Abstract
Background: The optimal age for surgery for infantile esotropia is controversial. Proponents of early surgery believe that further loss of binocular vision can be prevented by early surgery, a minority believes that binocular vision can even be restored by early surgery. The ELISSS compared early with late surgery in a prospective, controlled, non-randomized, multicenter trial. Methods: Fifty-eight clinics recruited children aged 6–18 months for the study. Each clinic operated all eligible children either ‘early’, i.e. at age 6–24 months, or ‘late’, i.e. at age 32–60 months. At baseline the angle of strabismus, refraction, degree of amblyopia and limitation of abduction were assessed. Intermediate examinations took place every six months. Children were evaluated at age six in the presence of independent observers. Primary endpoints were (i) level of binocular vision, (ii) manifest angle of strabismus at distance and (iii) remaining amblyopia. Secondary endpoints were number of operations, vertical strabismus, angle at near and the influence of surgical technique. Results: A total of 231 children were recruited for early and 301 for late surgery. Age at entry examination was 11.1 months (SD 3.7 months) in the early group and 10.9 (SD 3.7) months in the late group. Refraction, amblyopia and limitation of abduction were distributed equally in the early and late groups, but the angle of strabismus was slightly larger in the early group. Dropout-rates were 26.0% in the early and 22.3% in the late group. At age six, 13.5% of the early vs. 3.9% of the late group recognized the Titmus Housefly; 3.0% of the early and 3.9% of the late group had stereopsis beyond Titmus Housefly. No significant difference was found for angle of strabismus. 35.1% of the early group and 34.8% of the late group did not have an angle between 0° and 10°, the thresholds set for re-operation. For ratio of the visual acuities (remaining amblyopia) there was a small but significant advantage for the early group. There was hardly any correlation between the baseline parameters and the primary endpoints. Children scheduled for early surgery had first been operated at 20 (SD 8.4) months, but 8.2% had not been operated at age six. Children scheduled for late surgery had been operated at 49.1 (SD 12.7) months, but 20.1% had not been operated at age six. The number of operations per child was 1.18 (SD 0.67) in the early and 0.99 (SD 0.64) in the late group. Age at recruitment, age that strabismus reportedly had started and refraction at entry examination were similar among operated and non-operated children. Only the angle of strabismus at entry predicted, to some extent, whether a child had been operated at age six. Discussion: Children operated early had better gross stereopsis at age six as compared to children operated late. They had been operated more frequently, however, and a substantial number of children in both groups had not been operated at all.
INTRODUCTION
Congenital or infantile esotropia has been defined as esotropia with an onset before the age of six months, with a constant, large angle of strabismus (> 30 PD), no or mild amblyopia, small to moderate hyperopia, latent nystagmus, dissociated vertical deviation, limitation of abduction and absent or reduced binocular vision, in the absence of nervous system disorders ([Costenbader, Citation1961]; [Von Noorden, Citation1988]). Costenbader already found, however, that “infantile esotropes encompass the range of visual acuity, refractive errors and initial deviations, and do not adhere to a constant pattern”, a view confirmed recently by two prospective studies ([Meyer et al., Citation1998]; [Pediatric Eye Disease Investigator Group, Citation2002a]) reporting on baseline findings in their study cohorts.
Infantile esotropia is generally treated by surgery to align the eyes cosmetically and to minimize further loss of the remaining binocular vision. The optimal age for surgery is controversial. There are no consensus treatment guidelines, nor any randomized controlled trials that address this controversy ([Elliott & Shafiq, Citation2005]). In 1903, Worth had stated that the cause of strabismus was a retarded, insufficient or absent development of the ability to fuse the images of both eyes. The controversy about the optimal age for surgery started when, in 1939, Chavasse said that most infants with congenital squint were capable of developing fusion if the deviation could be fully corrected before the age of two years. Proponents of early surgery believe that further loss of binocular vision can be prevented by early surgery. A minority believes that binocular vision can even be restored by early surgery. The latter view implies that the abnormal position of the eyes is the prime culprit.
In detailed analyses of 256 children ([Berke, Citation1958]) and 500 children ([Costenbader, Citation1961]) with infantile esotropia, factors that influence cure were defined. Costenbader thought that the size and nature (intermittent or constant) of the initial deviation, the initial presence of hypertropia and the degree of amblyopia were factors with a negative influence on the chance of a functional cure. He thought that the age at first alignment, the initial refractive error and age at first presentation were less important. Costenbader disagreed with Berke, who had said that onset at birth and a long duration of the strabismus carried a worse prognosis. Children operated once reached “alignment and fusion” in 38.4%, children operated twice in 36% of cases. Overall, 12.6% attained Titmus Housefly positive, 2.8% had refined stereopsis. Ahead of his time, Costenbader included, in his analysis, 80 children who were not operated at all, conforming to the “intention-to-treat” principle. These had “alignment and fusion” in 76% of cases.
Citation[Hubel and Wiesel (1965)] were pessimistic about restoring binocular vision by strabismus surgery: “Perfect binocular fixation presumably depends also upon a normal set of neural connections in the visual pathway, possibly the very connections concerned with binocular interaction that are lost in these experiments. In that case even a perfect mechanical repair would not guarantee the realignment necessary to promote recovery of binocular vision.” In macaques it was shown recently that the period of delay in the correction of strabismus determines the severity of the defect in binocular vision ([Wong et al., Citation2003]). When comparing human with animal studies it seems important to realize, however, that these animals are normal to begin with. Infantile esotropia may well be a collection of causes with different causes. Some of these disorders may be amenable to early surgery to preserve binocular vision, whereas others, like neurological impairment ([Charles & Moore, Citation1992]), are not.
There has been no shortage of clinical studies advocating early surgery. In 1966, Ing reported on a series of 50 children with infantile esotropia selected from the practices of Costenbader, Parks and Albert. They had been operated before one-and-a-half years of age. Many had 100″, 400″ or 1000″ stereopsis on follow-up. They had been operated 1.8 times per child. In 1983, Ing reported on a series of 106 children operated upon and successfully aligned before the age of two years; 93% had Worth 4-dot fusion or gross stereopsis. This was reached by only 31% of the children aligned after this age. In 1995, Ing reported on 16 children from the practices of Lang, Von Noorden and others who had been operated at an average age of 4.2 months, all with a minimum of 4 years' follow-up, and concluded that binocularity remains a rare outcome of treatment for infantile esotropia. Citation[Wright et al. (1994)] reported on seven patients who had been operated between 13 and 19 weeks of age. In five, stereopsis could be demonstrated, three of whom had random dot stereopsis. None of these studies had a control group with late surgery, however, and it seems possible that the strabismus could have resolved spontaneously in some of the cases.
Spontaneous resolution of infantile strabismus occurs frequently ([Clarke & Noel, Citation1982]). In the Pediatric Eye Disease Investigator Group study Citation[(2002b)], 170 patients were recruited at a mean age of three months. In 2.4% of the children recruited with an angle of at least 40 prism diopters (PD) during by two examinations, the angle had reduced spontaneously to less than 8 PD at the age of seven months. This was 27% among children recruited with an angle of at least 20 PD at two examinations. The difficulty of establishing the diagnosis of strabismus in the first two months of life has been emphasized by Citation[Horwood & Williams (2001)] who related visual outcome in 1150 infants to occurrence of an ocular misalignment (neonatal squint) in the first 8 weeks of life. Frequent squinting in the neonatal period trebled the chances of developing a significant esodeviation or refractive error severe enough to require spectacles before 5 years of age, but the incidence of the abnormality still did not exceed 9%.
