ABSTRACT
Purpose
To identify factors associated with glaucoma surgery in pediatric uveitis.
Methods
Patients diagnosed with uveitis before their 18th birthday and with an observation period of at least one year were included in a retrospective case-control study.
Results
A total of 185 patients were included, 84 of whom had undergone glaucoma surgery. Juvenile idiopathic arthritis (JIA)-related uveitis was associated with undergoing glaucoma surgery (p = .002). In the JIA-subgroup, the presence of anterior segment complications (OR 3.1 (95% CI 1.0 to 9.6); P = .045) and an IOP > 21 mmHg during the first uveitis remission (OR 4.5 (95% CI 1.3 to 15.2); P = .015) were associated with an increased risk of glaucoma surgery. Sixty-eight percent of the cases needed glaucoma surgery within one year after they started IOP-lowering triple therapy.
Conclusion
The risk profile for undergoing glaucoma surgery as outlined in this study is a valuable help to recognize and treat secondary glaucoma in a timely manner.
The most common causes of vision loss in pediatric uveitis are cataract, papillitis, glaucoma, cystoid macular edema, and band keratopathy, of which the visual loss in secondary glaucoma is always irreversible.Citation1 The reported prevalence of secondary glaucoma in children with uveitis ranges between 5% and 25%Citation1–4 and elevated intraocular pressure (IOP) has been reported between 3% and 51%.Citation4
Secondary glaucoma occurs when uveitis is associated with elevated IOP and optic nerve damage, resulting in irreversible visual field loss. Multiple factors, such as damage of the trabecular meshwork due to inflammation, the use of steroids, or the development of peripheral anterior synechiae, can increase the IOP. Secondary glaucoma in pediatric uveitis has an unpredictable course, with large IOP fluctuations and varying responses to IOP-lowering medications.Citation5 Increased IOP is initially treated pharmacologically in a stepladder approach. If IOP remains unacceptably high, glaucoma surgery is required. To obtain the best long-term visual outcome, it is important to identify those children who are at increased risk for the development of secondary glaucoma at an early stage and to treat them adequately, before irreversible damage has occurred.Citation6
Two previous studies about pediatric uveitis reported a female preponderance, juvenile idiopathic arthritis (JIA) as the most common etiology, and anterior uveitis as a predictive anatomical site for developing ocular hypertension or glaucoma.Citation7,Citation8 Heinz et al. showed a significantly higher need for glaucoma surgery in pediatric uveitis compared to uveitis in adults.Citation7
The aim of this study is to identify risk factors for glaucoma surgery in pediatric uveitis. For this purpose, we evaluated a large group of children with uveitis and compared those who needed surgery to those who did not. Identification of factors associated with glaucoma surgery may contribute to the early detection of the need for glaucoma surgery in these patients and thereby prevent or reduce irreversible damage.
Patients and methods
Patients diagnosed with uveitis before their 18th birthday and with a minimal observation period of 1 year were included from the departments of ophthalmology of the University Medical Centers Groningen (UMCG), the Netherlands, and Utrecht (UMCU), the Netherlands. Patients were diagnosed with non-infectious uveitis between 1989 and 2016 and were identified from the uveitis databases of both centers. We designed a case-control study.Citation9 All patients with pediatric uveitis who had undergone glaucoma surgery were included as cases. Initially, in the UMCU, for each case, one random patient with pediatric uveitis and without a history of glaucoma surgery was included as a control. Because the UMCG-cohort appeared to have a lower number of cases, all patients without glaucoma surgery from the UMCG were included as controls, to increase the power of the study.Citation10 Data collection was done from the ophthalmic medical records until July 2018. The Medical Ethical Committees of the UMCG and the UMCU approved the conduction of the study.
Uveitis diagnosis
The diagnosis of uveitis was made by ophthalmologists specialized in pediatric uveitis. Classification of uveitis was done according to the Standardization of Uveitis Nomenclature (SUN) criteria.Citation11 Children were evaluated for the presence of an underlying systemic disease by pediatric rheumatologists. The diagnosis of posterior and panuveitis was made by fundoscopy; the diagnosis of macular edema was made by optical coherence tomography.
