ABSTRACT
Purpose
To evaluate corneal epithelial changes and related factors in chronic ocular graft-versus-host disease (oGVHD) patients.
Methods
21 patients (35 eyes) with chronic oGVHD and 8 patients (12 eyes) without oGVHD after bone marrow transplantation were recruited for assessment involving in vivo confocal microscopy (IVCM) analysis, ocular surface parameter determination and tear cytokine level analysis. The IVCM corneal epithelial scoring system was used to evaluate corneal epithelial changes.
Results
There was a significant difference in the corneal epithelial score (p = .001) between the two groups. The corneal epithelial scores were significantly correlated with the corneal fluorescein staining scores (CFS, r = 0.463, p < .001), Schirmer’s test (r = −0.389, p = .009) and tear cytokine levels of EGF (r = −0.491, p < .001) and APRIL (r = −0.318, p = .030).
Conclusions
The depth of corneal epithelial defects can be estimated by the CFS. Corneal epithelial changes of chronic oGVHD are considered to be associated with lacrimal deficiency and a lack of EGF.
Chronic graft-versus-host disease (cGVHD) is a major and severe complication after allogeneic hematopoietic cell transplantation (HCT) and has a significant impact on patients’ quality of life.Citation1,Citation2 Its clinical manifestations are similar to those of an autoimmune disease, and it may affect many organs, including skin, nails, mouth, eyes, muscles, fascia, joints, female genitalia, gastrointestinal tract, liver and lungs.Citation1,Citation3 A total of 60–90% of patients who receive hematopoietic stem cell transplantation present with ocular manifestations.Citation4 Up to 90% of chronic ocular GVHD (oGVHD) patients have symptoms similar to severe dry eye, such as irritation, dry eyes, photophobia, itching, redness, pain and foreign body sensation.Citation5–7 Due to tear deficiency, goblet cell loss and meibomian gland dysfunction (MGD), the tear film is unstable, resulting in ocular surface damage, including punctate keratitis, persistent epithelial defects, corneal ulceration, perforation and angiogenesis.Citation8–10 The pathophysiological process of ocular GVHD involves the participation of various cytokines. Previous studies have revealed significant differences in the levels of several tear cytokines between ocular GVHD patients and healthy individuals, dry eye patients and non-GVHD patients, which is expected to assist the screening and diagnosis of GVHD.Citation11–13 However, no study has been conducted to explore the correlation between inflammatory factors and epithelial injury in patients with GVHD.
Epithelial defect is a common lesion associated with GVHD. A previous study revealed that the prevalence of persistent epithelial defects (PEDs) in patients with chronic ocular GVHD was 8.1%.Citation14 In vivo confocal microscopy (IVCM) is a noninvasive examination tool that can be used to evaluate corneal epithelial morphological changes and defect depths. Comparing the central corneal images of GVHD patients with normal controls using IVCM, it was found that the density of superficial cells, wing cells and basal cells of the corneal epithelium was significantly decreased in patients with ocular GVHD.Citation15 In addition, changes in epithelial cells in patients with GVHD are similar to those in patients with dry eye, but more severe. Enlarged, hyperreflective epithelial cells in patients with ocular GVHD were observed in the IVCM images.Citation15 Pathological sections of the GVHD animal model showed atrophy of the corneal epithelium and vacuolization of epithelial cells in the basal layer and wing cell layer.Citation16 However, besides describing morphological changes and calculating epithelial cell density, there is a lack of clinical research that has comprehensively evaluated the epithelial changes in ocular GVHD patients.
Therefore, the purpose of this study was to use IVCM to explore the corneal epithelial cell changes in patients with ocular cGVHD after bone marrow transplantation and their correlation with ocular surface parameters and the level of tear inflammatory factors. It is hoped that this study will contribute to monitoring and assessing the severity of cGVHD and further elucidate the factors related to epithelial changes in patients with ocular cGVHD.
Material and methods
Patients
This study was a single-center, case-control study involving 35 eyes of 21 patients with oGVHD and 12 eyes of 8 patients without oGVHD (non-oGVHD) after bone marrow transplantation. The participants were recruited from the Cornea and Ocular Surface Disease Specialist Clinic.
Ocular chronic GVHD was diagnosed by several experts from Peking University Third Hospital based on the 2014 National Institutes of Health (NIH) Consensus Criteria,Citation2 the 2013 International Chronic Ocular GVHD Group ConsensusCitation17 and rich clinical experience.