The first prospective study ([Birch et al., Citation1990]) of surgery for infantile esotropia reported 35% gross random dot stereopsis (disparity equal or better than 400″) among 84 children first operated at 8.5 months of age, on average. Sixty-three were aligned within 10 PD. The average number of operations was 1.5. Three had full stereopsis, but these three were not operated. In another study ([Birch et al., Citation1995]), children were divided into three groups according to the age at which alignment within 8 PD had been reached. Children who did not reach alignment within 8 PD were excluded. In the group that reached alignment at 5–8 months of age, stereopsis better than 200″ was reached by 18.8%, at 9–12 months by 26.4% and at 13–16 months by none. However, in the third group, five of the 22 children had been operated twice and some were operated later because of longer amblyopia treatment. These two circumstances are both intrinsically related to a worse prognosis.
Although emphasis in the debate is on prevention of further loss or restoration of binocular vision, other arguments have been used. It has been said that the parent-child relation is disturbed by the cosmetic appearance of the child. The psychomotor development of the child is said to be better when the eyes are straight. An operation at age three would constitute a greater emotional trauma. Proponents of late surgery say that the correction of the angle of strabismus can be more precise at age three because the angle of strabismus, angles in up- and downshoot in adduction, and V- and A-pattern can be measured more accurately. The number of operations per child would be less. The treatment of amblyopia is said to be easier, because alternating use of both eyes is more easily assessed with a large angle. Finally, some say it is more difficult to convince parents to patch, when their child has a small, inconspicuous angle of strabismus.
To answer these questions, the ELISSS investigated whether early surgery (age at surgery 6–24 months) or late surgery (age at surgery 32–60 months) for infantile convergent strabismus is preferable.
METHODS
Study Design
The investigational plan was based on the study protocol (ELISSS group, 1993b), including the Instructions for the Final Examination dated 15 November 1998, the Amendment dated 1 December 1998 and the decisions made on the investigators' meeting in Heidelberg on 9 November 2002 which are laid down in the Statistical Analysis Plan dated 25 February 2003. Monitoring reports were published in 1994, 1995, 1996 and 1997, whereas in 1998 a report on the baseline characteristics of the study populations was published ([Meyer et al., Citation1998]). The ELISSS was a non-randomized, prospective, multicenter clinical trial carried out in 58 clinics in 11 European countries. It had been planned to enroll 380 patients in each of the two treatment groups.
During the planning phase of the study, the Study Committee concluded that randomization was not feasible because the treatment modalities of the two groups are extremely dissimilar: one would first have had to inform the patient's parents of the possibility of surgery next week, only to postpone surgery for three years if the randomization procedure prescribed late surgery. Even if some very considerate parents had cooperated, the referring ophthalmologists would have been hesitant to refer patients. A study design was therefore chosen whereby each participating clinic decided on its respective schedule of operation (early or late surgery) in advance, for the entire duration of the recruitment period. Patients were assigned to a treatment group according to the clinic in which they were first seen.
After a standardized entry examination at age 6–18 months (to be precise: > 6 months and < 18 months of age), patients were examined at six-month intervals. Patients in clinics operating early were operated at age 6–24 months, patients in clinics operating late at age 32–60 months. Approximately one month after surgery, the patient was examined in a standardized fashion. From this examination onwards, the intermediate examinations at six-month intervals were resumed. Guidelines were given for treatment of amblyopia, for the goals to be achieved by early surgery and for those to be achieved by late surgery. The final examination was to be performed at age six, and at least one year after a last re-operation. In order to reduce observation bias at the final examination, children were examined in a standardized fashion and in the presence of an independent observer.
Included were all children with infantile, convergent strabismus who first presented to the participating clinic between 6 and 18 months of age. Exclusion criteria were: onset at five months of age or later, pre- and dysmaturity, congenital nystagmus, neurological deficit, angle < 5° or > 30°, divergent, retardation, dysmorphia or motility disorders other than up- or downshoot in adduction, V- or A-patterns or limitation of abduction.
Study Endpoints
The three primary endpoints of the study concerned binocular vision, angle of strabismus, and visual acuity. The testing procedure for the three primary endpoints was described in the Instructions for the Final Examination and in the Statistical Analysis Plan.
For level of binocular vision, an ordinal variable in seven levels was defined: (1) Bagolini negative, (2) Bagolini positive, (3) both Bagolini and Housefly positive, (4) Titmus circles 200″ to 140″ recognized, (5) Titmus circles 100″ to 40″ recognized, (6) either all figures of the Lang Test or all those of TNO plate V (480″ to 240″) recognized and (7) at least two figures of TNO plate VI or VII (120″ to 15″) recognized.
For the manifest horizontal angle of strabismus, an ordinal variable in seven levels was defined: (1) angle < −4° or ≥ 15° (very bad result), (2) angle ≥ −4° and < 0° or ≥ 10° and < 15° (bad), (3) angle ≥ 6° and < 10° (moderately good), (4) angle ≥ 3° and < 6° (good), (5) angle ≥ 0° and < 3° (very good). In an exploratory fashion, the size of the angle was analyzed as a continuous variable.
As a measure of the degree of amblyopia that remained after treatment, the visual acuity of the worse eye relative to that of the better eye (ratio of the visual acuities) at the final examination was taken. The reason for primary analysis of the ratio of the visual acuities, and not of the visual acuity of the worse eye itself, was that differences in luminance, contrast and distance to the E-chart could easily occur in the 58 clinics where the final examinations took place, and the ratio would be less affected by these differences. In an exploratory fashion, the visual acuity of the worse eye was analyzed as a continuous variable.
Secondary endpoints were: number of operations, remaining vertical strabismus, manifest angle of strabismus during fixation at near with full corrected glasses in primary position, measured with unilateral cover and prisms, and differences between the results of primary endpoints caused by using different surgical techniques.
According to the principle “intention-to-treat”, all patients were to be analyzed in the treatment group that they were included in at the start of the study, determined by the participating clinic where they were first seen. This may seem strange, especially for the children who, in the end, were not operated at all, but the moment of inclusion at the start of the study resembles most the point where future ophthalmologists and orthoptists will decide whether to operate early or late, regardless of whether their diagnosis at that point is correct or whether the angle of strabismus will decrease spontaneously over time to the point that surgery becomes unnecessary.
Statistical Analysis
The statistical testing was performed strictly according to an a priori determined order. The hierarchically ordered hypotheses concerned (1) level of binocular vision, (2) angle of strabismus, (3) degree of amblyopia. Each test was to be performed at the level of significance α = 5%. At the first statistical test that could not reject the null hypothesis of no difference between the treatment groups at the level of significance α = 5%, the hierarchical testing procedure would stop. For all hypotheses rejected so far, the global level of significance α = 5% was retained ([Kieser et al., Citation1995]) as long as the strict a priori determined order for testing the null hypotheses (1), (2) and (3) was adhered to.
In the analysis of the secondary endpoints, Fisher's exact test was used for dichotomous data and the t-test (for approximately normally distributed data) or the Mann-Whitney-Wilcoxon-U-test for continuous data. Adjustment for baseline covariates (inclusion of potentially prognostic baseline parameters) was handled as for the primary endpoints. No imputation of missing values in secondary endpoints was done. All p-values calculated were two-sided.
For dealing with dropouts, first those baseline parameters were identified that were prognostic for the primary endpoints, independent of treatment group. Then, dropouts and completers were compared with regard to the identified baseline parameters that were prognostic for outcome. If dropouts and completers would not differ in that regard, primary analysis ignoring dropouts (Mann-Whitney U-test) and sensitivity analyses would be carried out. If they would differ, primary analysis including dropouts as treatment failures and sensitivity analyses would be carried out. This decision was supported by a committee that was not informed at the time about the results for each of the treatment groups, only about the results of the above analyses of baseline parameters.
