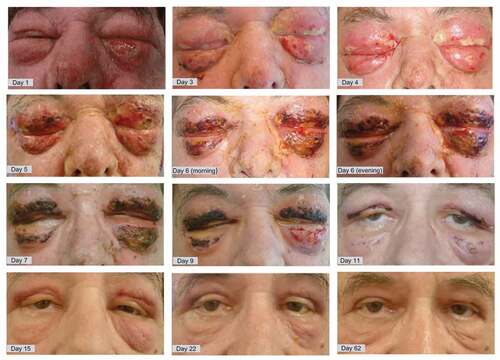
Despite these measures, the local orbital condition worsened within hours with increasing edema of both eyelids, skin blushing and the formation of blisters and purulent secretions along with necrotic spots on both upper and lower eyelids. A severe four-day continuous delirium developed, possibly aggravated in the context of blinding due to the progressive swelling with complete occlusion of the eyelids. S. aureus and a superantigen-producing S. pyogenes were grown from the conjunctival swab. After 5 days, debridement of necroses was initiated on a daily to bi-daily basis until day 10 after hospitalization. The patient was slowly recovering and was discharged after 15 days of hospitalization. As illustrated in , the eyelid area recovered without the need for primary skin grafts. In the following months, relevant scarring of the lids was unpreventable, resulting in lid malposition with brow ptosis, upper lid dermatochalasis and inferior lid ectropium that became a functional and cosmetic issue for the patient. Secondary surgical correction of the eyelids was performed in two stages: first, a direct brow lift with upper lid blepharoplasty and lateral tarsal strip was performed to restore the upper lid. Later a resection of the malar festoons and scarring tissue restored the normal appearance of the inferior lid.
Discussion
As our case indicates, the differential diagnosis of NF with its rapid periorbital spread is of considerable importance because regular surgical debridement on top of an antibiotic treatment strategy are crucial to ultimately achieve a satisfying outcome. Even after a supportive early control of infection, tissue response to the severe destructive process may require secondary interventions to correct functional anatomic changes during the healing process. From the clinical perspective, the diagnosis of PNF is a challenge, since there are several medical conditions with similar early presentations. As such, ophthalmic zoster must be excluded in the early stages. As the infection progresses, other less common differential diagnoses, including vasculitis of Wegener’s granulomatosis type, erysipelas, Quincke’s edema to preseptal cellulitis, and other inflammatory disorders affecting the lids and orbit have to be considered. Generally, acute fulminant skin infection and pyoderma gangraenosum, as well as other infectious etiologies, including orbital cellulitis, staphylogenic Lyell syndrome, endogenous endophthalmitis, cavernous sinus thrombosis, and rhino-orbital mucormycosis, have to be excluded.Citation2,Citation25,Citation26 Beyond the most important differences between NF of the periorbital region and other parts of the body is the frequently bilateral occurrence in the periorbital region.Citation2,Citation3,Citation5,Citation9 Despite rigid anatomical barriers, pathogens frequently seem to spread across the nasal bridge to the contralateral eyelids, which may best explain the frequent bilateral occurrence of PNF.Citation9
As such the proper diagnosis of PNF is generally not primarily established, as was the case with our patient. A delayed diagnosis may add to a systemic bacterial spread and septicemia, that may lead to multi-organ failure and increase mortality rates to 30%–70%.Citation27 The high mortality is, among other factors, associated with the production of bacterial toxins, including Streptococcal superantigens, which were detected in our patient’s case. Not surprisingly, a correct early diagnosis and subsequent early start of a proper antibiotic and debridement therapy can significantly reduce morbidity and fatality.Citation20
That the diagnosis was initially missed triggered us to revise and communicate the current knowledge of pathophysiology and clinical presentation of PNF. Mechanical damage to the skin as entry path is considered the most frequent cause of PNF,Citation3 though not reported in our case. After penetrating the dermis, the pathogens release their exotoxins, which fosters their rapid spread into the systemic circulation.Citation28–30 Group A beta-hemolytic Streptococcus and S. aureus are the most important causes of PNF.Citation3,Citation31,Citation32 Both pathogens express highly immunogenic surface proteins. These virulence factors, such as Group A streptococcal M1 as a neutrophil, monocyte and T cell activator, and M3, both offering protection against phagocytosis, the superantigens like exotoxin A and C, the cell pore forming streptolysin O that may lead neutrophils, macrophages and epithelial cells to apoptosis,Citation33,Citation34 substantially contribute to the immunopathogenesis of the invasive disease.Citation28 Regular microbial antigens are phagocytized by antigen-presenting cells such as B lymphocytes, upon which their peptide fragments are presented to T