Patients who met the following criteria were included in the experimental group: (1) diagnosed with ocular cGVHD; (2) first visit to the ophthalmology clinic; and (3) had not received topical immunosuppressant treatment. The inclusion criteria for the control group were as follows: (1) had undergone allogeneic HSCT; (2) no ocular rejection occurred; and (3) not diagnosed with ocular GVHD. Patients presenting with the following were excluded: (1) had ocular surgery in the past six months; (2) had other ocular diseases (autoimmune diseases, glaucoma, infection, retinopathy, allergy, cataract, ocular injury and so on); (3) were unable to undergo an IVCM examination because of poor physical and/or mental states; and (4) had incomplete medical records.
This study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee. The study complied with the Health Insurance Portability and Accountability Act of 1996 and adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Clinical examination
The clinical examination process was essentially the same as that described previously.Citation11 First, the patients’ demographic data, type of primary hematological disease, HLA matching, other organ rejections, and previous treatment were collected. The OSDI (Ocular Surface Disease Index) questionnaire was used to assess patients’ symptoms. In addition, the following data were collected for objective ocular examination: best visual acuity, intraocular pressure, corneal fluorescein staining (CFS) score, fluorescein tear film break-up time (T-BUT), and Schirmer’s test score without anesthesia.
The environment was controlled when samples and measurements were taken. Clinical examination was performed and tear samples were collected every Wednesday afternoon in the same consulting room, where the ambient temperature was controlled at 16–28 degrees centigrade, the humidity was controlled at 30–60%, and the light source is indoor and constant.
Tear collection and analysis
After obtaining the patient’s consent, 30 ml of sterile saline was instilled into the conjunctival sac of each eye, and a total of 15 ml tears were subsequently collected at the medial and lateral canthus with a capillary tear collector. The samples were then immediately stored at −80°C for later use. The concentrations of the following nine cytokines in the tears were examined: IL-6, IL-10, TNF-a, EGF, MMP-2, MMP-3, MMP-7, BAFF and APRIL. Microsphere-based immunoassay analysis was used to measure the cytokine concentrations.Citation11,Citation18,Citation19
In vivo confocal microscopy
The central cornea of all patients was scanned using in vivo confocal laser scanning microscopy (Heidelberg Retina Tomograph III [RCM/HRT III]; Heidelberg Engineering GmbH). The examination steps were as follows: First, the examiners applied an anesthetic to the patient’s ocular surface twice. The ophthalmic gel was dropped onto the surface of a corneal microscope objective, and a sterile corneal contact cap was then placed. The patient placed his jaw and forehead into the bracket and fixed his gaze at a point. The examiner adjusted the position of the laser scanning camera so that the laser beam was in the corneal lesion area. The camera was slowly advanced forward so that the contact cap was in slight contact with the central cornea. Corneal images at different depths were obtained by adjusting the focal plane. Each collected image was 400 × 400 μm in size.
Image analysis
Images from the epithelial layer of the patients’ cornea were selected for analysis. The two most representative images of the wing and basal cell layers of the corneal epithelium were selected for the quantitative analysis of each eye. ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) was used to count the number of cells, measure the area of the pictures and calculate the density of the wing cells and basal cells. The rectangular tool was used to intercept the clearest section of the cell in each picture for counting. The area of the region ranged from 17000 μm2 to 22000 μm2, and more than 100 cells were counted per graph. All intact epithelial cells and truncated cells at the top and right borders of the selected region were counted.
Based on the previous in vivo confocal microscopic scoring system established by Pauly et al,Citation20,Citation21 a corneal epithelial scoring system was established to comprehensively evaluate the changes in the corneal epithelium (). For the construction and specific operation of the scoring system, please refer to.Citation20 Briefly, the corneal epithelium were divided into three layers, the superficial, wing and basal cell layers, and each layer was observed and scored. The experimenter observed IVCM images of the epithelium to determine whether the epithelium was defective and whether the cells could be counted and subsequently chose the corresponding level of the presence (P) parameter. Then, it was determined whether the shape/size (S/S), reflectivity (R), and inflammation (I) parameters were abnormal. The clinical signs corresponding to the abnormal types of S/S, R and I are shown in . Finally, all the parameters of all three layers were combined to obtain the total score (max 42).
Table 1. IVCM scoring system for the evaluation of corneal epithelium.
