While nearly 30% of the uveitis entities remain uncharacterized even today,Citation1 ophthalmologists still must differentiate infectious uveitis from the more commonly occurring and perhaps better characterized immune-mediated non-infectious uveitis.
Infectious uveitis here is defined as inflammation of the uveal tract and surrounding structures caused by direct cytotoxic effect to ocular tissues by replicating infectious micro-organisms. This has always been one of the central tenets of the uveitis subspecialty: to always rule out infectious etiologies first.
The concept is straightforward but can be extremely challenging as the microorganisms causing intraocular infections are numerous and their manifestations protean. Depending on geographic and population characteristics, certain viruses, bacteria, fungi, or parasites may predominate. However, phenotypic expressions of even the same infectious organism can vary greatly, chronicity and recurrences can occur, and diagnostic modalities often are invasive yet non-definitive.
In addition, novel causes of infectious uveitis are increasingly reported during arthropod-borne seasonal epidemics of viral fever in the tropics, most of which are caused by single-stranded RNA viruses like Dengue, Chikungunya and Zika,Citation2 and the considerations of the host immune status with some being immunocompromised from retroviral infection or immunosuppressive therapy affecting how infectious uveitis presents and the odds for successful treatment of infectious uveitis seem small.
To that end, we are pleased to have invited a panel of highly experienced uveitis specialists (AD, AG, BT, SPC, JS, NR) from Australasia, the United Kingdom, and the United States to share their experience, expertise, and thoughts on the diagnosis and management of infectious uveitides at present and in the future.
Methods
The manuscript writing team consisting of 3 ophthalmologists selected for their different levels of uveitis training and experience ideate a list of critical questions encompassing 3 major areas in infectious uveitis: diagnosis, treatment, research and latest innovations. The final 10 questions were shortlisted from the pool of initial questions developed and were sent to the 6 panel experts electronically. Follow-up individual correspondence with the panel experts was done electronically for further clarification and insights. This was carried out from January 2022 to March 2022.
Results
Q1: Can you describe your experience with infectious uveitis, the proportion of infectious uveitis you see in your practice, any memorable case you can recall, and the lessons you learned from it?
Our groupCitation3 and others have published on the epidemiology of infectious uveitis in the United States. It makes up about 18% of all uveitides we see with an annual incidence of 18.9 per 100,000 persons. The most common pathogen based on our study is histoplasmosis, followed by viral etiologies (primarily herpetic), followed by toxoplasmosis. Bacterial endophthalmitis (both endogenous and exogenous) comprised a sizeable proportion.
One memorable case presented with clinical features mimicking acute viral retinitis. The lack of response to anti-viral treatment prompted further evaluation with ocular fluid examination and retina-choroidal biopsy. That finally revealed the primary large B-cell lymphoma diagnosis instead of an intraocular infection!
Excluding Toxoplasmosis, around 10% of cases I see have a clear infectious etiology. The causative organisms usually are herpetic viruses, HTLV-1, and necrotising toxoplasmosis infections in the immunocompromised and elderly. One must be mindful this might not be represented elsewhere as we have a major oncology and HIV centre here. We also as with an acute hospital see significant numbers of endogenous endophthalmitis – for example, most recent devastating aspergillus infection from bypass surgery and intravenous lines! Rare infections in our southwest United Kingdom cohort include atypical tuberculosis, pneumocystis, and various fungi like aspergillus.
More so in immigrants, we see tuberculosis-related ocular disease, and in travelers ocular manifestations of dengue, malaria, rickettsiae, and the occasional helminthic infection like in diffuse unilateral subacute neuroretinitis.
I don’t have any single memorable case to share but over the years, the challenging cases have taught me 3 things: 1) Undertake early diagnostics both through serum and ocular investigations (for bacterial, viral and fungal) and consider polymerase chain reaction analysis. 2) Always consider combined infections. We have had both active cytomegalovirus and herpes simplex virus together and also herpetic virus and toxoplasmosis together –where both treatment regimens were implemented to get full control of the disease. 3) Syphilis remains constant as a presentation!
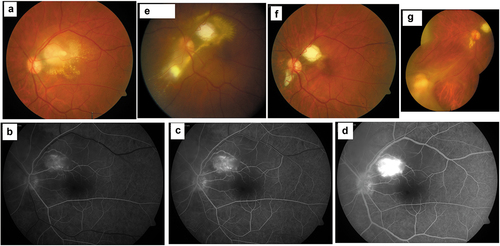
Based on the published data available from our centre in North India over the last two decades, nearly a threefold increase in the number of infectious uveitis cases was seen by us from 10.08% to 28.16% contributed majorly by viral uveitis (HSV, VZV and CMV) from 1.12% to 6.47% and TBU 10.08% to 19.25%. During the same timeline, the prevalence of toxoplasmosis has shown a significant decline from 1.69% to 0.66%, perhaps the lowest globally. More recently, we saw over three years patients with Toxocara (10 cases), Syphilis (8 cases), and endogenous endophthalmitis (14 cases) that were only rarely seen in the previous years.Citation4 For the uninitiated diagnosis of toxocara retinitis can be a major challenge ().
Figure 1. Case of a migrating Toxocara granuloma.