cells using MHC Class II.Citation35 Upon detection by T cell receptors, a specific immune response is mounted to the peptide antigen.Citation35 By contrast, superantigens, non-glycosylated low-molecular weight bacterial proteins resistant to heat, proteolysis and acid denaturation,Citation36 bind in parallel to MHC class II and T cell receptor, resulting, even at low superantigen concentrations, in a fast and exaggerated release of pro-inflammatory cytokines such as interleukin(IL)-1β, IL-2, tumor necrosis factor and interferon-γ.Citation36 Accordingly, antigen presentation is widely switched off, whereas an unspecific, but more aggressive immune response is triggered by unspecific T lymphocytes before a specific immune response by recruiting antigen-specific T cells is mounted.Citation25,Citation38 The superantigens are moreover powerful activators of the complement cascade, the bradykinin-kallikrein system, and the coagulation cascade, which leads to thrombosis of the small vessels and contributes to tissue ischemia and necrosis.Citation39 Streptococcal and staphylococcal superantigens are also capable to induce toxic shock syndromes. This non-specific manifestation in the whole body, independently of the location of the infection, is the result of a local depression of the neutrophil granulocytes and thus of the local signs and symptoms at the site of the infection. Staphylococcus-induced toxic shock syndrome is typically associated with fever, vomiting, diarrhea, flu-like symptoms such as headache, difficulty in swallowing and sore throat, as well as confusion and somnolence in younger patients.Citation40 Other factors that add to the fulminant course of NF include the initial rapid degradation of pro-inflammatory chemokines like IL-8 by bacterial proteases, which blunts the subsequent recruitment of neutrophils, necessary to mount an efficient anti-bacterial response.Citation41
These microenvironmental effects well explain the negative impact on the physiological antibacterial defense mechanisms and the insufficient penetration of antibiotics into the affected tissue,Citation39 which facilitates bacterial colonization at an early stage of infection.Citation42
Accordingly, debridement is essential in the treatment of NF, as antibiotics do not reach effective concentrations in the necrotic tissues,Citation14 while surgical debridement of the necrotic areas effectively decreases the bacterial load.Citation12
Risk factors increasing susceptibility to develop NF include diabetes mellitus, chronic renal failure, cardiovascular disease, drug abuse and alcoholism.Citation2,Citation3,Citation8,Citation43,Citation44 The patient’s general condition, risk factors and underlying comorbidities as well as pathogenic virulence factors determine the outcome of NF. Around 45% of patients with arterial hypotension and streptococcal-induced toxic shock syndrome develop acute respiratory distress syndrome.Citation45 Morbidity and mortality of the Streptococcus-induced toxic shock syndrome are higher than for the Staphylococcus-induced toxic shock syndrome and are in a range of 30–80%.Citation45,Citation46 Patients who have neutralizing antibodies against superantigens are less likely to develop NF.Citation37 If superantigens however enter the bloodstream of patients devoid of neutralizing antibodies from previous exposures, they can trigger a sudden, significant and non-specific T cell stimulation and consequently a cytokine storm,Citation37 resulting in systemic toxicity, multi-organ failure and septic shock.Citation38,Citation47 The Streptococcus-induced toxic shock syndrome can present with locally invasive Streptococcal infections such as pharyngitis or more violent diffuse disorders like arthritis, bacteremia, endocarditis, meningitis, pneumonia, sinusitis, cellulitis, myositis and necrotizing fasciitis.Citation48
Our patient ultimately experienced a favorable outcome of his bilateral PNF though this was initially misdiagnosed as angioedema. The rapid clinical evolution allowed to exclude most differential diagnoses and recognize the underlying infectious etiology, triggering a multidisciplinary approach with antibiotics and repeated debridement, followed by a plastic surgical reconstruction of the eyelids to achieve a virtually complete anatomic recovery. In a similar case reported by Leonardo et al. the patient’s primary symptoms were bilateral acute painful swelling and redness of the eyelids.Citation32 PNF due to group A Streptococcus was diagnosed,Citation32 but a determination of superantigens was not reported. Similar to our case, intravenous antibiotic treatment and surgical debridement of the necrotic tissue resulted in a successful outcome.Citation32 Another case of methicillin-resistant S. aureus (MRSA)-associated PNF was reported by Cameron et al.Citation49 