Sample size calculation and statistical analysis
Before the experiment, we calculated the required sample size based on previous literature to have enough statistical power. Since non-oGVHD patients after bone marrow transplantation were relatively difficult to be enrolled, we adopted a 3:1 enrollment method between experimental group (N1) and control group (N2). According to the data reported by Ahmad Kheirkhah et alCitation22 (CFS, 7.1 ± 2.4 in GVHD group and 5.4 ± 1.8 in DED group), the calculated sample size was N1 = 26, N2 = 8 (α = 0.05, power (1-β) = 0.9). According to the data reported by Jingliang He et alCitation23 (CFS, 5.17 ± 2.29 in GVHD group and 0.20 ± 0.63 in non-GVHD group), the calculated sample size was N1 = 6, N2 = 2 (α = 0.05, power (1-β) = 0.9). According to the data reported by Tudor C. Tepelus et alCitation15 (basal cell density, 7851 ± 724 cells/mm2 in oGVHD group and 8570 ± 913 cells/mm2 in DED group), the calculated sample size was N1 = 35, N2 = 11 (α = 0.05, power (1-β) = 0.8). Finally, 35 eyes were included in the experimental group and 12 eyes were included in the control group.
Statistical analysis was performed using SPSS for Windows version 24 (SPSS, Inc., Chicago, IL, USA). The χ2 test was used for the comparison of qualitative variables. The Shapiro–Wilk test was conducted to determine whether the continuous variables were normally distributed. Normally distributed variables are expressed as the mean ± standard deviation (SD), nonnormally distributed variables are expressed as the median ± interquartile range, and categorical variables are expressed as frequencies and percentages. For normally distributed variables, a t test was used, and for nonnormally distributed variables, the Mann–Whitney U test was used to compare whether there were significant differences between the two groups. In addition, Spearman’s rank-order correlation test was conducted to verify the correlations between variables. p values were two-sided, and less than 0.05 was considered statistically significant.
Results
Demographic details and clinical characteristics of the study subjects
Thirty-five eyes from 21 chronic oGVHD patients (19 males and 16 females) and twelve eyes from 8 non-oGVHD patients after HSCT (9 males and 3 females) were included in this study. The demographic details of the patients in both groups are shown in . All patients had received allogeneic hematopoietic stem cell transplantation. There were no significant differences in age (p = .282), sex (p = .207), donor sex (p = .190), HLA matching (p = .195), topical eye drops or systemic drugs used in the past month (p > .05) between the oGVHD and non-oGVHD groups. The primary hematological diseases of the oGVHD group were acute myeloid leukemia (AML, n = 8), acute lymphocytic leukemia (ALL, n = 6), lymphoma (n = 1), plasma cell leukemia (PCL, n = 1), myelodysplastic syndrome (MDS, n = 3) and aplastic anemia (AA, n = 2), and those in the non-oGVHD group were AML (n = 2), ALL (n = 2), chronic myeloid leukemia (CML, n = 1), acute lymphoblastic transformation of CML (n = 1) and MDS (n = 2).
Table 2. Demographic details of the study subjects.
The patients’ clinical characteristics and details of the tear analysis in both groups are shown in [median ± interquartile range]. There was no significant difference in visual acuity [(Log MAR), p = .595], intraocular pressure [mmHg, (p = .864)] or OSDI scores (p = .415) between the two groups. The CFS scores in the oGVHD group were significantly higher than those in the non-oGVHD group (p < .001***). In contrast, the opposite was observed in the tear film break-up time [BUT, secs, (p = .031*)] and Schirmer tear secretion scores [mm, (p < .001***)] between the two groups. In addition, nine cytokines were analyzedL IL-6, IL-10, TNF-a, EGF, MMP2, MMP3, MMP7, BAFF and APRIL. The results showed that EGF (p = .001**), MMP7 (p = .032*) and APRIL (p = .002**) levels were significantly different between the two groups. Detailed information on the levels of tear cytokines is shown in .
Table 3. Clinical characteristics of the study subjects.
Details of confocal analysis and the relationship between clinical and IVCM parameters
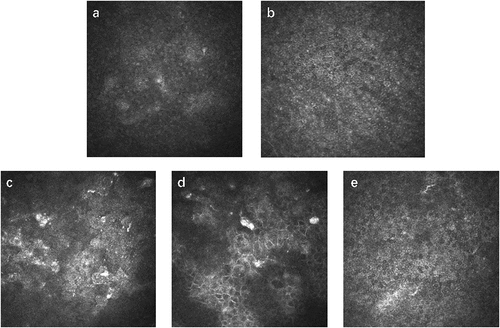
shows representative IVCM pictures of ocular cGVHD patients’ corneal epithelia. Epithelial defects, morphological changes, abnormal reflectivity or inflammatory cell infiltration were observed in the corneal epithelium of some patients.