Like other uveitis specialists who work in the tertiary referral setting, my practice is dominated by patients with non-infectious uveitis requiring treatment with systemic immunosuppression. In the community practice setting, however, infectious uveitis is relatively more common. In Australia, common types of infectious uveitis are toxoplasmic retinochoroiditis, herpetic anterior uveitis and retinopathy, syphilitic uveitis and tubercular uveitis. Endogenous bacterial and fungal endophthalmitis occurs in specific settings. Without drawing out a specific patient, over the years, I have encountered several atypical presentations of toxoplasmic retinochoroiditis. As a result, I often offer the advice that if a patient presents with a unilateral posterior uveitis, based on the statistics alone, ocular toxoplasmosis is the most likely diagnosis and should always be considered.
We found that about 30% of uveitis cases seen at the Singapore National Eye Centre are infectious in origin.Citation5 Cytomegalovirus anterior uveitis was the most common (27%) infectious uveitis. I recall an interesting middle-aged patient with fever and malaise, who presented with bilateral non-necrotizing anterior scleritis, anterior uveitis and vitritis.Citation6 She had small diffuse dendritiform keratic precipitates in both eyes suspicious of an infectious etiology. Aqueous sample grew Propionibacterium Acnes after 2 weeks, and she responded to intravenous antibiotics. I learned the importance of paying close attention to the type and distribution of keratic precipitates, which help to identify the infectious case. We were unable to identify the source of infection but were able to cure the patient.
Q2: Many people are exposed to infectious microbes yet only some develop overt systemic disease and even fewer result in aninfectious uveitis which itself may have variable outcomes. What do you think is responsible for such variability?
The host-pathogen response plays a significant role in determining whether exposure to an infectious agent will progress to active infection and furthermore whether recurrent or chronic uveitis may subsequently result.
Failure to clear an infection may lead to dormancy or latent infection, such as with herpes viruses, toxoplasmosis, and tuberculosis. Reactivation may occur with perturbation of host factors that control infection, such as in immunodeficiency (HIV, older age, diabetes, host cell stress) and immunosuppression (corticosteroid use, chemotherapy, immunomodulatory agents [particularly anti-TNF and other biologics]). Adding further complexity is that latent infection may cause a maladaptive, chronic, low-grade, clinically unrecognized inflammation resulting in autoimmunity (i.e. molecular mimicry in TB-associated uveitis).
Advances in and wider availability of personalized medicine approaches may help elucidate biomarkers of varying degrees of relative immunocompromise that confer risk of developing infectious uveitis, including cytokine profiles, immunoreactive cellular function, and genetic/epigenetic factors.
As we know the eye is very good at protecting itself through its various ocular barriers, highly competent immune systems, and ability to prevent overt inflammation – the numerous pathways of which I will not go into detail.
We regulate common infections well – herpetic viruses being a prime example. The retina can encyst toxoplasmosis tachyzoites spectacularly well too. These barriers are very effective unless compromised through high replication and inflammation or systemic drive to breaking down barriers (infection, ageing, immunocompromise, other diagnoses of immune-mediated inflammatory diseases and so on).
Let me approach this question from an epidemiological viewpoint with lessons from tuberculosis related uveitis. Dr Alan C Woods, the father of the uveitis subspecialty reported a declining prevalence from 1941 to 1960 of tubercular uveitis in his clinic in the Wilmer eye institute, Baltimore from 80% to 20%.Citation7 This decline paralleled the discovery of sarcoidosis, brucellosis, toxoplasma and histoplasmosis as aetiologies of uveitis indicating that prevalence data in the clinics is highly biased by the current endemicity of the infectious diseases in the population, the discovery of new infectious agents and characterization of the clinical phenotype. One such case of necrotizing retinitis attributed to TB even on histopathologyCitation8 was recognized 20 years later as the classical presentation of toxoplasmosis retinitis.Citation9
There is hardly any reliable population-based data to show whether incidence or prevalence of infectious uveitis is changing over time. If there is a change it may reflect instead better documentation, greater recognition or more standardized laboratory, ancillary testing facilities and the diagnostic criteria. In short, it may have always been this way, just that we as ophthalmologists have yet to fully elucidate the disease fully.
Using polymerase chain reaction from the ocular fluids of suspected TBU in the last 25 years we characterized a variety of phenotypes of tuberculous uveitis including the most commonly seen serpiginous-like choroiditis or multifocal serpiginoid choroiditis, retinal periphlebitis, choroidal granuloma, panuveitis, anterior uveitis and even the intermediate uveitis.Citation10–12 Compared to in-house multiplex PCR tests, we found the commercially available tests the GeneXpert and the Line probe assay have poor sensitivity to play any useful role in routine clinical practice. The diagnosis of TBU is often made by recognizing the clinical phenotype in vulnerable populations (High burden TB countries) who show immunological or radiological evidence of tuberculosis.