General descriptives
Measurements at the presentation were based on the first consultation in the hospital of expertise for uveitis (UMCU/UMCG). The following information at presentation was recorded: age at onset of uveitis, sex, bilaterality of the uveitis, anatomical classification of uveitis, visual acuity in LogMAR, IOP, etiology of the uveitis, ocular complications, antinuclear antibodies (ANA) status, and the use of ‘disease modifying anti-rheumatic drugs’ (DMARDs). Anterior segment complications included band keratopathy, cataract, and posterior synechiae. Posterior segment complications included macular edema and papillitis. Different subtypes of DMARDS were reported: systemic steroids, conventional synthetic (cs)DMARDs (like methotrexate, mycophenolic acid and salazopyrin), and biological (b)DMARDs (like adalimumab and infliximab). The DMARDs used in the first presentation were divided into subgroups: the first presentation before 2000 (before the introduction of methotrexate and mycophenolic acid), between 2000 and 2010, and after 2010 (after the introduction of bDMARDs). The following information was recorded during the observation period: IOP, use of IOP-lowering medication, use of DMARDs and daily use of topical steroids during the first episode of uveitis remission, highest IOP, start of IOP-lowering medication, maximum IOP-lowering medication, moment of starting triple therapy of IOP-lowering medication, and cataract surgery. To get an impression of the impact of a steroid-induced IOP response in quiescent uveitis, the topical steroid use during the first episode of uveitis remission was reported. The term ‘remission’ was used, when quiescence of uveitis was achieved, with or without medication, for at least three months. The amount of topical steroid use per day was counted as follows: use of topical prednisolone (drops or ointment) and dexamethasone was counted as 1 unit per drop, use of rimexolone (Vexol, Alcon bv) or fluorometholone (FML liquifilm, Abbvie bv) as 0.5 units per drop, and use of subconjunctival triamcinolone (Kenacort, Bristol-Myers Squibb) as 4 units. In order to get an insight into the DMARD use later during the observation period, the use of DMARDS was scored in the period before surgery in the cases. In the controls, the DMARD use was reported 2.5 years after their first visit, based on the median time period of glaucoma surgery in the cases. The observation period ended when glaucoma surgery was performed or, in the control group, at the last ophthalmic examination. In both centers, the goal of uveitis treatment was to obtain disease remission, based on the SUN criteria,Citation11 with a maximum daily maintenance dosage of topical steroids of 2 times per day, with or without systemic immunosuppressive medication.
Indication for glaucoma surgery
The indication for glaucoma surgery was made by glaucoma specialists and was mostly based on an IOP > 21 mmHg with maximum tolerated IOP-lowering medication and individual patient characteristics, such as age, visual acuity, changes in optic disc cupping, and the extent, duration, and speed of the IOP increase. This is in line with recommendations in the literature, where the diagnosis of secondary glaucoma in children is most often based on a substantial increase in IOP over a relatively short period of time.Citation12–14 Unfortunately, in young children, reliable measurements of changes in optic disc cupping and perimetry are usually not possible, and thus a diagnosis of glaucoma cannot be based on a definition that includes reproducible visual field defects.Citation15 In practice, most children with ongoing substantial IOP rise in our study group underwent glaucoma surgery, recognizing the risk of a significant reduction in visual acuity due to damage to the optic disc with suboptimal IOP control.
Data analysis
One eye per patient was analyzed. If a patient underwent glaucoma surgery in both eyes, the eye that first underwent surgery was included. In the control group, if the uveitis was bilateral, the eye with the first presentation of uveitis was used. If both eyes were affected at the same time, the worse eye, based on the visual acuity, complications, and IOP was chosen. When both eyes were equally affected, a random eye was chosen.
Descriptive statistics were used to present the ratio variables with median and interquartile range (IQR) because of the non-parametric distribution. Medians were compared using the Wilcoxon test for paired samples and the Mann-Whitney U-test for independent samples. For the differences between the nominal data groups, we used the Pearson chi-square test. Kaplan-Meier curves were used to graphically display at which time-point during the observation period the event (glaucoma surgery) occurred in the cases.