Figure 1. Representative IVCM images of superficial, wing and basal corneal epithelial layers in two ocular cGVHD patients. a Wing epithelium of patient 1 with fuzzy cell boundaries and enlarged, hyperreflective nuclei. b Basal epithelium of patient 1 with fuzzy cell boundaries and visible, hyperreflective nuclei. c Superficial epithelium of patient 2 showing enhanced desquamation with invisible nuclei. d Wing epithelium of patient 2 with enlarged and irregularly shaped cells. e Basal epithelium of patient 2 with mostly normal cells.
The results of the analysis of the corneal epithelial score and cellular density are shown in . In vivo confocal microscopy showed that there was a significant difference in the corneal epithelial score (p = .001**) and the basal cell density (p = .049*) between the two groups. However, epithelial cell density in the wing cell layer (p = .804) did not differ between the two groups. The wing cell density and the basal cell density were 4973.86 ± 1106.72 cells/mm2 and 6416.89 ± 1478.93 cells/mm2 in the oGVHD group and 5066.55 ± 1129.66 cells/mm2 and 7734.29 ± 1607.35 cells/mm2 in the non-oGVHD group, respectively [median ± interquartile range].
Table 4. Results of the IVCM analysis.
The correlations between ocular surface parameters and the confocal analysis are shown in . The corneal epithelial scores significantly correlated with corneal fluorescein staining scores (p = .001**, r = 0.463), central corneal fluorescein staining scores (p < .001***, r = 0.491) and Schirmer tear secretion scores (p = .009**, r = −0.389). We also analyzed the correlation between the corneal epithelial score and tear cytokines and found that the corneal epithelial score was significantly correlated with EGF (p < .001***, r = −0.491) and APRIL (p = .030*, r = −0.318).
Table 5. Correlations between ocular surface parameters, tear cytokines and confocal analysis.
Discussion
Our study recruited 21 patients (35 eyes) with chronic ocular GVHD and 8 patients (12 eyes) without ocular GVHD after HSCT. The patients underwent clinical examination, tear collection for cytokine analysis and in vivo confocal microscopy. We evaluated the corneal epithelium of the two groups through IVCM and explored the correlations between these data and clinical observations and tear cytokine levels, aiming to develop methods for assessing the severity and treatment of GVHD and to explore the relative factors of corneal epithelial defects and morphological changes in patients with chronic oGVHD.
Morphological changes of epithelial cells under IVCM
IVCM is an important noninvasive tool for observing the morphology of the corneal layer.Citation24 Under an in vivo confocal microscope, the three layers of the corneal epithelia are clearly visible, from shallow areas to deep areas. Normal superficial cells are large and polygonal with a bright cytoplasm, small, centered, highly reflective nuclei and perinuclear dark halos. Wing cells, which form the middle layers of epithelia, are uniformly polygonal, closely arranged and smaller than superficial cells, with highly reflective cell boundaries, low reflective cell bodies, and nuclei that are generally difficult to see. Basal cells are light and dark polygonal cells with the smallest cell bodies, closely arranged, highly reflective cell boundaries and invisible nuclei.Citation25
Profound changes were observed in the partial corneal epithelium of oGVHD, consistent with the findings of previous studies.Citation15 The superficial epithelial cells showed fuzzy cell boundaries and invisible nuclei. Wing cells and basal cells exhibited morphological changes, nuclear hyperreflexia and inflammatory infiltration (). These microstructure changes were similar to those in severe DED patientsCitation15 and may be related to tear film hyperosmolality, enhanced tear evaporation, ocular surface inflammation and ocular surface homeostasis disorder.Citation26
Changes in epithelial cell density under IVCM
Previous researchers have used IVCM to study corneal epithelial cell density, sub-basal nerves, globular immune cells (GICs) and dendritic cells (DCs) in patients with oGVHD.Citation15,Citation22,Citation23,Citation27 They found that the density of corneal epithelium in patients with ocular GVHD was significantly lower than that in normal subjects but similar to that in dry eye patients with the same disease severity and patients with hematological malignancies without cGVHD.Citation15,Citation22,Citation27
By observing IVCM images, researchers counted the cell density of the wing and basal epithelial layer and performed a statistical analysis. We demonstrated that the density of wing cell layers was not significantly different between the two groups, but the density of basal cell layers in patients with ocular cGVHD was significantly lower than that in patients without GVHD after HSCT. This result shows that the density of basal cells is decreased in patients with ocular GVHD, which is consistent with the relatively more serious clinical examination indexes of cGVHD patients.