Recurrences in uveitis due to herpes viruses and toxoplasmosis are not uncommon. As yet it is not possible to either predict or stop recurrences as these organisms remain latent throughout life and cannot be eradicated with the current therapeutic agents. On the other hand, while MTB also remains latent in a variety of tissues and specifically in the eye speculated to be sequestrated in the RPE cells, use of standard 4-drug anti-tuberculous treatment (ATT) in TBU in our experience was highly effective and only about 15% of the eyes showed recurrences of the inflammation over long follow-up. Using molecular techniques like multiplex polymerase chain reaction with gene sequencing, line probe assay and GeneXpert we have shown that nearly 5% of the eyes with TBU may harbor, at least, the DNA of resistant strains of MTB if not the organisms per se.
Many factors govern whether or not exposure to an infectious pathogen will result in clinical disease: host health and disease, some of which is determined by genetics and some of which is determined by environmental factors, are important, but so too are pathogen-related factors. The latter has received relatively little attention to date but is likely to be quite important. Different strains of a pathogen may have different virulence and add to this the fact that pathogens have different geographical ranges and exist in variable environments. Take Toxoplasma gondii as an example. There are many considerations, but a few pathogen-driven factors include predominance of parasites with atypical genetics in regions outside the US and Western Europe; lack of covered reservoirs and/or treatment of drinking water in some parts of the world; large feral cat populations in certain countries, including Australia; and tradition for eating undercooked or raw meat in some cultures.
I believe that the phenotypic expression is dependent on the balance between the microbial load and the host defense system. Thus, we see a spectrum of clinical manifestations with a single infectious agent.
Q3: Many infectious uveitides are presumed diagnoses especially if they present in less classical ways i.e. ocular tuberculosis, toxoplasmosis. Even if there are tests, they are invasive like paracentesis and might not be sensitive enough. What do you think is the next frontier for better diagnostics?
Koch’s postulate of a pathogen detected within diseased tissue remains the gold standard to confirm the diagnosis of infection. Therefore, it is appropriate that novel techniques of pathogen detection have been at the forefront in the diagnosis of infectious uveitis. To date, successful methods have focused on obtaining samples of intraocular fluid and tissue to detect pathogenic organisms by special stains, cultures, and molecular techniques. Metagenomic next generation sequencing perhaps represents the most promising iteration of these techniques for pathogen detection.Citation13
Another approach to satisfy Koch’s postulate would be to detect a unique host response to a given pathogen such as cytokine profiles and “omic” markers. Specific circulating microRNAs (miRNAs), which can be detected in blood, without invasive intraocular biopsy, have been identified for tuberculosis, malaria, herpesviruses, and other infectious diseases.Citation14 Furthermore, as an advantage over PCR, miRNA expression profiles appear promising in differentiating latent infection from active disease.Citation15
These data may also provide valuable targets of specific derangements within the inflammatory pathway, guiding treatment toward specific biologic agents. In this sense, these investigations may also benefit non-infectious uveitis management.
The issue in the short term is not so much the worry of the invasive nature of diagnostic taps. Rather, it is the ability to make diagnosis on small samples that needs to be improved. For sure we also need to continually improve on our ability to perform safely retinal and/or choroidal fine needle biopsies for minute cell numbers to determine molecular diagnosis of infection and full genomic analysis and so on (as currently for COVID).
Possible avenues to explore and research include diagnosis through blood/ peripheral molecular analysis such as miRNA as biomarkers for diagnosis. And though we assume the infection is localized to the eye, it still may be detected systemically through immune cell traffic.
Finally, combining all these – molecular, miRNA, ocular imaging and clinical signs into a machine learning domain to facilitate complex diagnoses such as necrotising retinitis and fungal disease with a point of care device with rapid readouts.
The holy grail will be to obtain definitive diagnosis of infectious uveitis and its causative organism(s) in an efficient, affordable, and safe manner. For example, necrotizing infections of the retina especially in immunosuppressed individuals are difficult to differentiate clinically whether they are caused by herpes viruses, toxoplasmosis, or syphilis. With prespecified pathogen-directed polymerase chain reaction (PCR) from the ocular fluids, (obtained from micro-incisional pars plana vitreous surgery using either 23/25 or 27 gauge) these can be diagnosed with more than 95% sensitivity and nearly 100% specificity.Citation16–19
The future is likely to be a further advancement of such molecular techniques in the diagnostics of infectious uveitis. Next-generation sequencing (NGS) for metagenomics has begun to find its way into clinics to diagnose infections where it can help detect hitherto known or even unknown microbes with significantly higher rates than conventional cultures.Citation20 In addition, the long-suspected rubella infection in patients of Fuchs’ uveitis has also been confirmed by the MDS technique.Citation21
The major challenge however remains to get a sample not contaminated by bystander environmental organisms, but MDS has been one of the most exciting developments in the field of microbial diagnostics and in the coming times, we expect to see a more extensive application of these techniques to detect offending microbes in the field of infectious uveitis as well as the yet unknown microbes that drive non-infectious uveitis.
Today, my practice still is grounded in clinical evaluation: I make most diagnoses based on the history and examination, and while I seek confirmation by investigation, if that is not possible, I generally feel confident to move ahead with treatment based on the evaluation. I use qPCR in my practice, but the microbial panel is small and not discovery-based. Cytokine analysis has proved valuable in diagnosing vitreoretinal lymphoma, and potentially cytokine profiles could be employed to diagnose different infections. Large numbers of samples linked to well-defined phenotype uveitis presentations would be essential to further investigation in this area.In addition to more sophisticated molecular testing, with increasingly small samples and from sites remote from the eye, I believe that advances in ophthalmic imaging are going to help clinicians pinpoint infectious diagnoses. For example, there appear to be characteristic optical coherence tomography findings in syphilitic uveitis, focused on the retinal pigment epithelium. Ultimately it may become possible to harness imaging methodologies to recognise pathogens in situ: for parasites, this should already by possible.