RESULTS
Recruitment
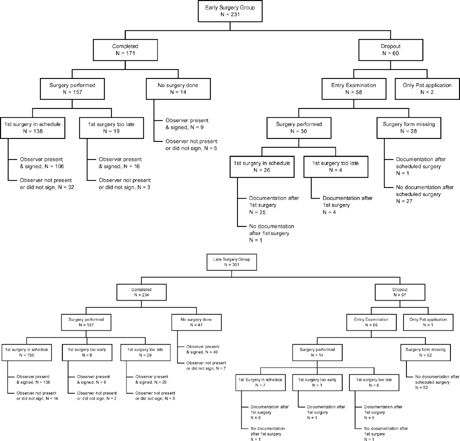
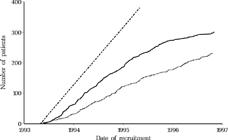
Between 3 May 1993 and 31 October 1996, 532 patients were included into the study, 231 into the early and 301 into the late group ().
FIGURE 1 Patient recruitment over time. Straight interrupted line: target recruitment. Striated line: early group, Continuous line: late group.
An additional 171 patients screened for inclusion in the early group and 271 in the late group were excluded because of age limit (13 early, 10 late), onset after 4 months (33, 93), prematurity (14, 18), congenital nystagmus (11, 38), neurological deficit (35, 66), angle < 5° (7, 18), angle > 30° (33, 39), divergent (3, 8), and other medical reasons like dysmorphia (4, 10), organizational reason such as a language barrier (2, 4), formal reason such as application after surgery (57, 40) and lack of cooperation by the parents (0, 7). In some children, there was only a marginal violation of the exclusion criteria and these children were included: 28 children were recruited in the 5th month of life and seven were between 18 and 19.5 months of age. The angle of strabismus was 8 PD (4.57°) in one patient and 60 PD (30.96°) in 10 patients.
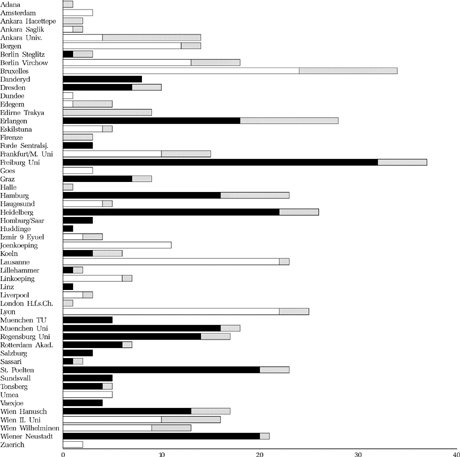
The numbers of patients recruited in the participating clinics are shown in , with the dropout rates. Most participating clinics were university clinics.
FIGURE 2 Number of recruited and dropout children per clinic. White: to be operated early, Black: to be operated late, Striated: dropout.
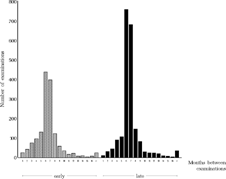
The study protocol prescribed that examinations were to be performed every six months and one month after an operation. The actual intervals are depicted in . To assess the completeness of follow-up examinations, the number of examination and surgery forms per patient was calculated. The total number of case report forms completed for the early group was 11.3 (SD 5.2) per patient, while for the late group it was 11.4 (SD 4.6), including the children who later became dropouts.
FIGURE 3 Intervals between successive examinations of all children who underwent the final examination.
In some cases, the final examination was lacking, although intermediate examinations had taken place. Imputation was used for missing final examinations of those dropouts who were at least five years old at their last regular examination, performed at six-month intervals, but who did not attend the final examination. The data from the regular examination was used instead if the last operation had been performed at least 12 months previously. This applied to nine patients of the early group. Three other patients, two in the late group and one in the early group, had not been operated at all, and they were included with the data obtained at their last regular examination at age five. These 12 patients were included as completers (see patient flow chart, ). Another 12 patients (four in the early group, eight in the late group) who did undergo a final examination had to be counted as dropouts because the time between their last operation and the final examination was 8.5 months or less.
It was decided to perform all final examinations in the presence of an independent external observer in order to reduce assessment bias. The Instructions for the Final Examination prescribed that the final examination forms would be signed by the external observer. In 335 of the 405 final examinations of the completers, the forms were signed by the external observer, giving proof of his presence at the examination (82.7%; 76.0% early surgery group, 87.6% late surgery group). In other cases, like the imputed cases mentioned above, the external observer was not present or did not sign.
Baseline Parameters
In non-randomized trials, the patient selection may differ between treatments. Therefore, it was investigated whether baseline imbalances between treatment groups existed. Baseline parameters that were analyzed for their prognostic relevance for the primary endpoints, and their relationship to dropouts were: sex, age at entry examination, spherical equivalent of the refraction obtained by retinoscopy, horizontal angle of strabismus, degree of amblyopia and limitation of abduction. A pilot study had shown that, among 15 parameters assessed, these parameters could be obtained reliably and reproducibly in a one-year-old child (ELISSS, 1993a). Mean age at entry examination was 11.1 (SD 3.7) months in the early group and 10.9 (SD 3.7) months in the late group. Hence, the age at entry examination was approximately equal, this having consequences for the other parameters that vary with age, like refraction. It was found that the two treatment groups differed only in respect to the size of the angle of strabismus (large angles of strabismus were slightly over-represented in the early group), not in respect to sex, age, refraction, degree of amblyopia or limitation of abduction ([Meyer et al., Citation1998]).
Groups were compared using Fisher's exact test for dichotomous data and the unpaired t-test for approximately normally distributed data or the Mann-Whitney-Wilcoxon U-test for continuous data. For assessment of homogeneity between the treatment groups, descriptive p-values were calculated. Approximate 95% confidence intervals for the difference of proportions (Method 11, pp. 873–890 in Citation[Newcombe (1998)], based on the Wilson Score with continuity correction) and 95% confidence intervals for the differences of means or medians were calculated.
The proportion of male children was 45% in the early group and 51% in the late group (p = 0.190). Spherical equivalents, angle of strabismus, degree of amblyopia and limitation of abduction were compared using the Mann-Whitney-Wilcoxon U-test. For ordered categorical data (degree of amblyopia and limitation of abduction) this test was asymptotically equivalent to the mean score test and led to similar p-values. The horizontal angle of strabismus, measured with prisms and corneal reflexes during fixation of an object with a light at 50 cm or by estimation of the location of the corneal reflexes, differed significantly, with a median of 21.8° (mean 21.2°) in the early and 20.0° (mean 19.1°) in the late group (p < 0.0001, 95% CI for the differences between the medians [1.6; 3.8]). To handle this discrepancy, the following was decided: if, at the conclusion of the study, the angle of strabismus would be identified as a baseline parameter that was prognostic for any of the primary endpoints, then the baseline angle of strabismus would be included as a covariate into the primary analysis.
The angle of strabismus was measured by estimation of the location of the corneal reflexes in 117 children from the early vs. 208 from the late group. Prisms and corneal reflexes were used in 92 vs. 90 children, whereas prisms and covertest were used in 20 vs. 2 children.
In the early group the spherical equivalent, as measured with retinoscopy, had a median value of 2.25 diopters and a mean of 2.46 diopters. In the late group, the median value was 2.00 diopters and the mean 2.19 diopters (p = 0.032).
For degree of amblyopia at baseline, an ordinal variable in five levels had been defined: (1) Cross-fixating or freely alternating were 84 children from the early vs. 82 from the late group. (2) Alternating with a fixation preference were 89 vs. 138 children. (3) Failing to maintain fixation, but with central fixation in fundus, were 42 vs. 65 children. (4) Poorly fixating with eccentric fixation in fundus were 13 vs. 4 children. (5) Poorly fixating with far eccentric fixation in fundus were 1 vs. 7 children (p = 0.141).