The following analyses were performed in the entire study group (cases versus controls): First, univariable analyses of characteristics at presentation were performed and covariates with P-values ≤0.2 were entered into a Cox survival analysis in a backward stepwise conditional method. Glaucoma surgery was defined as the event. Second, univariable analyses of factors present during the observation period were done. Subgroup analyses: The Cox regression analysis showed that JIA was the most important factor associated with glaucoma surgery. Therefore, an additional series of univariable analyses of factors present during the observation period was done in this subgroup, followed by a logistic regression analysis of covariates with a P-value ≤0.2. Data was statistically analyzed with SPSS 23.0.0 (SPSS Inc, Chicago, Illinois, USA). A P-value <0.05 was considered statistically significant. A Bonferroni correction was applied where needed.
Results
In total, 84 cases and 101 controls were included in the study. shows the univariable analyses of the covariates at presentation. After dichotomization of the categorical covariates, the following characteristics at presentation were entered into the Cox analysis: age (P = .06), anatomic location of the uveitis (anterior versus other, P = .06), anterior segment complications (present versus absent, P = .17), etiology (JIA versus non-JIA, P = .002), and ANA status (positive versus negative, P = .15). To correct for possible differences between the two centers, ‘center’ was added as a covariate to the multivariable analyses. All covariates used in the Cox survival analysis had below 10% missing data points. The final step of the backward stepwise model enclosed only covariates with a P-value of ≤ 0.1. In the final model of the Cox survival analysis, the etiology (JIA) was the most important independent covariate at presentation (HR 1.5 (95% CI 1.0 to 2.4); P = .067) associated with glaucoma surgery. We additionally performed the Cox regression analysis in the non-JIA subgroup. However, no significant risk factors were seen in this analysis.
Table 1. Characteristics at presentation.
Univariable analyses of covariates during the observation period in the entire group () showed a significantly higher IOP (21 mmHg in cases versus 18 mmHg in controls, P = .009) and more topical steroids use in the cases (3 units versus 1 unit, P < .001) during the first remission. During the observation period, the highest IOP was measured later in the cases than in the controls (after 27 versus 14 months, P = .007), resulting in a higher event-rate during the first years of the observation period (). In 68% of the cases, glaucoma surgery was required within 1 year after increasing the IOP-lowering medication to triple therapy (). As a primary surgical procedure, a trabeculectomy was performed in 36 cases, a Baerveldt drainage implant in 35 cases and an Ahmed drainage implant in 13 cases.
Figure 1. Kaplan-Meier curve shows the time when cases underwent glaucoma surgery after presentation.
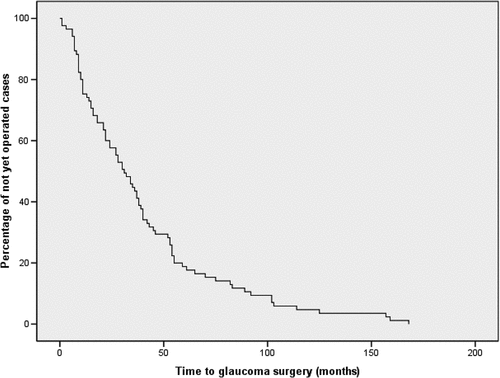
Table 2. Variables during observation period.
Table 3. Interval between the start of a third type of IOP-lowering medication and glaucoma surgery.
At presentation, systemic disease-modifying anti-rheumatic drugs (DMARDS) were significant more frequently used in the control group (49% versus 68%, P = .007; ), which was mainly based on the differences in the use of systemic steroids (P = .007) in patients with their first presentation between 2000 and 2010 (P = .01). The indication to start DMARDs was (partly) based on the uveitis in 61% of cases and 77% of controls (P = .08). In the other patients, DMARDs were started because of the underlying systemic disease (such as JIA). The differences in the use of DMARDs were no longer seen during the first uveitis remission (P = .2; ). In the period around glaucoma surgery (or around 2.5 years after presentation in the controls), bDMARDs were started apparently more often in the controls (P = .05). A few patients used systemic steroids during this period, most often at a low dose in a slowly tapering schedule in combination with a DMARD. All cases temporarily received systemic steroids around the surgical intervention to limit the inflammation caused by the surgery.