primarily presenting with left eye and nostril redness and swelling after opening of a small nasal skin abscess a few days before.Citation49 Despite a timely diagnosis of facial cellulitis antibiotic therapy, the situation worsened with bilateral leg pain and redness, and shortness of breath progressing to pneumonia, sepsis and bilateral thigh cellulitis.Citation49 Finally, MRSA was recovered from blood cultures and treatment changed to meropenem, vancomycin and clindamycin.Citation49 Though an indurated swelling, but no crepitus or necrosis were presentCitation49 surgical exploration and debridement of the face and thighs were implemented along with wound monitoring on a daily basis until the wounds were clean. This case highlights the importance of imaging, rigorous exploration of the tissue and regular debridement of necrotic tissue.Citation49
Surgical removal of the infected or damaged tissue limits the spread of the infection and enables to maintain a maximal amount of tissue. This facilitates local healing and thus maintains the highest amount of neighboring healthy tissue. In such a case, the affected areas should initially be inspected and probably debrided at least every 1–2 days until no more necrotic tissue can be found.Citation20 In conjunction with surgical and antibiotic therapy, hyperbaric oxygenation and intravenous gamma globulins have been suggested in specifically severe instances.Citation50,Citation51 The latter has also been discussed with the aim of neutralizing extracellular toxins.Citation20,Citation22,Citation52 The effectiveness of both, hyperbaric oxygenation and intravenous immunoglobulins has been suggested, but also due to missing randomized studies in a rather infrequent disease, not been clearly demonstrated.Citation53,Citation54
A limitation of the current study is that microbiological analysis was only performed from a conjunctival swab, but not from the necrotic tissue. As such, histological investigations have not been accomplished. Both analyses are not considered specific standards of care. Whether they might foster a better understanding of this severe infection remains to be investigated.
In conclusion, early establishment of an aggressive antibiotic and debridement therapy, driven by the pathophysiological observation of a severe inflammatory and ischemic tissue destruction reduce morbidity and mortality in PNF and serve the basis for a functionally and anatomically satisfying outcome of this infection.
Acknowledgments
The authors are indebtedly grateful to Dr. phil. II Angelika Ströhle, MCL, Niederwangen, Switzerland, and M.P.G. van der Linden, PhD, German National Reference Center for Streptococci, Department of Medical Microbiology, University Hospital RWTH Aachen, Germany, for their support with identification of the pathogens and the efforts in identifying the superantigens of S. pyogenes contributing to the severity of this case and for critical reading and commenting on this manuscript which clearly contributed to the quality of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shindo ML, Nalbone VP, Dougherty WR. Necrotizing fasciitis of the face. Laryngoscope. 1997;107:1071–1079. doi:10.1097/00005537-199708000-00013.
- Lazzeri D, Lazzeri S, Figus M, et al. Periorbital necrotising fasciitis. Br J Ophthalmol. 2010;94:1577–1585. doi:10.1136/bjo.2009.167486.
- Amrith S, Hosdurga Pai V, Ling WW. Periorbital necrotizing fasciitis – a review. Acta Ophthalmol. 2013;91:596–603. doi:10.1111/j.1755-3768.2012.02420.x.
- Chou PY, Hsieh YH, Lin CH. Necrotizing fasciitis of the entire head and neck: literature review and case report. Biomed J. 2020;43:94–98. doi:10.1016/j.bj.2019.08.002.
- Pasternack MS, Schwartz MN. Skin and Soft Tissues Infections., Aus Madnell, Douglas and Benett’s Principles and Practice of Infectious Diseases. 8th ed. 2015.
- Tong Y-T, Mak M-S, Ho H-C, Poon T-L, Mak Y-W, Choi W-K. Necrotizing fasciitis of bilateral eyelids: a case report and review of the literature. Surg Pract. 2019;23:117–120. doi:10.1111/1744-1633.12381.
- Eiben P, Rodriguez-Villar S. A case of periorbital necrotizing fasciitis rapidly progressing to severe multiorgan failure. J Surg Case Rep. 2018;2018:rjy083. doi:10.1093/jscr/rjy083.
- Sud R, Sharma P, Garg G, Takkar B, Khanduja S. Periorbital necrotizing fasciitis due to klebsiella pneumoniae in an immunocompetent patient. Indian J Ophthalmol. 2019;67:1721–1722. doi:10.4103/ijo.IJO_360_19.
- Elner VM, Demirci H, Nerad JA, Hassan AS. Periocular necrotizing fasciitis with visual loss pathogenesis and treatment. Ophthalmology. 2006;113:2338–2345. doi:10.1016/j.ophtha.2006.06.037.
- Kronish JW, McLeish WM. Eyelid necrosis and periorbital necrotizing fasciitis. Report of a case and review of the literature. Ophthalmology. 1991;98:92–98. doi:10.1016/s0161-6420(91)32334-0.