For limitation of abduction at baseline, an ordinal variable in four levels had been defined: (1) Freely abducting with pursuit eye movement were 78 children from the early vs. 117 from the late group. (2) Freely abducting with vestibular eye movement were 67 vs. 55 children. (3) Limited abduction but passing midline had 59 vs. 109 children. (4) Not passing midline applied to 25 vs. 19 children (p = 0.753).
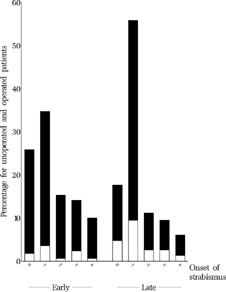
Reported age at onset of strabismus was 1.5 (SD 1.3) months for the early group vs. 1.4 (SD 1.1) months in the late group (p = 0.993, ). Periodic strabismus as opposed to constant strabismus was reported in 25 of 209 children in the early vs. 42 of 277 children in the late group (p = 0.353).
FIGURE 5 Reported age (months) at onset of strabismus for all patients who underwent the final examination, stratified according to whether the children had been operated (black) or not (white) at age six.
Glasses had been prescribed previously in 44 of 230 children in the early group vs. 73 of 300 in the late group (p = 0.170). They had been prescribed on average 0.1 months (range 0-4) months before the entry examination in the early vs. 0.2 months (range 0–8) before the entry examination in the late group. The spherical equivalent of the glasses was 3.0 (SD 1.2) diopters in the early vs. 2.9 (SD 1.9) diopters in the late group.
Occlusion had been prescribed in 81 of 230 children in the early vs. 125 of 299 children in the late group, for a period of 5.7 (SD 3.7) and 5.6 (SD 3.4) months, respectively. Six patients in the early and seven patients in the late group had been prescribed atropine, for 7.7 (SD 2.6) months and 5.3 (SD 2.7) months, respectively.
It had been found in the pilot study (ELISSS Group, 1993a) that latent nystagmus, DVD, torticollis, hypertropia, up- or downshoot in adduction and V- or A-pattern could not be assessed reliably and reproducibly in children under age one. Most of these disorders of ocular motility were found more frequently in the late group ([Meyer et al., Citation1998]), it remains unclear why. We have to be cautious in interpreting the detected differences, however, as the proportions of missing data differ between the two groups, probably because the fields were optional in the examination forms.
Latent nystagmus was reported in 33 of 211 children in the early group and 82 of 246 children in the late group (p < 0.0001). Torticollis was reported in 20 of 190 children in the early group and 48 of 246 children in the late group (p = 0.012). DVD was reported in 15 of 196 children in the early group and 33 of 233 children in the late group (p = 0.045).
Thirteen of 228 children in the early group had a manifest hypertropia of either eye, 9 had an alternating manifest hypertropia, while 38 of 298 children in the late group had a manifest hypertropia of either eye and 9 had an alternating manifest hypertropia. Thirty-six of 228 children in the early group had an upshoot in adduction in right or left gaze, three had a downshoot in adduction, while 89 of 298 children in the late group had an upshoot in adduction in right or left gaze and seven had a downshoot in adduction. V-pattern motility > 5° was found in 7 of 220 children in the early group and 26 of 297 children in the late group. A-pattern motility was found in 1 of 220 children in the early group and 7 of 297 children in the late group.
Surgery
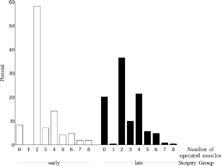
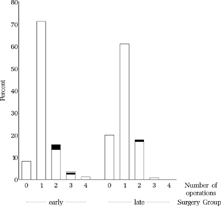
For those 405 patients who completed the study and underwent the final examination, the number of operations performed is shown in . The mean number of operations was 1.18 (SD 0.67) in the early group and 0.99 (SD 0.64) in the late group; 8.2% of the early group and 20.1% of the late group had not been operated at age six.
FIGURE 6 Number of operations. 8.2% from the early group and 20.1% from the late group had not been operated at age six. Among the children operated upon two or three times, only a few were operated upon for consecutive divergence (black), although consecutive divergence occurred frequently (). One child from the early group was operated upon twice for consecutive divergence (striated).
The average number of operated muscles for 403 completers (), including the 61 patients who were not operated at all, was 2.69 (SD 1.64) in the early and 2.54 (SD 1.76) in the late group (p = 0.83).
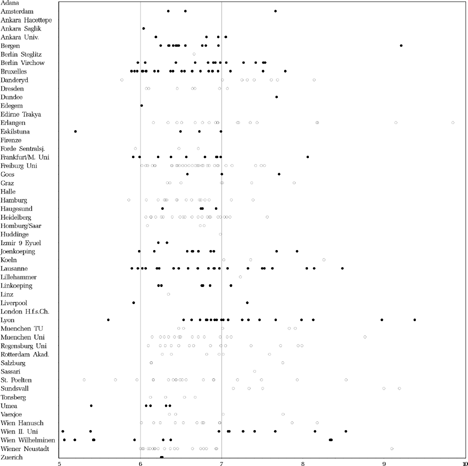
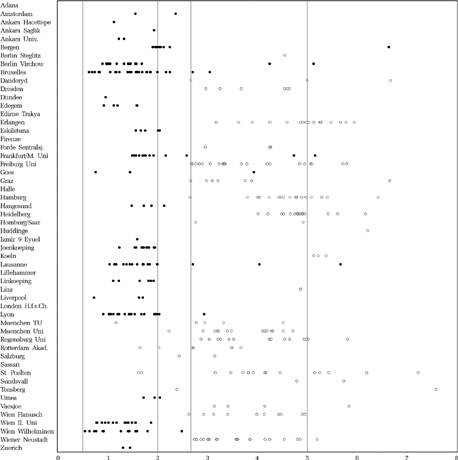
The average age at the first operation was 20.0 (SD 8.4) months in the early and 49.1 (SD 12.7) months in the late group for all 344 patients (157 early and 187 late) who were operated at least once and excluding children who dropped out later. The age at the last operation was 28.1 (SD 19.1) months in the early and 53.5 (SD 12.7) months in the late group for these 344 patients. shows the age at the first operation per clinic and per child. In the following, details of surgical procedures and operated muscles are given for those 342 patients of the 405 completers who were operated and underwent the final examination, but only for all first operations, not for re-operations.
FIGURE 8 Age (years) at first surgery. Dots: scheduled for early surgery, Circles: scheduled for late surgery.
Of the 200 operations performed in the early group, 156 were first operations. Of these 156, 24 were bilateral Faden and six were unilateral Faden operations on the medial rectus muscles. Two of these 30 Faden operations were combined with bilateral and one with unilateral recession of the inferior oblique.
Forty-eight recession-resections were performed; one of these was bilateral, six of these were in combination with a contralateral medial rectus recession and three in combination with a lateral rectus resection. Ten of these 48 were combined with bilateral and four with unilateral recession of the inferior oblique.
Seventy-seven bilateral recessions were performed. Nine of these were combined with bilateral and two with unilateral recessions of the inferior oblique, two with bilateral recessions of the superior rectus and one with recessions of all four oblique muscles. One unilateral lateral rectus resection was performed.
Of the 232 operations performed in the late group, 186 were first operations. Of these 186, 26 were bilateral Faden and 12 were unilateral Faden operations on the medial rectus muscles. Seven of these 38 Faden operations were combined with bilateral and three with unilateral recession of the inferior oblique.
Of the 110 recession-resections that were performed, 15 were in combination with a contralateral medial rectus recession, 18 were combined with bilateral and 12 with unilateral recession of the inferior oblique, six with bilateral recession of the superior oblique and one with unilateral plication of the superior oblique.