Because JIA-uveitis was the most predictive covariate for needing glaucoma surgery, additional analyses were performed in the JIA-subgroup. shows the univariable analyses of the covariates at presentation and covariates during the observation period in this subgroup. A logistic regression analysis was performed, with glaucoma surgery as the dependent variable. After dichotomization of the categorical covariates, the following characteristics were entered as independent covariates into the analysis: age at presentation (P = .13), anterior segment complications at presentation (present versus absent, P = .06), IOP during uveitis remission (below 22 versus above 21 mmHg, P = .001), and the use of more than 2 units of topical steroids during uveitis remission (P = .054). The presence of anterior segment complications at presentation (OR 3.1 (95% CI 1.0 to 9.6); P = .045) and an IOP > 21 mmHg during uveitis remission (OR 4.5 (95% CI 1.3 to 15.2); P = .015) were found to be significant risk factors for glaucoma surgery in the JIA-subgroup.
Table 4. Characteristics of JIA-uveitis.
Discussion
In the present study, JIA-associated uveitis was the most important baseline characteristic associated with glaucoma surgery in pediatric non-infectious uveitis. Within the JIA-subgroup, the presence of anterior segment complications and an IOP > 21 mmHg during the first uveitis remission were found to be significant risk factors for glaucoma surgery. After the start of triple therapy of IOP-lowering medication, most cases underwent glaucoma surgery within 1 year. Our results are consistent with the findings in previous studies that JIA-associated uveitis is a risk factor for the development of ocular hypertension and glaucoma.Citation7,Citation8,Citation16,Citation17 It is known that chronic inflammation in the anterior segment, as in JIA-uveitis, increases aqueous outflow resistance due to the accumulation of inflammatory debris and damage to the trabecular meshwork.Citation16
Our results show an increased median IOP in cases and controls during the observation period (37 mmHg in cases and 27 mmHg in controls), indicating that an IOP rise is very common in these children. Significant differences of the IOP between cases and controls have already emerged during the first uveitis remission, with similar results in the JIA-subgroup. During the first uveitis remission, the IOP is no longer suppressed by reduced ciliary body functioning as seen in active uveitis, and therefore the IOP might rise significantly.Citation8 Regular IOP monitoring is therefore important during the first months of treatment to diagnose and treat ocular hypertension early. Another important aspect is the higher number of topical steroids used in the cases during the first uveitis remission. It is assumable, that steroids add to the early IOP increase in the cases as such a steroid-induced response is known to be frequent, severe, and rapidly occurring in this age group.Citation3,Citation18,Citation19 The elevation of IOP due to steroid use can be found as early as the first or second weekCitation20–22 and depends on the dose. These observations underscore that efforts should be made to avoid the use of topical steroids and start early with DMARDs as much as possible in these children.
In our total study group, the IOP continues to rise after the first uveitis remission episode, with the highest IOP after 27 and 14 months in cases and controls, respectively. Our results therefore cannot purely be explained by steroid use alone, suggesting that a multifactorial nature of the IOP increase is more likely, as described earlier. Not surprisingly, in the cases, the IOP increased to higher values during the observation period and more IOP-lowering medications were used. It is noticeable that the majority of the cases underwent glaucoma surgery within one year of increasing IOP-lowering medication to three types of medication (). For this reason, the start of a third type of IOP-lowering medication can be regarded as a tipping point. A clinically relevant aspect is the interval between presentation and glaucoma surgery (median of 31 months), which indicates the need for prolonged IOP surveillance in these patients. An equal observation was published by Stroh et al., in which ocular hypertension had developed within the first three years of the observation period in the majority of patients.Citation17
The larger amount of DMARD use in the controls during their first visit is consistent with the finding that the uveitis in the controls is less often (only) anteriorly located. The frequent use of DMARDs in both groups during the observation period (76% in cases and 73% in controls) reflects the chronic course of pediatric uveitis. During the observation period, csDMARDs (most often methotrexate) were generally the DMARDs of choice, and bDMARDs (most often adalimumab) were used sporadically. In theory, low-threshold starting of systemic immune suppression reduces the ocular inflammation and thus the need for high doses and prolonged use of topical steroids.Citation23–25 Preventing damage to the trabecular meshwork and reducing the chance of a steroid-induced IOP increase may contribute to reducing the likelihood of developing ocular hypertensionCitation17,Citation26,Citation27 and needing glaucoma surgery. In recent decades, DMARDs, especially biologics, are more commonly used in the treatment of uveitis, based on new insights and developments in the field of ophthalmology, rheumatology, and inflammatory disease. It would be interesting to evaluate the effect of different pharmacological treatment regimens of uveitis, especially the role of anti-TNF-alpha blockers, on the need for glaucoma surgery in a randomized, prospective study design in the future.