Corneal epithelial score
An analysis of the existing literature shows that most researchers have focused on describing epithelial images and calculating cell density; there is a lack of indicators for the comprehensive evaluation of epithelia and epithelial defects. In this study, the corneal epithelial score was used to evaluate epithelial defects and cell morphological changes for the first time. Our study referred to the corneal evaluation system established in the previous literatureCitation20,Citation21 and made some modifications to obtain an epithelial evaluation system suitable for GVHD patients. Compared with simply counting epithelial cell density, this method can more comprehensively quantify the changes in epithelial cells. In addition, based on this quantitative parameter, this study permitted analysis of the degree of epithelial changes and highlighted the relationship between clinical indicators and morphological changes in a statistically significant manner.
Our results revealed a significant difference in the corneal epithelial score between the experimental group and the control group, indicating that epithelial defects and morphological changes are more serious in patients with ocular GVHD.
The relationship between clinical parameters and IVCM corneal epithelial score
The corneal epithelial scores were compared with the clinical examination data, and the results showed that the corneal epithelial score was significantly correlated with the corneal fluorescence staining score and Schirmer tear secretion score.
Clinically, we typically evaluate the degree of epithelial defects through CFS; however, this method can only reveal whether there is an epithelial defect and the area of the defect and cannot demonstrate the depth of the defect and cell microscopic changes. Moreover, in most cases, a large area of shallow defects can still quickly restore transparency after treatment, which has little impact on patient prognosis. The depth of infiltration of the defect lesions, on the other hand, substantially impacts prognosis. When the defect reaches a certain depth or causes a certain level of damage to the deep epithelium, healing is challenging, and corneal cloudiness that affects vision can be present. Our results revealed a significant correlation between the epithelial score and corneal fluorescence staining, suggesting that the depth of epithelial defects can be roughly understood from the corneal fluorescein staining score. This result shows that the histological changes in the corneal epithelium obtained by CFS can reflect the degree of cytological changes in the corneal epithelium. It would be more convenient for clinicians to evaluate the severity and prognosis of epithelial lesions for ocular GVHD patients and is of clinical significance for primary hospitals without IVCM.
In addition, the finding that the corneal epithelial score is significantly correlated with the Schirmer tear secretion score indicates that epithelial changes in patients with ocular GVHD may be related to lacrimal deficiency.
The relationship between tear cytokines and IVCM corneal epithelial score
Tear cytokines related to GVHD and the corneal epithelia were selected for detection and analysis, including IL-6, IL-10, TNF-a, EGF, MMP-2, MMP-3, MMP-7, BAFF and APRIL. IL-6, IL-10 and TNF-a are all tear cytokines that have been mentioned in the previous literature as having diagnostic ability for chronic GVHD and are correlated with ocular surface parameters.Citation11–13,Citation28 EGF and MMPs are tear cytokines closely related to corneal epithelium.Citation29,Citation30 APRIL and BAFF are both important regulators of B cell survival and proliferation.Citation31,Citation32 There have been few reports concerning changes of APRIL and BAFF in tears of patients with chronic oGVHD.
The results showed that EGF, APRIL and MMP-7 were significantly decreased in ocular cGVHD patients compared to in non-cGVHD patients after HSCT, and EGF (r = −0.491, p < .001) and APRIL (r = −0.318, p = .030) were significantly correlated with the corneal epithelial score.
EGF (epidermal growth factor) is mainly secreted by the lacrimal gland.Citation33,Citation34 Studies have shown that the lacrimal gland is damaged and tear secretion is reduced in patients with GVHD.Citation35 This could explain why the level of tear EGF in patients with chronic oGVHD decreased significantly compared with patients without oGVHD after HSCT. In addition, EGF has been shown to stimulate the proliferation and migration of corneal epithelial cells, thus playing important roles in promoting corneal epithelial self-renewal and wound healing.Citation29,Citation36–41 Our study revealed that EGF levels were significantly negatively correlated with the corneal epithelial score. Therefore, we speculate that the significant decrease in EGF levels in tears is related to corneal epithelial defects and morphological changes in patients with chronic oGVHD. The decrease in EGF levels might slow the repair of epithelial cells, resulting in more serious corneal epithelial changes in patients with oGVHD. Exogenous EGF supplementation may be helpful for the treatment of epithelial defects in chronic oGVHD patients.