Thirty-one bilateral recessions were performed. Ten of these were combined with bilateral recession of the inferior oblique, one with bilateral recession of the superior oblique. Two unilateral medial rectus recessions in combination with bilateral recession of the inferior oblique were performed.
Interestingly, five children in the late group never had surgery on the medial or lateral rectus muscles, although four of these underwent bilateral and one unilateral recession of the inferior oblique. None of these five was re-operated.
The total operative displacement (mm) of the medial and lateral rectus (first and re-operations added together) of all completers who were operated on the medial and/or lateral rectus was 11.4 (SD 4.5) mm for 143 children in the early group and 11.9 (SD 4.5) mm for 167 children in the late group (p = 0.313).
Adverse peroperative advents included a generalized muscle spasm after administration of Trapenal (pain medication), venous hemorrhages during surgery, a conversion of surgical technique from anteroposition to a myectomy, a postoperative infection treated with eyedrops, a suture reaction, a chest infection four days postoperatively, and difficulty opening the eyes after surgery. Only in the case of “venous hemorrhage” did the surgeon think that the problem may have influenced the result of surgery.
The final examinations were performed at age of 6.8 (SD 0.8) years, on average, in the early group and 6.8 (SD 0.7) years in the late group. The interval between the last operation and the final examination was 4.4 (SD 1.5) years in 157 children from the early group and 2.3 (SD 1.1) years in 187 children from the late group.
The final examination took place on schedule (at age six and at least one year after the last operation) in 119 children out of 175 in the early group and in 182 of 242 children in the late group ().
Prognostic Value of the Baseline Parameters Assessed at the Entry Examination
The baseline parameters spherical equivalent, angle of strabismus, degree of amblyopia and limitation of abduction were checked for their assumed prognostic relevance with regard to the primary endpoints. The distribution of these four baseline parameters and of age at the entry examination in both groups is depicted in , , , and .
FIGURE 10 Age (months) at entry examination for all operated (black) and unoperated (white) patients who underwent the final examination. Mean age at entry examination was 11.1 SD 3.7 months in the early group and 10.9 SD 3.7 months in the late group.
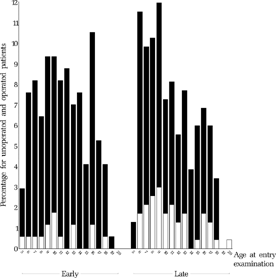
FIGURE 12 Baseline spherical equivalent (averaged and rounded) at approx. 11 months for all operated (black) and unoperated (white) patients who underwent the final examination.
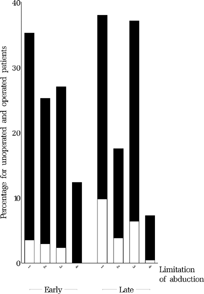
FIGURE 14 Baseline angle of strabismus at approx. 11 months for all operated (black) and unoperated (white) patients who underwent the final examination.
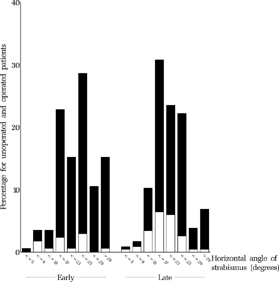
FIGURE 16 Baseline degree of amblyopia at approx. 11 months for all operated (black) and unoperated (white) patients who underwent the final examination. Categories: (1) cross-fixation or alternating freely, (2) preference of fixation, (3) failure to maintain fixation, (4) poor fixation behavior and eccentric fixation in fundus, (5) poor fixation behavior and far eccentric fixation.
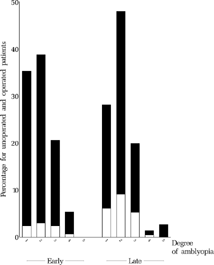
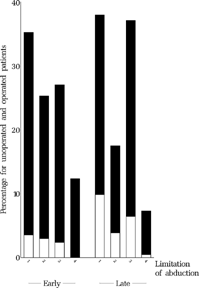
FIGURE 18 Baseline limitation of abduction at approx. 11 months for all operated (black) and unoperated (white) patients who underwent the final examination. Categories: (1) free abduction, using pursuit, (2) free abduction, using doll's-head, (3) passing midline but not free, (4) not passing midline.
The early and late groups are both stratified according to whether the children had been operated at age six (black) or not (white) in these figures. Scatter plots (, , , and ) visualize the relationship between the potentially prognostic baseline parameters and the primary endpoints. Mean age at entry examination was 11.1 (SD 3.7) months in the early group and 10.9 (SD 3.7) months in the late group.
FIGURE 11 Relationship between age at entry examination and outcome at age six: binocular vision (left upper panel), angle of strabismus (upper right), relative acuity (lower left) and absolute acuity (lower right) of the amblyopic eye. Although no primary endpoint, visual acuity of the worse eye has been added in a fourth scatter plot because there was a large variation of the visual acuity of the better eye. For more easy comparison, the same linear scale has been used for the ordinate in the latter two scatter plots. For level of binocular vision, see Methods or legends of .
FIGURE 13 Relationship between baseline angle of strabismus at approx. 11 months and outcome at age six.
FIGURE 15 Relationship between baseline angle of strabismus at approx. 11 months and outcome at age six.
FIGURE 17 Relationship between baseline degree of amblyopia at approx. 11 months and outcome at age six. For the categories of amblyopia, see in Methods or the legend to .
FIGURE 19 Relationship between baseline limitation of abduction at approx. 11 months and outcome at age six. For the categories of limitation of adduction, see in Methods or the legend to .
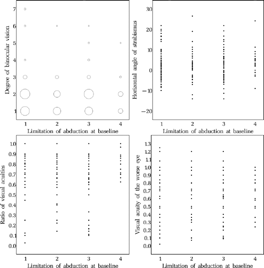
Although no primary endpoint, visual acuity of the worse eye has been added in a fourth scatter plot because there was a large variation in the visual acuity of the better eye. For more easy comparison, the same linear scale has been used for the ordinate in the latter two scatter plots. For the level of binocular vision, see in Methods or the legend to .
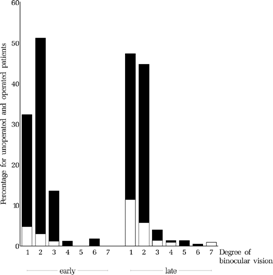
FIGURE 22 Level of binocular vision at the final examination. It was significantly better in the early group than in the late group (p = 0.001). Categories: (1) Bagolini negative, (2) Bagolini positive, (3) Housefly positive, (4) Titmus circles 200″ to 140″, (5) Titmus circles 100″ to 40″, (6) all figures of Lang Test or TNO 480″ & 240″, (7) TNO 120″–15″.
As a statistical measure for assessing the relationship between the primary endpoints and the potentially prognostic baseline parameters, Spearman's rank correlation coefficients including the corresponding p-values are presented in . A rank correlation coefficient of ±1 indicates the strongest correlation between parameters, while a correlation coefficient of zero indicates no correlation at all between parameters. The p-value is calculated to test the hypothesis whether the correlation coefficient is different from zero.
TABLE 1 Relationship Between Potentially Prognostic Baseline Parameter's (left) and Primary Endpoints (top)—Spearman's Rank Correlation Coefficients (rs) Including Corresponding p-Values.
, , , , , , , , , and very clearly reveal that there seems to be hardly any correlation between the potentially prognostic baseline parameters and the primary endpoints (i.e., we could not demonstrate it, but a potential correlation may have been obscured by statistical noise caused by events that happened between the ages of one and six). Furthermore, a preliminary analysis of the dichotomized endpoints confirmed that none of the baseline parameters was a confounder for the treatment effect, i.e. the estimated treatment effect does not change meaningfully when the baseline parameter is accounted for in a logistic regression model. Therefore, these baseline parameters were not included as covariates in the final analysis of the primary endpoints.