Future diagnostic tests will be focused on improving the precision and speed at which an organism is identified. We have currently been using molecular polymerase chain reaction-based assays. These tests are unable to identify a causative agent in up to 30–50% of our presumed infective cases. Current advances in metagenomics next generation sequencing (NGS) technology have demonstrated its potential application in the field of ocular infectious disease and is likely to be the next frontier for better diagnostics. We have been conducting research in cytokine profiles and multi-omics in infectious uveitis but currently not using it in clinical practice. When diagnosing certain infectious uveitis remains challenging, using integrated omics analysis to identify markers that could constitute a diagnostic signature is an attractive approach. It may help differentiate the infectious from non-infectious case that presents with similar clinical features. However, we will still need to depend on ocular samples for testing.
Q4: Treatment for infectious uveitis is often systemic despite frequently being isolated to the eye. This causes issues with compliance, side effects, and require blood monitoring. Do you foresee more effective and long-lasting local therapy being developed and what are the newer modalities that we can import from the medical retina field?
Systemic therapies are likely still to play some role in the treatment of infectious uveitis. The benefit of systemic control of pathogens was demonstrated in the mortality benefit of systemic anti-CMV treatment in AIDS patients with CMV retinitis. Local ocular therapies nevertheless can play a role in patients who are unable to tolerate systemic treatment (i.e., pancytopenia as a side effect of ganciclovir treatment). The Vitrasert ganciclovir implant was one example of a device that could achieve durable therapeutic drug levels within the eye. There is potential to leverage newer technologies for intravitreal (polyimide eluting implants, pars plana port delivery systems) or suprachoroidal delivery of antimicrobial therapies. Another option that could be explored would be gene therapy technologies that have shown promise in the treatment of retinal degenerations.
I agree – I entirely get the need for local therapies if there is no evidence of systemic infection. This will no doubt help reduce adverse effects from treatment and increase compliance. However, bear in mind this may only be for a subset of uveitis patients who needs longer term treatment for prevention of recurrence i.e. in cases of herpetic or toxoplasmosis infection. Two issues need to be considered in local therapeutics development: firstly, the technology for appropriate release perhaps in the form of a slow release implant and secondly, specific pharmacokinetics of the drug formulation that enables anti-infectious activity without local toxicity.
I believe that except for the MTB as stated above, the other two common causes of infectious uveitis, the Herpes viruses and the toxoplasma gondii cannot be eliminated by systemic therapy. Local delivery ensures effective dosage where it is needed the most avoiding the systemic toxicity of antibiotics. We have used intravitreal clindamycin (1 mg/0.1 ml) injections very effectively for controlling toxoplasmic retinochoroiditis. Compared to nearly 50 grams of clindamycin, a potentially highly toxic antibiotic, required to be delivered systemically over 6 weeks, we get away by using less than 10 mg total dose when used as intravitreal injection. As an example of the host directed therapies, we have used intravitreal injections of anti-VEGF agents as an adjunct to the systemic ATT for a very effective and quick regression of the TB choroidal granulomas.
The medical and surgical retinal fields are delivering novel forms of local drug delivery that could be exploited to treat infections locally. Many infections are systemic, however, even if the manifestations are ocular when the patient presents to a uveitis specialist. One of the arguments for treating presumed tubercular uveitis with standard anti-tubercular therapy is the potential to reduce risk of subsequent pulmonary disease, for example. Thus overall, my preference is to treat systemically except in exceptional circumstances, such as reactivation of ocular toxoplasmosis in a pregnant patient.
The development of drug delivery systems for ocular infections is not novel. In the pre-HAART era, CMV retinitis was managed by suturing a non-biodegradable drug delivery device into the vitreous cavity at the pars plana, which eluted ganciclovir for at least 3 months. Such delivery systems are useful for infections that require frequent repeated injections into the posterior segment of the eye, especially those which cause recurrent infection or infections which need prolonged drug administration. Drug delivery systems have the advantage of bypassing the blood-retinal and blood-aqueous barrier, reducing the dose administered, minimizing systemic toxicity. Isolated ocular infections that currently require systemic therapy or prophylaxis, such as viral infections, toxoplasmosis, and certain cases of tuberculous uveitis for example, would greatly benefit from long-lasting local drug delivery systems. In the medical retina field, various routes and numerous ocular drug delivery platforms are being explored. The most promising modality to be considered for infectious uveitis now is the port delivery system (for ranibizumab). This is a permanent refillable ocular implant which will allow for in-clinic refill-exchange procedures that will greatly help in alleviating the management burden, patient compliance and financial implications in patients with infectious uveitis.
Q5: While infectious uveitis always must be first treated as so, there is also a need to control collateral damage from intense inflammation. Any pearls to start anti-inflammatory therapy be it local or systemic across the various types of microorganism?