APRIL (a proliferation-inducing ligand), belonging to the TNF (tumor necrosis factor) family, is an important regulator of B cell survival and proliferation.Citation31,Citation32,Citation42 It is mainly secreted by immune cells, such as monocytes, neutrophils, dendritic cells, macrophages and T cells.Citation43–45 Previous studies mostly focused on its role in cancer and autoimmune diseases such as systemic lupus erythematosus and Sjögren’s syndrome.Citation32,Citation46,Citation47 Several studies have shown that B cells also take part in the pathogenesis of acute and chronic GVHDs.Citation48,Citation49 One study revealed a strong correlation between acute GVHD and the serum concentration of APRIL, which further suggested that B cells are involved in acute GVHD and that the serum APRIL concentration may be an important predictor of the severity of acute GVHD.Citation50 However, there have been few reports concerning changes in APRIL in the tears of patients with chronic GVHD. Recently, our research group discovered for the first time that APRIL levels in tears was lower in patients with chronic oGVHD than DED patients. Besides, APRIL/BAFF shows satisfactory diagnostic capabilities.Citation51 This study improve that the level of APRIL in tears of ocular cGVHD patients is significantly lower than that in the tears of non-GVHD patients. It also revealed that the corneal epithelial score was significantly correlated with APRIL levels. Thus, we speculate that APRIL is a protective factor that might play a role in inhibiting the local immune response or epithelial repair. Further studies should be performed to explore the role of APRIL and B cells in ocular GVHD.
MMPs (matrix metalloproteinases) are enzymes that degrade the structural components of the ECM (extracellular matrix).Citation52 In the cornea, MMPs are involved in epithelial wound healing and interstitial remodeling.Citation30,Citation53,Citation54 Interestingly, our study revealed that the levels of EGF and MMP-7, tear inflammatory factors that are upregulated in corneal wound healing, were significantly lower in patients with ocular cGVHD than in non-GVHD patients after HSCT, suggesting that patients with cGVHD might have adverse reactions to injury, which might affect the healing of epithelial defects.
In addition, our study revealed that there is no significant difference in specific inflammatory factors, such as IL-6 and IL-10, in ocular cGVHD patients compared with the control group. Based on our overall results, we speculate that the cause of epithelial damage is not an acute specific inflammatory reaction but lacrimal gland damage, which leads to tear deficiency, resulting in nonspecific inflammation caused by ocular surface dryness and poor repair of epithelial defects caused by the decrease in EGF levels in tears. However, neither this nor previous studies could confirm whether the changes in tear inflammatory factors and corneal epithelia were due to the GVH disease process itself or to a consequence of dry eye, which requires further and more rigorous experiments to explore.
This study was limited by the relatively small sample size. Relatively few patients experience GVHD, and even fewer met our inclusion and exclusion criteria. Further studies with a larger sample size are needed to verify our results. Besides, we didn’t test all the inflammatory factors related to chronic oGVHD because of our limited funds and the difficulty in purchasing some antibodies due to the outbreak of COVID-19.
Conclusion
In summary, using the IVCM corneal epithelial score, our study revealed that cGVHD patients have more severe epithelial changes than non-GVHD patients after HSCT. In addition, the corneal epithelial score was correlated with the corneal fluorescence staining score, Schirmer tear secretion score and level of tear cytokines EGF and APRIL. These results suggest that the depth of corneal epithelial defects can be estimated by the corneal fluorescence staining score. In addition, the corneal epithelial changes in ocular cGVHD patients are presumed to be related to the lack of tear and repair factors rather than a specific inflammatory response.
Author contributions
Design of the study (SW Liu, RM Peng, J Hong); conduct of the study (SW Liu, RM Peng); collection and management of data (J Ma, Z Shen, BH Hu, YH Zhao); analysis and interpretation of data (SW Liu, RM Peng); writing of manuscript (SW Liu); and review or approval of manuscript (J Hong).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author Jing Hong.
Additional information
Funding
References
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi:10.1053/bbmt.2003.50026.
- Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi:10.1016/j.bbmt.2014.12.001.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi:10.1016/S0140-6736(09)60237-3.
- Franklin RM, Kenyon KR, Tutschka PJ, et al. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983;90(1):4–13. doi:10.1016/S0161-6420(83)34604-2.
- Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol. 2013;58(3):233–251. doi:10.1016/j.survophthal.2012.08.004.
- Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol. 2012;12(5):540–547. doi:10.1097/ACI.0b013e328357b4b9.
- Townley JR, Dana R, Jacobs DS. Keratoconjunctivitis sicca manifestations in ocular graft versus host disease: pathogenesis, presentation, prevention, and treatment. Semin Ophthalmol. 2011;26(4–5):251–260. doi:10.3109/08820538.2011.588663.
- Claes K, Kestelyn P. Ocular manifestations of graft versus host disease following bone marrow transplantation. Bull Soc Belge Ophtalmol. 2000;(277):21–26.
- Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2004;15(6):503–507. doi:10.1097/01.icu.0000143684.22362.46.
- Yeh PT, Hou Y-C, Lin W-C, et al. Recurrent corneal perforation and acute calcareous corneal degeneration in chronic graft-versus-host disease. JFormos Med Assoc. 2006;105(4):334–339. doi:10.1016/S0929-6646(09)60125-X.
- Hu B, Qiu Y, Hong J. Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease(GVHD). Ocul Surf. 2020;18(2):298–304. doi:10.1016/j.jtos.2019.12.005.
- Nair S, Vanathi M, Mahapatra M, et al. Tear inflammatory mediators and protein in eyes of post allogenic hematopoeitic stem cell transplant patients. Ocul Surf. 2018;16(3):352–367. doi:10.1016/j.jtos.2018.04.007.
- Cocho L, Fernández I, Calonge M, et al. Biomarkers in ocular chronic graft versus host disease: tear cytokine- and chemokine-based predictive model. Invest Ophthalmol Vis Sci. 2016;57(2):746–758. doi:10.1167/iovs.15-18615.
- Sinha S, Singh RB, Dohlman TH, et al. Prevalence of persistent corneal epithelial defects in chronic ocular graft-versus-host disease. Am J Ophthalmol. 2020;218:296–303. doi:10.1016/j.ajo.2020.05.035.
- Tepelus TC, Chiu GB, Maram J, et al. Corneal features in ocular graft-versus-host disease by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):2389–2397. doi:10.1007/s00417-017-3759-x.
- Pérez R, Pérez-Simón JA, Caballero-Velazquez T, et al. Limbus damage in ocular graft-versus-host disease. Biol Blood and Marrow Transplant. 2011;17(2):270–273.
- Ogawa Y, Kim SK, Dana R, et al. International chronic ocular Graft-vs-Host-Disease (GVHD) consensus group: proposed diagnostic criteria for chronic GVHD (Part I). Sci Rep. 2013;3:3419. doi:10.1038/srep03419.
- Kellar KL, Iannone MA. Multiplexed microsphere-based flow cytometric assays. Exp Hematol. 2002;30(11):1227–1237. doi:10.1016/S0301-472X(02)00922-0.
- Huang XJ, Liu D-H, Liu K-Y, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38(4):291–297. doi:10.1038/sj.bmt.1705445.
- Pauly A, Labbe A, Baudouin C, et al. In vivo confocal microscopic grading system for standardized corneal evaluation: application to toxic-induced damage in rat. Current Eye Research. 2008;33(10):826–838. doi:10.1080/02713680802381460.
- Pauly A, Brignole-Baudouin F, Labbe´ A, et al. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007;48(12):5473–5483. doi:10.1167/iovs.06-0728.
- Kheirkhah A, Qazi Y, Arnoldner MA, et al. In vivo confocal microscopy in dry eye disease associated with chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2016;57(11):4686–4691. doi:10.1167/iovs.16-20013.
- He J, Ogawa Y, Mukai S, et al. In vivo confocal microscopy evaluation of ocular surface with graft-versus-host disease-related dry eye disease. Sci Rep. 2017;7(1):10720. doi:10.1038/s41598-017-10237-w.
- Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13(5):569–576. doi:10.1097/ACI.0b013e328364ec92.
- Guthoff R, Zhivov A, Stachs O, et al. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Exp Ophthalmol. 2009;37(1):100–117. doi:10.1111/j.1442-9071.2009.02016.x.