Analysis of Dropouts
The characteristics of dropouts as compared to those of completers were assessed on the basis of the baseline parameters. A total of 127 (23.9%) of the 532 patients included into the study were dropouts (dropout early group: 26.0%, late group: 22.3%, p = 0.356). The dropout rate varied per clinic. It is possible that cases with a bad outcome were more likely to drop out (because they sought a second opinion elsewhere). In an effort to detect such a tendency, it was checked whether the distribution of any of the potentially prognostic baseline parameters measured at baseline differed between dropouts and completers of the study ().
TABLE 2 Potentially Prognostic Baseline Parameters Measured at Baseline—Differences Between Dropouts and Completers.
No differences between dropouts and completers with regard to the potentially prognostic baseline parameters could be identified. Therefore, it seemed reasonable to analyze completers only and to exclude dropouts from primary analyses. The decisions described above were supported by a committee (listed in the Appendix) that, at the time, was not informed about the results for the treatment groups, only about the results of the analyses of potentially prognostic baseline parameters.
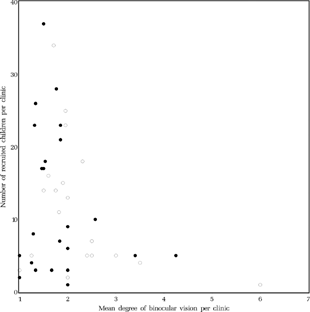
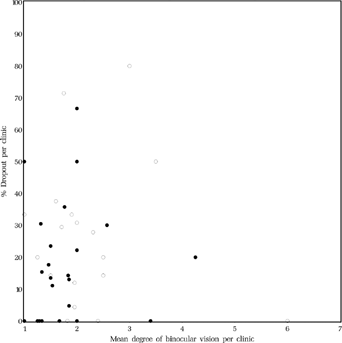
It seemed possible that dropouts did not return to the clinic because of poor results of the surgery. Then, on average, the results for the remainder of the patients from that clinic should be better. It could not be demonstrated, however, that clinics with higher dropout rates had better results regarding binocular vision (Spearman rank correlation coefficient rs = 0.122, p = 0.407, ).
FIGURE 20 Relationship between mean level of binocular vision at age six and mean dropout rate per clinic. Dots: early surgery, Circles: late surgery. For the level of binocular vision, see in Methods or the legends to .
To detect selection bias resulting from clinics preferentially recruiting infants with a favorable outcome, (note that we found no relation between baseline and endpoints) the relationship between level of binocular vision at age six and number of recruited children per clinic was calculated (). There were five clinics with an average level of binocular vision at age six of level three (Housefly positive) or better. They had each recruited five children or less, 10 patients in the early and 10 patients in the late group in total.
Analysis of the Primary Endpoints
Binocular vision at the final examination was significantly better in the early group than in the late group (p = 0.001, (Mann-Whitney-Wilcoxon test) and (Mean score test), plus ).
TABLE 3 Mean and Median Level of Binocular Vision at the Final Examination. For Levels of Binocular Vision, See in Methods, the Legend to or .
TABLE 4 Level of Binocular Vision—Frequencies of Categories.
The proportion of children who reached Bagolini positive was 51.2% in the early vs. 44.7% in the late group. The proportion of children who reached Housefly positive was 13.5% in the early vs. 3.9% in the late group. Only 3.0% in the early and 3.9% in the late group reached stereopsis beyond Housefly positive. They are listed in . The relation between age at first operation and the level of binocular vision at the final examination is depicted in . The relation between angle of strabismus and the level of binocular vision at the final examination is depicted in .
FIGURE 23 Age at first operation vs. level of binocular vision at the final examination, for all children operated once or repeatedly. For the level of binocular vision, see in Methods or the legend to .
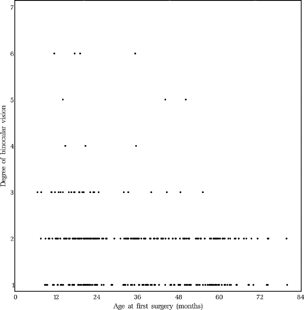
FIGURE 24 Relation between level of binocular vision and angle of strabismus at distance fixation. Black dots represent the patients who had not been operated at age six.
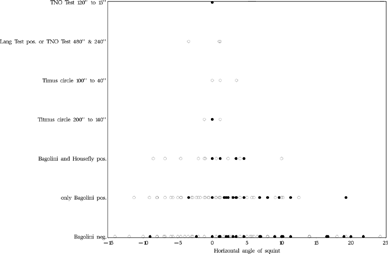
TABLE 5 Lising of All Patients (3.0% in the Early and 3.9% in the Late Group) Who Attained Stereopsis Beyond Housefly Positive at the Final Examination.
For the purpose of statistical analysis, the size of the manifest horizontal angle of strabismus at distance fixation was classified by defining a five-level ordinal variable (). The classified angle of strabismus at the final examination did not differ between the treatment groups (p = 0.967; Mean score test, ). The average score was 3.4 in both groups, which is between a “moderately good” and a “good” result.
TABLE 6 Classified Angle of Strabismus: Frequency of Classes.
The distribution of angles of strabismus in 4-degree categories at the final examination is shown for both treatment groups in . No statistically significant differences were found between clinics within either the early or the late group.
FIGURE 25 Horizontal angle of strabismus for both groups at the final examination at age six. The angle at baseline at approx. 11 months for the same group of children is shown in .
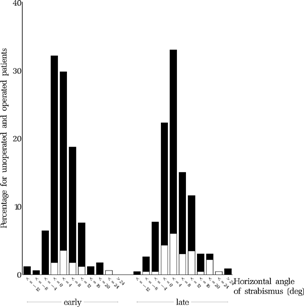
According to the Guidelines for surgery (ELISSS group, 1993b), which dictated: “Re-operation should be undertaken in cases with a residual esotropia of greater than 10°, or in case of overcorrection”, 35.1% of the early group and 34.8% of the late group should have been re-operated, but was not. However, the angle may have increased during the interval between the evaluation of the result of surgery in the clinic and the final examination, in some cases several years later. In addition, it became evident that many parents had declined surgery despite the fact that their child had a large convergent or divergent angle.
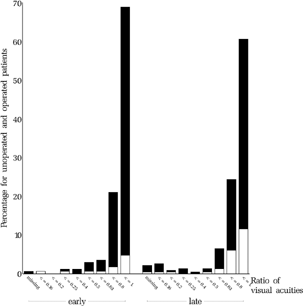
For the treatment of amblyopia, the guidelines for amblyopia treatment in the protocol (ELISSS group, 1993b) prescribed that “Cases with strongly established fixation preference and/or significant anisometropia will, after entry in the study, undergo appropriate and effective occlusion therapy to the point of near spontaneous alternation and central fixation of the worse eye.” As a measure of the degree of amblyopia remaining after treatment, the visual acuity of the worse eye relative to that of the better eye was analyzed. The ratio of visual acuities was larger in the early group than in the late group (p = 0.023, , ). No statistically significant differences were found between clinics within either the early or the late group. shows the mean visual acuities in the better and the worse eye, respectively. shows the distributions of the visual acuity of the worse eye for both groups.
FIGURE 27 Visual acuity of the amblyopic eye at age six. The degree of amblyopia at the entry examination is shown for the same group of children in .
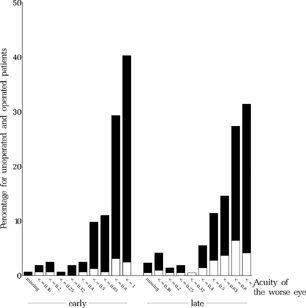
TABLE 7 Ratio of the Visual Acuities (Worse Eye Relative to Better Eye).