To analyze the differences between the two groups at presentation and in the course of the disease, we followed the patients for as long as possible. Despite the fact that most glaucoma surgery was performed within 3 years after the presentation of uveitis, and the median observation period of the controls was 6 years, we cannot exclude that there are patients in the control group who will need glaucoma surgery in the future. The results of the current study are also limited by the fact, that after inclusion, there was a difference in the distributions of cases and controls between the two centers due to a relatively higher number of controls in the UMCG. To correct for this, we could randomly remove 20 of the UMCG controls, but this would reduce the power of the study and it would be a post hoc modification. For this reason, we would prefer to retain the chosen approach and acknowledge its limitations. Additionally, the study is retrospective, which is reflected in for example the variability in the observation period, and missing data. Another limitation is the presumably moderate representativeness of our study group for the general pediatric uveitis patients because our patients were included from two centers of expertise for uveitis. In addition, personal experiences or preferences of ophthalmologists and pediatric rheumatologists may have influenced the choice and course of treatment. The strengths of this study are its cohort size, the systematic way in which the data was collected and the sharing of expertise in a difficult and challenging patient population.
Conclusion
This study displays a risk profile for developing pharmacologically uncontrollable high IOP in non-infectious uveitis in children needing glaucoma surgery. This risk profile helps the clinician to recognize and thereby treats secondary glaucoma at an early stage. Our study shows that starting a third type of IOP-lowering medication is a tipping point for needing glaucoma surgery in these patients in the near future. Due to the considerable time span of the surgical interventions during the observation period, the authors emphasize the importance of long-term IOP monitoring and risk assessment.
Acknowledgments
Part of this work has been presented at the Dutch Ophthalmology Conference in Maastricht, The Netherlands, on March 29, 2019. In association with this presentation, the abstract was published in 2019 in a special issue of Acta Ophthalmologica (Abstracts of the Netherlands Ophthalmological Society (NOG) Annual Congress).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- de Boer J, Wulffraat N, Rothova AJ. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87:879–884. doi:10.1136/bjo.87.7.879.
- Paroli MP, Speranza S, Marino M, Pirraglia MP, Pivetti-Pezzi P. Prognosis of juvenile rheumatoid arthritis-associated uveitis. Eur J Ophthalmol. 2003;13(7):616–621. doi:10.1177/112067210301300704.
- Kaur S, Kaushik S, Singh Pandav S. Pediatric uveitic glaucoma. J Curr Glaucoma Pract. 2013;7(3):115–117. doi:10.5005/jp.journals.10008.1147.
- Kothari S, Foster CS, Pistilli M, et al. The risk of intraocular pressure elevation in pediatric noninfectious uveitis. Ophthalmology. 2015;122(10):1987–2001. doi:10.1016/j.ophtha.2015.06.041.
- Muñoz-Negrete FJ, Moreno-Montañés J, Hernández-Martínez P, Rebolleda G. Current approach in the diagnosis and management of uveitic glaucoma. Biomed Res Int. 2015;2015:1–13. doi:10.1155/2015/742792.
- Abu Samra K, Maghsoudlou A, Roohipoor R, Valdes-Navarro M, Lee S, Foster CS. Current treatment modalities of JIA-associated uveitis and its complications: literature review. Ocul Immunol Inflamm. 2016;24(4):431–439. doi:10.3109/09273948.2015.1115878.
- Heinz C, Koch JM, Zurek-Imhoff B, Heiligenhaus A. Prevalence of uveitic secondary glaucoma and success of nonsurgical treatment in adults and children in a tertiary referral center. Ocul Immunol Inflamm. 2009;17(4):243–248. doi:10.1080/09273940902913035.