- Zhang X, Chen Q, Chen W, et al. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118(5):902–907. doi:10.1016/j.ophtha.2010.08.033.
- Steger B, Speicher L, Philipp W, et al. In vivo confocal microscopic characterisation of the cornea in chronic graft-versus-host disease related severe dry eye disease. Br J Ophthalmol. 2015;99(2):160–165. doi:10.1136/bjophthalmol-2014-305072.
- Jung JW, Han SJ, Song MK, et al. Tear cytokines as biomarkers for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(12):2079–2085. doi:10.1016/j.bbmt.2015.08.020.
- Brazzell RK, Stern ME, Aquavella JV, et al. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci. 1991;32(2):336–340.
- Gordon GM, Austin JS, Sklar AL, et al. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and timps, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J Cell Physiol. 2011;226(6):1461–1470. doi:10.1002/jcp.22306.
- Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756. doi:10.1084/jem.189.11.1747.
- Kimberley FC, Medema JP, Hahne M. April in B-cell malignancies and autoimmunity. Results Probl Cell Differ. 2009;49:161–182.
- Wilson SE, Lloyd SA, He YG. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–1995.
- Wilson SE, He YG, Lloyd SA. EGF, EGF receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:1756–1765.
- Carreno-Galeano JT, Dohlman TH, Kim S, Yin J, Dana, R. A review of ocular graft-versus-host disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflamm. 2021;29:1–10.
- Gospodarowicz D, Mescher AL, Brown KD, et al. The role of fibroblast growth factor and epidermal growth factor in the proliferative response of the corneal and lens epithelium. Exp Eye Res. 1977;25(6):631–649. doi:10.1016/0014-4835(77)90142-7.
- Petroutsos G, Courty J, Guimaraes R, et al. Comparison of the effects of EGF, pFGF and EDGF on corneal epithelium wound healing. Curr Eye Res. 1984;3(4):593–598. doi:10.3109/02713688409003059.
- Wilson SE, He Y-G, Weng J, et al. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59(6):665–678. doi:10.1006/exer.1994.1152.
- Wilson SE. Corneal wound healing. Exp Eye Res. 2020;197:108089. doi:10.1016/j.exer.2020.108089.
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi:10.1016/j.preteyeres.2015.07.002.
- Loureiro RR, Gomes JÁ. Biological modulation of corneal epithelial wound healing. Arq Bras Oftalmol. 2019;82(1):78–84. doi:10.5935/0004-2749.20190016.
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi:10.1038/nri2572.
- Craxton A, Magaletti D, Ryan EJ, et al. Macrophage- and dendritic cell–dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101(11):4464–4471. doi:10.1182/blood-2002-10-3123.
- Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and April. Nat Immunol. 2002;3(9):822–829. doi:10.1038/ni829.
- Hardenberg G, Planelles L, Schwarte C, et al. Specific TLR ligands regulate April secretion by dendritic cells in a PKR-dependent manner. Eur J Immunol. 2007;37(10):2900–2911. doi:10.1002/eji.200737210.
- Maślińska M, Przygodzka M, Kwiatkowska B, et al. Sjögren’s syndrome: still not fully understood disease. Rheumatol Int. 2015;35(2):233–241. doi:10.1007/s00296-014-3072-5.
- Ryan MC, Grewal IS. Targeting of BAFF and April for autoimmunity and oncology. Adv Exp Med Biol. 2009;647:52–63.
- Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93(11):1702–1711. doi:10.3324/haematol.13311.
- Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, et al. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–4927. doi:10.1182/blood-2008-10-161638.
- Kim JS, Kim S-J, Cheong J-W, et al. Clinical significance of B cell-activating factor (BAFF) and a proliferation-inducing ligand (April) in acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Korean J Hematol. 2011;46(3):175–179. doi:10.5045/kjh.2011.46.3.175.
- Shen Z, Ma J, Peng R, et al. Biomarkers in ocular graft-versus-host disease: implications for the involvement of B cells. Transplant Cell Ther. 2022;28(11):749.e1–749.e7. doi:10.1016/j.jtct.2022.07.023.
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi:10.1038/nrm2125.
- Fini ME, Girard MT, Matsubara M. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta Ophthalmol. Suppl 1992;(202):26–33. doi:10.1111/j.1755-3768.1992.tb02165.x.
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290:S12–S23. doi:10.1007/PL00007449.