TABLE 8 Visual Acuity of the Better and of the Worse Eye. The Average Values of the Visual Acuities are Not Logarithmic Averages But, Due to the Nature of the Statistical Test, the p-Value Remains the Same If Logarithmic Values are Taken.
‘As Treated’ Analysis
As a sensitivity analysis, an ‘as treated’ analysis of the completers was performed. For this analysis, patients whose first operation took place at an age of 5.5 to 24.5 months were counted as belonging to the early group (n = 138) and patients with a first operation between 31.5 and 60.5 months were counted in the late group (n = 150). Those completers who were not operated at all (n = 61) and those patients whose first operation took place outside the ranges specified above (n = 56) were excluded from this analysis. , , show the results of the ‘as treated’ analysis for the primary endpoints. These results confirmed the results of the primary intention-to-treat analysis.
TABLE 9 ‘As Treated’ Analysis: Level of Binocular Vision (Frequencies of Categories).
TABLE 10 ‘As Treated’ Analysis: Classified Angle of Strabismus (Frequencies of Categories).
TABLE 11 ‘As Treated’ Analysis: Ratio of the Visual Acuities.
Secondary Endpoints
As shown in , the number of operations was higher in the early group. The average number of operations was 1.18 (SD 0.67) in the early as compared to 0.99 (SD 0.64) in the late group. Differences between children operated once or more and children not operated at all were found for the angle of strabismus (2.26° (SD 5.66°) vs. 5.52° (SD 6.74°), p = 0.000), but not for level of binocular vision (1.78 (SD 0.85) vs. 1.78 (SD 1.26), p = 0.141) or ratio of the visual acuities (0.85 (SD 0.18) vs. 0.80 (SD 0.22), p = 0.118).
Each child was examined one month postoperatively before returning to the six-month schedule. In the early group, the horizontal angle of strabismus was 4.6° (SD 5.1°) (N = 74) one month postoperatively vs. 4.7° (SD 5.6°) (N = 126), six months postoperatively. In the late group, the horizontal angle of strabismus was 4.6° (SD 5.1°) (N = 93) one month postoperatively vs. 4.9° (SD 5.8°) (N = 94) six months postoperatively.
The manifest angle during fixation at near was 3.73° (SD 5.64°) in the early group (N = 157) and 5.66° (SD 6.66°) in the late group (N = 207, p = 0.012). For comparison, the manifest angle during fixation at distance, one of the primary endpoints, was 2.15° (SD 5.45°) in the early group (N = 167) and 3.21° (SD 6.29°) in the late group (N = 231, p = 0.062).
No significant difference between the early and late groups with regard to the vertical angle of strabismus was found. The vertical deviation in gaze ahead was 0.56° (SD 3.07°) in the early group (N = 109) and 0.24° (SD 2.84°) in the late group (N = 199). The vertical deviation in 25° left gaze was 2.38° (SD 4.14°) in the early group (N = 83) and 2.44° (SD 4.48°) in the late group (N = 123). The vertical deviation in 25° right gaze was −1.84° (SD 3.96°) in the early group (N = 78) and −1.78° (SD 3.44°) in the late group (N = 120).
Torticollis was found in 15.2% of the early group (N = 164) vs. 20.9% of the late group (N = 225, p = 0.186).
Latent nystagmus was found in 35.4% of the early group (N = 164) vs. 61.8% of the late group (N = 225, p = 0.000).
DVD was found in 57.5% of the early group (N = 167) vs. 53.8% of the late group (N = 223, p = 0.474), even though DVD had been found more often in the late group at baseline: in 7.7% of the early group and 14.2% of the late group ([Meyer et al., Citation1998]). The DVD-rates were analyzed in relation to the horizontal angle of strabismus at the final examination (). Decompensated DVD was found to be associated with overcorrection of convergent strabismus.
TABLE 12 DVD-Rate vs. Horizontal Angle of Strabismus.
Faden Operation
Surgery consisted of bilateral medial rectus recessions, combined recession-resection surgery or Faden surgery. The surgical technique could, unfortunately, not be dictated. It would have greatly limited the number of clinics willing to participate. If the surgical technique had been dictated, the dosage and other aspects of surgery would have had to be dictated as well. It was thought at the time that there were too many other variables in the relation between dose and effect of surgery that could not be controlled. Moreover, the study investigated strabismus surgery as it is currently practiced in Europe. It was decided, however, to analyze Faden surgery separately.
There were 74 patients (32 early group, 42 late group) who had at least one Faden operation. The rate of patients with Faden operations did not differ between the early (22.5%) and late (22.6%) surgery groups (p = 0.693).
The level of binocular vision at the final examination did not differ between patients who had Faden surgery and those who did not have Faden surgery (p = 0.213, ).
TABLE 13 Faden Surgery: Level of Binocular Vision (Frequencies of Categories).
Among the operated children in the early group, the level of binocular vision did not differ between patients who had Faden surgery and those who did not have Faden surgery (p = 0.577).
The angle of strabismus was 2.27° (SD 4.82°) in the Faden group (N = 73) vs. 2.26° (SD 5.89°) in the recession and/or resection group (N = 268, p = 0.732).
The ratio of the visual acuities was 0.84 (SD 0.17) in the Faden group (N = 70) vs. 0.85 (SD 0.19) in the recession and/or resection group (N = 267, p = 0.410).
DISCUSSION
In our study, among children first operated at the age of 20.0 (SD 8.4) months, i.e. early, 13.5% could recognize the Titmus Housefly at age six. This was 3.9% among children operated at the age of 49.1 (SD 12.7) months, i.e. late. It is likely that this difference is caused by age at the time of surgery and not caused by an unknown confounder like better treatment in the early group, because binocular vision beyond Titmus Housefly occurred in 3.0% of the early and 3.9% of the late group. The average number of operations was 1.18 (SD 0.67) in the early and 0.99 (SD 0.64) in the late group. At age six, 8.2% of the early group and 20.1% of the late group had not been operated.
Considering the larger proportion of children reaching Titmus Housefly and Bagolini test positive, early surgery for large-angle esotropia seems warranted. Almost all children with an angle > 25° () and a limitation of abduction at midline () at entry examination had been operated at age six. The situation is more complex for small-angle esotropia, as a considerable number of these children in our study had not been operated at age six ().
A cost-effectiveness analysis is beyond the scope of our study but the unexpectedly large number of not-operated children, most of whom had a spontaneous reduction of the angle of strabismus, would be an important factor in such a balance. It is important to note that a spontaneous reduction in the angle of strabismus does not imply a cure in the sense of better binocular vision — better than what, possibly, could be achieved by early surgery.
Age at recruitment, age at which strabismus reportedly had started, degree of amblyopia at entry and refraction at entry did not seem to predict which children would not be operated at age six. Future studies should be directed at characteristics that identify these children at an early age.
In a non-randomized clinical trial, we must be aware of the possible limitations of the validity of our results. As all participating clinics committed themselves to operating all eligible children during the course of the study, either early or late, there was no bias due to confounding by indication, i.e. the clinics could not, for example, enter large angles for early and small angles for late surgery. The number of children recruited differed per clinic, and a selection bias may have arisen when clinics that did not include all eligible infants, as prescribed in the protocol, preferentially recruited infants with a favorable prognosis. Considering the fact that we found hardly any correlation between the potentially prognostic baseline parameters and the primary endpoints, it is difficult to imagine how the clinician would have been able to discern such cases at entry but other, unknown, confounders may have been present. There were five clinics that reached an average level of binocular vision at age six of Titmus Housefly positive or better (). As this happened to concern 10 patients in the early and 10 patients in the late group, the influence of such a bias, if really present, would be negligible.
Most children were operated within the limits set, but not all (). When the results were analyzed according to the actual date of operation (‘as treated’ analysis, , , ), the outcome was the same.