Some infectious agents induce inflammation from cytolytic response (HSV, VZV, CMV) and others by a combination of cellular damage and robust host immune response to the infectious agent (TB and toxoplasmosis).
In general, it is preferable to allow antimicrobials to reach a steady state of therapeutic concentration before starting anti-inflammatory therapy, which can be tailored based on the mechanism of inflammation. Topical or oral corticosteroids, capped at a maximum dose of prednisone 0.5 mg/kg/day or equivalent, are the mainstay of treatment, and can be started 48–72 hours after appropriate antimicrobials are on board. This can be taken as a rule of thumb.
It is very infectious aetiology dependent. Conceptually, it would be wise to reduce infectious replication first and then treat with anti-inflammatories as to prevent inflammation arising from the innate immune system acting against the dying infectious agent. The timing would be optimised by for example being able to test small samples to check ‘infectious load’ enabling safer and more effective use of immunosuppression – locally or systemic, without risk of causing fulminating infections. Fungi is the most difficult and tricky to manage, toxoplasmosis – if not necrotising less so and anti-inflammatory treatment can be added early, herpetic viruses – again load first with anti-viral treatment prior to adding the steroids (which I believe is what most are currently doing anyway)
So currently, I treat locally (in the form of eyedrops) but withhold systemic steroids until the diagnosis is made, next stabilise the patient with anti-infectious agents (not talking of fungi here) and in general add systemic steroids 48–72 hours later.
Reactive inflammation may cause considerable damage to the eye in the setting of infection. However, the underlying cause of the inflammation is the infection, and anti-inflammatory treatment can promote replication of an ocular pathogen due to suppression of the immune response. Thus, the first principle for treatment remains control of the infection. In general, if I see a role for anti-inflammatory treatment with systemic prednisolone – for example, in toxoplasmic retinochoroiditis – I like to see a response to the antimicrobial drug(s) or at least allow time for antimicrobial therapy to have had sufficient time to take effect before starting prednisolone, and I watch carefully to ensure there is no progression after starting this agent.
Generally, I prefer to start the antimicrobial therapy 48–72 hours ahead of the corticosteroid/immunomodulatory therapy to allow time for the former to take effect. In addition, this would provide sufficient time to ensure that the patient is able to tolerate the antimicrobial, especially if a depot steroid injection is being considered. However, if the inflammation is severe, giving the antimicrobial a 24-hour head start to begin working before the anti-inflammatory therapy would be the minimum for me.
Q6: Some patients have “chronic” uveitis and often we wonder whether are we missing something or is this just a truly recalcitrant disease. How do you deal with patients whose disease you cannot fully resolved/partially control?
It is always prudent to reconsider the differential diagnosis when a patient is not responding as expected to treatment. In cases where the diagnosis is uncertain, pausing therapy and obtaining a sample of ocular fluid can be helpful to guide treatment. For instance, vitreous biopsy for cytology can identify masquerade syndromes. Anterior chamber paracentesis with PCR testing on the aqueous can be helpful to confirm an infectious agent and to follow titers down with treatment. If inflammation persists and PCR titers are not falling despite treatment, one may consider viral mutations conferring resistance. Examples include testing for ganciclovir resistance and switching to Foscarnet in HSV/VZV infection, escalating from sulfamethoxazole-trimethoprim to quadruple therapy in toxoplasmosis and so on.
We must also consider and optimize the systemic immune status of the host, and finally, there may be a role for judicious use of anti-inflammatory agents, such as corticosteroids, in combination with antimicrobial therapy.
I agree. In a nutshell, sometimes stop the current treatment, re-analyse the case for potential differentials that might have been overlooked, and if none, then start appropriately and slowly increase immunosuppressive therapy (under cover of antimicrobials).
Traditionally, CMV retinitis was seen in patients with HIV infection and manifested as a perivascular chronic retinitis that progressed rather slowly and was accompanied by minimal anterior chamber or vitreous reaction. On the other hand the HSV and VZV cause necrotizing retinitis often accompanied by severe inflammatory reaction in immunocompetent individuals. Although rare, even the immunocompetent patients, especially the elderly with diabetes mellitus, may develop CMV retinitis mimicking acute retinal necrosis. Precious time is wasted trying to treat these patients with the conventional antiviral therapy without any response. Thus it may be prudent to subject ocular fluids to PCR to look for the offending virus.
When one cannot resolve uveitis, either presumed infectious or presumed non-infectious, it is essential to re-review, which may involve more history taking, another meticulous examination of all parameters and further investigations, as well as discussion with one’s uveitis colleagues who may be able to offer new insights.
Taking the example of a chronic viral AU, treatment response is monitored mainly by clinical means. If the disease appears recalcitrant, I will repeat the real-time polymerase chain reaction (PCR) to determine whether the viral load has reduced in response to the therapy. Switching to a prodrug with better bioavailability or changing the route of drug administration may help in a case of slow response. Genotypic detection of CMV drug resistance using the Sanger sequencing of PCR-amplified gene segments UL97 and UL54 gene segments is helpful for detecting ganciclovir resistance when treating chronic CMV AU. If present, this would be an indication for switching to a different antiviral drug. If the virus is no longer detectable on PCR, I will step up the anti-inflammatory therapy while maintaining the antiviral prophylaxis.