- Deneubourg DL, Catherine Z, Lejuste P, Breton P. Periorbital necrotizing fasciitis induced by streptococcus pyogenes: a case report and clarification. J Oral Maxillofacial Surg. 2018;76:154.e151–154.e155. doi:10.1016/j.joms.2017.09.004.
- Nadal J, Galatoire O, Laouar K, et al. Periorbital necrotizing fasciitis without initial trauma: a rare case report. J Francais D’ophtalmologie. 2019;42:e209–e211. doi:10.1016/j.jfo.2018.09.022.
- Harrison WD, Kapoor B. Necrotizing soft tissue infection: principles of diagnosis and management. Orthop Trauma. 2016;30:223–231. doi:10.1016/j.mporth.2016.05.001.
- Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279–288. doi:10.1016/j.jamcollsurg.2008.10.032.
- Liu M, Lei B. Pathogenesis of hypervirulent group a streptococcus. Jpn J Med. 2018;1:269–275. doi:10.31488/jjm.1000127.
- Würtz NS, Mikkelsen LH, Jørgensen JS, et al. Periocular necrotizing soft tissue infection in greater Copenhagen. Acta Ophthalmol. 2020;98:207–212. doi:10.1111/aos.14205.
- Anand A, Sharma A, Ravins M, et al. Unfolded protein response inhibitors cure group a streptococcal necrotizing fasciitis by modulating host asparagine. Sci Transl Med. 2021;13. doi:10.1126/scitranslmed.abd7465.
- Hansen MB, Rasmussen LS, Svensson M, et al. Association between cytokine response, the lrinec score and outcome in patients with necrotising soft tissue infection: a multicentre, prospective study. Sci Rep. 2017;7:42179. doi:10.1038/srep42179.
- Palma Medina LM, Rath E, Jahagirdar S, et al. Discriminatory plasma biomarkers predict specific clinical phenotypes of necrotizing soft-tissue infections. J Clin Invest. 2021;131. doi:10.1172/jci149523.
- Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med. 2017;377:2253–2265. doi:10.1056/NEJMra1600673.
- Ting CF, Lam J, Anastas C. Subgaleal haematoma as a cause of periorbital necrotising fasciitis: a case report. Orbit. 2020;39:143–146. doi:10.1080/01676830.2019.1606834.
- Herr M, Grabein B, Palm HG, et al. Nekrotisierende fasziitis. Unfallchirurg. 2011;114:197–216. doi:10.1007/s00113-010-1893-6.
- Chander J, Stchigel AM, Alastruey-Izquierdo A, et al. Fungal necrotizing fasciitis, an emerging infectious disease caused by apophysomyces (mucorales). Revista iberoamericana de micologia. 2015;32:93–98. doi:10.1016/j.riam.2014.06.005.
- Abdul Kadir N, Ahmad SS, Abdul Ghani S, Paramananda M. A case of acute periorbital necrotizing fasciitis. J Acute Dis. 2016;5:174–176. doi:10.1016/j.joad.2015.10.005.
- Sachsenweger M. Augenheilkunde. Thiemen Verlag; 2003:16–27, 60–64.
- Jensen SL, Amato JE, Hartstein ME, Breer WA. Bilateral periorbital necrotizing fasciitis. Arch Dermatol. 2004;140:664–666. doi:10.1001/archderm.140.6.664.
- Stevens DL. Streptococci and enterococci. In: Oxford Textbook of Medicine. 5th ed. 2005:671–674.
- Commons RJ, Smeesters PR, Proft T, Fraser JD, Robins-Browne R, Curtis N. Streptococcal superantigens: categorization and clinical associations. Trends Mol Med. 2014;20:48–62. doi:10.1016/j.molmed.2013.10.004.
- Proft T, Fraser JD. Streptococcal superantigens: biological properties and potential role in disease. In: Ferretti JJ, Stevens DL, Va F, eds. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016 Feb 10. https://www.ncbi.nlm.nih.gov/books/NBK333435/. [Internet].
- King P. Haemophilus influenzae and the lung (haemophilus and the lung). Clin Transl Med. 2012;1:10. doi:10.1186/2001-1326-1-10.
- McCabe Ga, Hardy T, Campbell TG. Bilateral periorbital necrotising fasciitis associated with invasive group: a Streptococcus infection. BMJ Case Rep. 2020;13:e236800. doi:10.1136/bcr-2020-236800.