- Gautam Seth N, Yangzes S, Thattaruthody F, et al. Glaucoma secondary to uveitis in children in a tertiary care referral center. [ published online February 2, 2018]. Ocul Immunol Inflamm. 26:1–9. doi:10.1080/09273948.2017.1411517.
- Altman DG . Practical Statistics for Medical Research. 1st ed. New York: Chapman and Hall/CRC; 1990. doi:10.1201/9780429258589.
- Krzywinski M, Altman N. Points of significance: power and sample size. Nat Methods. 2013;10(12):1139–1140. doi:10.1038/nmeth.2738.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi:10.1016/j.ajo.2005.03.057.
- Kanski JJ, Shun-Shin GA. Systemic uveitis syndromes in childhood: an analysis of 340 cases. Ophthalmology. 1984;91(10):1247–1252. doi:10.1016/S0161-6420(84.
- Kotaniemi K, Sihto-Kauppi K. Occurrence and management of ocular hypertension and secondary glaucoma in juvenile idiopathic arthritis-associated uveitis: an observational series of 104 patients. Clin Ophthalmol. 2007;1(4):455–459. http://www.ncbi.nlm.nih.gov/pubmed/19668522%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2704536
- Leinonen S, Kotaniemi K, Kivelä T, Majander A. Potential effect of tumor necrosis factor inhibitors on trabeculectomy with mitomycin C for patients with juvenile idiopathic arthritis-related uveitic glaucoma a retrospective analysis. JAMA Ophthalmol. 2015;133(11):1323–1328. doi:10.1001/jamaophthalmol.2015.3387.
- Spaeth GL. European glaucoma society terminology and guidelines for glaucoma, 5th ed. British J Ophthalmol. 2021;105:1–169. doi:10.1136/bjophthalmol-2021-egsguidelines.
- Sijssens KM, Rothova A, Berendschot TTJM, de Boer JH. Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology. 2006;113(5):853–859.e2. doi:10.1016/j.ophtha.2006.01.043.
- Stroh IG, Moradi A, Burkholder BM, Hornbeak DM, Leung TG, Thorne JE. Occurrence of and risk factors for ocular hypertension and secondary glaucoma in juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm. 2017;25(4):503–512. doi:10.3109/09273948.2016.1142573.
- Ng JSK, Fan DSP, Young AL, et al. Ocular hypertensive response to topical dexamethasone in children: a dose-dependent phenomenon. Ophthalmology. 2000;107(11):2097–2100. doi:10.1016/S0161-6420(00.
- Al Hanaineh AT, Hassanein DH, Abdelbaky SH, El Zawahry OM. Steroid-induced ocular hypertension in the pediatric age group. Eur J Ophthalmol. 2018;28(4):372–377. doi:10.1177/1120672118757434.
- Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70:500–507. doi:10.1001/archopht.1963.00960050502012.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11(6):478–483. doi:10.1097/00055735-200012000-00016.
- Kaur S, Dhiman I, Kaushik S, Raj S, Pandav SS. Outcome of ocular steroid hypertensive response in children. J Glaucoma. 2016;25(4):343–347. doi:10.1097/IJG.0000000000000209.
- Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res (Hoboken). 2014;66(7):1073–1084. doi:10.1002/acr.22214.
- Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;376(17):1637–1646. doi:10.1056/nejmoa1614160.
- Bitossi A, Bettiol A, Silvestri E, et al. Adalimumab accounts for long-term control of noninfectious uveitis also in the absence of concomitant DMARD treatment: a multicenter retrospective study. Mediators Inflamm. 2019;2019:1–8. doi:10.1155/2019/1623847.
- Utz VM, Bulas S, Lopper S, et al. Effectiveness of long-term infliximab use and impact of treatment adherence on disease control in refractory, non-infectious pediatric uveitis. Pediatr Rheumatol. 2019;17:1. doi:10.1186/s12969-019-0383-9.
- Wennink RAW, Kalinina Ayuso V, Pameijer EM, Dekkers CC, Bozkir I, de Boer JH. Improved clinical outcomes in patients with juvenile idiopathic arthritis associated uveitis in the last decade. Acta Ophthalmol. 2022;100:781–787. doi:10.1111/aos.15097.