Q7: Research into infectious uveitis has always been difficult. The cases are rare, and case definitions are also vague. Current research centers significantly on consensus papers and guidelines. What barriers do you think are limiting infectious uveitis research and how best to overcome them?
Consensus and guidelines publications are primarily based on anecdotal evidence and may be subject to personal bias based on the clinical environment. Our understanding of infectious uveitis would benefit significantly from improved detection of infectious pathogens (i.e., by novel next-generation sequencing) and a better understanding of host response to infection (i.e. biomarkers). Prospective, multicenter studies of patients with clinical infectious uveitis, including robust approaches to detection of known and unknown infectious agents in ocular samples and detection of host biomarkers, will help advance our understanding of underlying pathogenesis and guide treatment.
Cross institution collaboration will be necessary to overcome the scarcity of cases. For example, a global tissue and/or case repository will be useful to collate cases worldwide with all the required clinical and imaging data. Fuller genomic analysis (as above) of infectious uveitis is also imperative for us to better understand the pathophysiology of infectious uveitides and make sense of response to treatments especially in complex cases. Machine learning can also have a big part to play in the future diagnosis and treatment algorithms of these diseases.
Multicentre collaborations that cross national borders should be developed for high-quality clinical trials. There are many examples of how the uveitis community comes together for clinical care: The SUN collaborative is the grandest example. Our international societies, including the International Ocular Inflammation Society and the International Uveitis Study Group, can play important roles here.
Classification criteria of infectious uveitis based on clinical phenotype may not have the same precision as when PCR is added for the criteria. However, PCR is costly and may not be widely available, and this may limit the number of centers and cases that can be involved in this study. In addition, the virulence, genotype, and microbial load may vary in different parts of the world, leading to challenges in study design, especially for therapeutic trials. Furthermore, it is difficult to differentiate between infective and inflammatory responses during treatment without repeating the PCR for the microbial load at different time points. This necessitates repeated ocular fluid sampling, which not all researchers would be comfortable with. Finally, funding is a huge limiting factor. Having access to point-of-care diagnostics to confirm infectious cases may facilitate research and enable collaborative multicenter studies.
Q8: Artificial intelligence (AI) is a rapidly growing area in medicine, and we see significant applications of AI in image analysis and OCT reading. While this is still in its infancy, where do you think AI can come into play in our management of infectious uveitis. Can it even surpass the clinician in the future if the dataset is large enough with inputs from local epidemiology, infectious disease colleagues, and so on?
Machine learning and artificial intelligence have tremendous power to integrate multimodal inputs and model an outcome. The SUN working group has successfully employed these techniques to standardize the nomenclature of clinical findings in various uveitides. As deep learning is not biased, any model will only be as effective as the data on which it is built; hence the adage, “Garbage in, garbage out.” To the extent that we can curate and annotate robust imaging and clinical datasets for uveitis, we believe machine learning can serve a valuable role in aiding telemedicine, particularly in areas without local uveitis expertise.
Regarding treatment, our impression is that time will tell, and we believe the clinician’s role will remain important in monitoring disease response and side effects from interventions. Moreover, clinical expertise will be crucial in the detection of evolving infectious agents, as recent epidemics of viral infections in Africa, Brazil, and now COVID are good examples.
Yes, there is great potential in this area. The challenge will be the validation process in machine learning. We need to be able to diagnose infectious uveitis in its myriad of presentations more definitively before a good model can be built. And for this to happen, the molecular diagnosis must improve.
Artificial intelligence (AI) is part of the future of uveitis, like the rest of ophthalmology and indeed all of medicine. And it can be applied to many aspects of our practice, not only ophthalmic imaging. Like ophthalmic images, history or examination features can be used to build diagnostic algorithms. There is the potential to develop treatment algorithms to identify patients who would benefit from lower versus higher intensity therapy. As uveitis care is individualised, it is hard to imagine that a clinician will not be required to oversee management. Still, I foresee that AI-based tools will augment uveitis practice for the better.
AI in the future is likely to assist in diagnosing infectious uveitis as clinicians continue to digitize diagnostic information such as scans and images. This will help pave the way for to AI identify diagnostic biomarkers in uveitis cases. However, this requires the input of thousands of definite case examples. Indeed, AI has been shown to surpass the clinician in speed and accuracy in specific fields of medicine. It has already been successful in helping in drug development, such as identifying targets for intervention and discovering drug targets. Importantly, AI can help to personalize treatment. These advances in modern medicine may also be imported into the field of uveitis if we collaborate with our colleagues locally and internationally.
Q9: The last question - we cannot let you go in this era without a COVID-related question. COVID-related infectious uveitis, COVID vaccination-related uveitis, COVID induced non-infectious uveitis: what are your thoughts and pearls?
We have little experience with COVID-related uveitis or COVID vaccine-induced uveitis. However, it is not surprising given reported cases of post-viral uveitis and post-vaccination uveitis from various other vaccines. COVID vaccine-related uveitis is a mixed bag and could depend on whether mRNA vaccine or others and the components of vaccine and manufacturing.