- Leonardo FHL, Anabuki M, Gonçalves ACP. Bilateral periorbital necrotizing fasciitis: case report. Arq Bras Oftalmol. 2018;81:239–241. doi:10.5935/0004-2749.20180047.
- Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. Streptolysin s and necrotising infections produced by group g streptococcus. Lancet. 2002;359:124–129. doi:10.1016/s0140-6736(02)07371-3.
- Shumba P, Mairpady Shambat S, Siemens N. The role of streptococcal and staphylococcal exotoxins and proteases in human necrotizing soft tissue infections. Toxins (Basel). 2019;11:332. doi:10.3390/toxins11060332.
- Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of phagocytosed antigens by mhc class i and ii. Traffic. 2013;14:135–152. doi:10.1111/tra.12026.
- Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DYM, and Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26:422. doi:10.1128/CMR.00104-12.
- Muldrew KL, Simpson JF, Stratton CW, Tang YW. Molecular diagnosis of necrotizing fasciitis by 16s rrna gene sequencing and superantigen gene detection. J Mol Diagn. 2005;7:641–645. doi:10.1016/s1525-1578(10)60599-5.
- Fraser JD. Clarifying the mechanism of superantigen toxicity. PLoS Biol. 2011;9:e1001145. doi:10.1371/journal.pbio.1001145.
- Salcido RS. Necrotizing fasciitis: reviewing the causes and treatment strategies. Adv Skin Wound Care. 2007;20:288–293; quiz 294–285. doi:10.1097/01.ASW.0000269317.76380.3b.
- Quach GT, Frisby J, Kralovich K, Bohra M. First necrotizing fasciitis caused by haemophilus influenza serotype a. J Investig Med High Impact Case Rep. 2017;5:2324709617736791. doi:10.1177/2324709617736791.
- Hidalgo-Grass C, Dan-Goor M, Maly A, et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group a streptococcal necrotising soft-tissue infections. Lancet. 2004;363:696–703. doi:10.1016/s0140-6736(04)15643-2.
- Siemens N, Norrby-Teglund A. Shocking superantigens promote establishment of bacterial infection. Proc National Acad Sci. 2017:201713451. doi:10.1073/pnas.1713451114.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ. 2005;330:830–833. doi:10.1136/bmj.330.7495.830.
- Pal B, Evans S, Walters RF. Necrotising fasciitis of the periorbital region. Eye. 2001;15:676–677. doi:10.1038/eye.2001.216.
- Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9:281–290. doi:10.1016/s1473-3099(09)70066-0.
- Gottlieb M, Long B, Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J Emerg Med. 2018;54:807–814. doi:10.1016/j.jemermed.2017.12.048.
- Walker MJ, Barnett TC, McArthur JD, et al. Disease manifestations and pathogenic mechanisms of group a streptococcus. Clin Microbiol Rev. 2014;27:264–301. doi:10.1128/cmr.00101-13.
- Stevens D, Bryant A. Severe group a streptococcal infections. In: Ferretti JJ, Fischetti VA, eds. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016.
- Cameron CA, Juniat V, Pyragius M, Selva D. Conservative management of periorbital necrotizing fasciitis caused by methicillin-resistance staphylococcus aureus. Can J Ophthalmol. Journal canadien d’ophtalmologie. 2021;56:e86–e88. doi:10.1016/j.jcjo.2020.10.011.
- Singam NV, Rusia D, Prakash R. An eye popping case of orbital necrotizing fasciitis treated with antibiotics, surgery, and hyperbaric oxygen therapy. Am J Case Rep. 2017;18:329–333. doi:10.12659/ajcr.902535.
- Krenk L, Nielsen HU, Christensen ME. Necrotizing fasciitis in the head and neck region: an analysis of standard treatment effectiveness. Euro Arch Oto-rhino-laryngol. 2007;264:917–922. doi:10.1007/s00405-007-0275-3.
- Raithatha AH, Bryden DC. Use of intravenous immunoglobulin therapy in the treatment of septic shock, in particular severe invasive group a streptococcal disease. Indian J Crit Care Med. 2012;16:37–40. doi:10.4103/0972-5229.94433.
- Bruun T, Rath E, Oppegaard O, Skrede S. Beta-hemolytic streptococci and necrotizing soft tissue infections. Adv Exp Med Biol. 2020;1294:73–86. doi:10.1007/978-3-030-57616-5_6.
- Soh CR, Pietrobon R, Freiberger JJ, et al. Hyperbaric oxygen therapy in necrotising soft tissue infections: a study of patients in the United States nationwide inpatient sample. Intensive Care Med. 2012;38:1143–1151. doi:10.1007/s00134-012-2558-4.