It is not surprising we see with such a large number of cases and vaccination a SMALL number of patients that have the potential of, firstly, inflammation in the eye (do note it is just temporal association with vaccines, rather than evidence of causality), secondly, direct COVID infecting inducing inflammation within the eye or thirdly, indirect consequences of COVID disease such as inflammatory storms, coagulopathies resulting in other ocular complications.
I think it is pretty reasonable that we will potentially see a relapse or new cases of non-infectious diseases with such immune stimulation of both innate and T cell systems. However, what is impressive is that this seems relatively low in frequency?
Several viruses including the parvoviruses, CMV, EBV, Herpes, Hep A and C, HTLV, etc. have been implicated in triggering autoimmune diseases like rheumatoid arthritis and lupus by disturbing the self-tolerance. There are several reports in the literature of either the infection with SARS CoV-2 or even the use of vaccine against this virus that led to the initiation, recurrence, or aggravation of several autoimmune diseases, including lupus, dermatomyositis, vasculitis, autoimmune hemolytic anemia, immune thrombocytopenic purpura, autoimmune thyroid disease, and many more such autoimmune disorders. Likewise, several cases of VKH disease have been reported that show a temporal relation with either the infection or its vaccine. For yet unexplained reasons, several fungal endogenous endophthalmitis cases have been reported from India due to COVID-19 even in some of the patients who suffered from a disease not requiring hospitalization. The hypotheses include immunosuppression from pre-existing comorbidities like diabetes and steroids/immunomodulators used while treating cytokine storms predisposing them to these infectionsCitation22
Two years into the pandemic, we still have much to learn, and as the work advances, SARS-CoV-2 continues to evolve. For uveitis specialists, the most significant challenges are delivering optimal care to our patients around the various changes in the way we live and work due to COVID-19 and ensuring our patients on systemic immunosuppression are adequately protected. This involves complete vaccination and, should a patient become infected with the virus, additional measures such as neutralising monoclonal antibodies to reduce the risk of severe disease.
COVID-related infectious or noninfectious uveitis seems to be less common than vaccination-related uveitis. Vaccine-related uveitis has been reported following various types of COVID vaccine and is likely the result of a dysfunctional immune response. The most common manifestation is acute anterior uveitis, although intermediate and posterior uveitis have also been reported. The uveitis may be of new onset or a recurrence. It is important to enquire about recent covid infection and vaccination during history taking to clinch the diagnosis—treatment with steroids and cycloplegics, where appropriate, usually result in resolution of the uveitis.
Summary and take-home pearls
The infectious causes form a significant minority of uveitis patients presenting to clinical practice. Careful attention to details such as morphology of keratic precipitates may yield diagnostic clues. While epidemiology will depend on geographic location and practice setting, common causes to consider include toxoplasma (which is possibly the commonest cause of infectious uveitis),Citation23 herpetic viruses, syphilis, and tuberculous.
Phenotypic expression of infectious uveitis is often a balance between the virulence of the invading microorganism and the robustness of the immune response mounted by the host. This gives rise to a range of possible disease presentations.
Current laboratory diagnostic methods are imperfect and might be a major factor impeding accurate diagnosis and classification of infectious uveitis. Improving medical technology in terms of ocular imaging for infectious organisms insitu or characteristic signs, genomic sequencing for molecular diagnosis, and improved sensitivities from small ocular samples might be essential to more definitive diagnoses. One challenge will be separating truly etiologic agents from bystander organisms when using molecular diagnostics as these methods become more commonly employed.
Systemic therapy will likely still be necessary for patients with infectious uveitides, but improved local drug delivery systems might be a way of supplementing the arsenal with enhanced compliance and cost-effectiveness.
Anti-inflammatory therapy is a double edge sword in cases of infectious uveitis. Ideally, there must be a response/initiation of anti-microbial therapy for 48–72 hours before initiating anti-inflammatory therapy.
For recalcitrant cases, always reconsider the diagnosis, repeat investigations if necessary, consider anti-microbial resistance as a factor, and cautiously escalate treatment (anti-microbial ± anti-inflammatory)
Further research into infectious uveitis is essential and this will require cross-institution collaboration to overcome the scarcity of cases. Colleagues from other specialties like microbiology and public health can be a valuable resource in this endeavor with their knowledge of endemic infections and trends. Another important prerequisite is the improvement of diagnostic methods for such cases with better pathogen characterization to lend strength to such studies. Artificial intelligence and molecular diagnostics are potential technologies that can advance this area of uveitis.
Conclusion
Our sincerest gratitude to the experts for sharing with us their personal experience in the field of infectious uveitis and their insights into the future of diagnosing and managing these cases. It is indeed exciting times ahead with molecular diagnosis, machine learning, and novel therapeutics and delivery systems on the horizon. The potential for better diagnostics and treatment is enormous and we have no doubt that the future is bright for our patients and the uveitis field.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Choi RY, Rivera-Grana E, Rosenbaum JT. Reclassifying idiopathic uveitis: lessons from a tertiary uveitis center. Am J Ophthalmol. 2019;198:193–199. doi:10.1016/j.ajo.2018.10.018.
- Kuthyar S, Anthony CL, Fashina T, Yeh S, Shantha JG. World Health Organization high priority pathogens: ophthalmic disease findings and vision health perspectives. Pathogens. 2021;10:442. doi:10.3390/pathogens10040442.
- Zhang Y, Amin S, Lung KI, Seabury S, Rao N, Toy BC. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: a claims-based analysis. PLoS One. 2020;15(8):e0237995. Published 2020 Aug 25. doi:10.1371/journal.pone.0237995.
- Dogra M, Singh R, Agarwal A, et al. Epidemiology of uveitis in a tertiary-care referral institute in North India. Ocul Immunol Inflamm. 2017;25(sup1):S46–S53. doi:10.1080/09273948.2016.1255761. Epub 2016 Dec 12. PMID: 27937033.
- Siak J, Jansen A, Waduthantri S, Teoh CS, Jap A, Chee SP. The pattern of uveitis among Chinese, Malays, and Indians in Singapore. Ocul Immunol Inflamm. 2017;25(sup1):S81–S93. doi:10.1080/09273948.2016.1188968.
- Ang M, Chee SP. Propionibacterium acnes endogenous endophthalmitis presenting with bilateral scleritis and uveitis. Eye (Lond). 2010 May;24(5):944–945. doi:10.1038/eye.2009.194. Epub 2009 Jul 31. PMID: 19648894.
- Woods AC. Modern concepts of the etiology of uveitis. Am J Ophthalmol. 1960;50(6):1170–1187. doi:10.1016/0002-9394(60)91006-0.
- Verhoeff FH. Histologic findings in a case of localized tuberculous chorioretinitis. Trans Am Ophthalmol Soc. 1928;26:53–61.
- Wilder HC. Toxoplasma chorioretinitis in adults. AMA Arch Ophthalmol. 1952;48(2):127–136. doi:10.1001/archopht.1952.00920010132001.
- Gupta V, Arora S, Gupta A, Ram J, Bambery P, Sehgal S. Management of presumed intraocular tuberculosis: possible role of the polymerase chain reaction. Acta Ophthalmol Scand. 1998 Dec;76(6):679–682. doi:10.1034/j.1600-0420.1998.760609.x. PMID: 9881551.
- Gupta A, Gupta V, Arora S, Dogra MR, Bambery P. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina. 2001;21(5):435–444. doi:10.1097/00006982-200110000-00004. PMID: 11642371.
- Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin. 2005 Spring;45(2):71–88. doi:10.1097/01.iio.0000155934.52589.e3. PMID: 15791159.
- Van Gelder RN. Molecular diagnostics for ocular infectious diseases: LXXVIII Edward Jackson memorial lecture. Am J Ophthalmol. 2022;235:300–312. doi:10.1016/j.ajo.2021.12.002.
- Verma P, Pandey RK, Prajapati P, Prajapati VK. Circulating MicroRNAs: potential and emerging biomarkers for diagnosis of human infectious diseases. Front Microbiol. 2016;7:1274. doi:10.3389/fmicb.2016.01274.
- Tribolet L, Kerr E, Cowled C, et al. MicroRNA biomarkers for infectious diseases: from basic research to biosensing. Front Microbiol. 2020;11:1197. doi:10.3389/fmicb.2020.01197.
- Montoya JG, Parmley S, Liesenfeld O, Jaffe GJ, Remington JS. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology. 1999;106(8):1554–1563. doi:10.1016/S0161-6420(99)90453-0.
- Fenner T, Garweg J, Hufert FT, Böhnke M, Schmitz H. Diagnosis of human cytomegalovirus-induced retinitis in human immunodeficiency virus type 1-infected subjects by using the polymerase chain reaction. J Clin Microbiol. 1991;29:2621–2622. doi:10.1128/jcm.29.11.2621-2622.1991.
- Fox GM, Crouse CA, Chuang EL, et al. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol. 1991;109:266–271. doi:10.1001/archopht.1991.01080020112054.
- Zhao X-Y, Xia S, Chen Y-X. Role of diagnostic pars plana vitrectomy in determining the etiology of uveitis initially unknown. Retina. 2020 February;40(2):359–369. doi:10.1097/IAE.0000000000002372.
- Deshmukh D, Joseph J, Chakrabarti M, et al. New insights into culture negative endophthalmitis by unbiased next generation sequencing. Sci Rep. 2019;9(1):844. Published 2019 Jan 29. doi:10.1038/s41598-018-37502-w.
- Valdes L, Bispo P, Sobrin L. Application of metagenomic sequencing in the diagnosis of infectious uveitis. Semin Ophthalmol. 2020;35(5–6):276–279. doi:10.1080/08820538.2020.1818795.
- Dutta Majumder P. Endogenous fungal endophthalmitis in COVID-19 patients: an unexplored possibility. Indian J Ophthalmol. 2022 Apr;70(4):1083–1085. doi:10.4103/ijo.IJO_510_22. PMID: 35325989.
- Patel A, Kelgaonkar A, Kaza H, et al. Recent advances in diagnosis and treatment of infectious uveitis prevalent in Asia-Pacific region. Asia-Pac J Ophthalmol. 2021 January-February;10(1):99–108. doi:10.1097/APO.0000000000000367.