The referring ophthalmologists may have sent different kinds of patients to early and late operating clinics, causing an indirect bias due to confounding by indication. However, the baseline parameters, the degree of amblyopia, the refraction and the limitation of abduction were distributed equally between the early and late groups. Only the angle of strabismus was slightly larger in the early group at entry at 11 months ().
Unfortunately, the two therapeutic modalities, early or late surgery, could not be blinded. Instead of blinding, the final examinations were done in the presence of an independent external observer, an ophthalmologist or an orthoptist, to avoid assessment bias. In 82.7% of cases the final examination forms had been signed by the external observer, thereby giving proof of his presence. No observer was present at the imputed cases, i.e. where no formal final examination had taken place and previously obtained data was used, and in a few other cases the observer did not sign.
The quality of data was good with 11.4 (SD 4.9) case report forms per patient, including the children who dropped out during the course of the study.
The cogency of the methods was also good: None of the participants in the study was informed about any of the results of the study before the hypotheses to be tested were formulated in the highest possible detail. In- and exclusion criteria and composition of the two groups were not manipulated in any way during the course of the study.
It is conceivable that children with an unfavorable outcome preferentially were lost to follow-up, because they sought a second opinion. However, the baseline parameters were distributed equally among dropouts and completers (), and clinics with a higher dropout rate did not have better results, as could be expected from elimination of bad cases ().
Treatment bias was minimized by standardization of treatment procedures. The goals for early and late surgery and for the treatment of amblyopia were formulated in guidelines. The guidelines for surgery were not strictly adhered to, however: 35% of the children were not within 0–10° of esotropia at age six (in both groups) although the guidelines for surgery instructed that surgery should be continued until this range had been reached.
This study examined surgery for infantile strabismus as it is currently practiced throughout Europe. Other studies may find that higher levels of stereopsis can be reached under strictly controlled circumstances and by operating more frequently. It also seems likely that higher levels of stereopsis could be reached by operating earlier, in the first year of life. However, at that age, the fraction of children who are operated but would, without surgery, have a spontaneous reduction of the esotropia to microstrabismus would be even higher than the 8.2% and 20.1% found in our study. This could only be assessed adequately in a prospective, randomized, controlled trial.
The following papers regarding the study have been published previously by the study group or members thereof (presented in chronological order):
Simonsz HJ. How accurate is examination at age one? Pilot study of the Early vs Late Strabismus Surgery Study Group. In: Kaufmann H, et al. Trans 20thMeet European Strabismological Assoc., Brussels, 1992, pp. 127–132.
Early vs. Late Infantile Strabismus Surgery Study Group. How accurate is orthoptic examination at age one? Strabismus. 1993a; 1:75–83.
Early vs. Late Infantile Strabismus Surgery Study Group. The protocol for the Early vs. Late Infantile Strabismus Surgery Study. Strabismus. 1993b; 1:135–157.
Early vs. Late Infantile Strabismus Surgery Study Group. The Early vs. Late Infantile Strabismus Surgery Study: First monitoring report. Strabismus. 1994; 2:87–102.
Early vs. Late Infantile Strabismus Surgery Study Group. The Early vs. Late Infantile Strabismus Surgery Study: Second monitoring report. Strabismus. 1995; 3:131–142.
Early vs. Late Infantile Strabismus Surgery Study Group. Früh- oder Spätoperationen beim frühkindlichen Innenschielen. Eine prospektive, europäische, multizentrische Studie. Z prakt Augenheilkd. 1995; 16:320–323.
Early vs. Late Infantile Strabismus Surgery Study Group. Früh-oder Spätoperationen beim frühkindlichen Innenschielen. Eine prospektive, europäische, multizentrische Studie. Orthoptik-Pleoptik. 1995; 19:30–32.
Early vs. Late Infantile Strabismus Surgery Study Group. Current state of the Early vs. Late Infantile Strabismus Surgery Study. In: Spiritus M (ed.). Trans 22nd Meet European Strabismological Assoc. Buren: Æolus-Press, 1996; pp. 175–185.
Early vs. Late Infantile Strabismus Surgery Study Group. The Third Monitoring Report of the Early vs. Late Infantile Strabismus Surgery Study. Strabismus. 1996; 4:99–110.
Early vs. Late Infantile Strabismus Surgery Study Group. The Fourth Monitoring Report of the Early vs. Late Infantile Strabismus Surgery Study. Strabismus. 1997; 5:133–147.
Haag U. The Early vs. Late Infantile Strabismus Surgery Study—Experiences from a non-randomised clinical trial. In: Abel U, Koch A (eds.). Proc Conf “Nonrandomized Comparative Clinical Studies”, Heidelberg, 1998, pp. 129–134.
Meyer K, Simonsz HJ, Kolling G, Haag U, Dinkel H. State of the Early vs. Late Infantile Strabismus Surgery Study. In: Spiritus M (ed). Trans 24th Meet European Strabismological Assoc. Buren: Æolus-Press, 1998; pp. 27–32.
Meyer K, Breitschwerdt H, Kolling GH, Simonsz HJ. The Early vs. Late Infantile Strabismus Surgery Study: Do sources for bias exist in this non-randomized trial? Br J Ophthalmol. 1998; 82:934–938.
Unnebrink K, Breitschwerdt H, Kolling G, Simonsz HJ. The Early vs. Late Infantile Strabismus Surgery Study: Quality management for the final examination procedure. In: Spiritus M (ed.). Trans 25thMeet European Strabismological Assoc., Jerusalem, 1999. Lisse: Swets & Zeitlinger Publishers, 2000; pp. 242–247.
Unnebrink K, Bauer C, Kolling G, Simonsz HJ. The Early vs. Late Infantile Strabismus Surgery Study: State of the final examinations and some preliminary findings. In: De Faber JT (ed.). Trans 26th Meet European Strabismological Assoc., Barcelona, 2000. Lisse: Swets & Zeitlinger Publishers, 2001; pp. 57–60.
Kolling G, Unnebrink K, Bauer C, Simonsz HJ, Studiengruppe “Früh- oder Spätoperationen beim frühkindlichen Innenschielen”. Wie sehen Schielpatienten im Alter von einem Jahr und von vier Jahren aus? Z prakt Augenheilkd. 2001; 22:292–298.
Unnebrink K, Bauer C. The Early vs. Late Infantile Strabismus Surgery Study: Biometrical report. Schriftenreihe des Koordinierungszentrums für Klinische Studien (KKS) Heidelberg, 2003.ISSN 1617–4453.
APPENDIX
Members of the Early vs. Late Infantile Strabismus Surgery Study Group
The Early vs. Late Infantile Strabismus Surgery Study (ELISSS) was a non-randomized, prospective, multicenter clinical trial carried out in 58 clinics in 11 European countries. The study was planned in 1992, patient recruitment started in May 1993. H.J. Simonsz and G.H. Kolling designed and coordinated the study. All authors contributed to the interpretation of the data and writing of the report. Biometrical support was given by H. Noack, H. Schäfer, U. Haag (1992–1996) and K. Unnebrink (1996–2003). Data management, including analysis programming and correspondence with the participating clinical centers, was performed by H. Breitschwerdt (1992–1999) and C. Bauer (2000–2003).
The following is a list of the active participants per country. For each country, the country coordinator is marked with “CC”. Each entry consists of the clinic's name and the names of the clinic coordinators.
Austria: Graz (A. Langmann, S. Lindner), Linz (S. Priglinger, M. Raab), Salzburg (H. Thaller-Antlanger (CC), D. Koschkar-Moser), St. Pölten (H. Gruber-Luka (CC), R. Führer), Vienna: Wien Hanusch-Krankenhaus (S. Harrer, K. Rigal), Wiener Neustadt (R. Pelz, B. Puchhammer), Wien Universitäts-Augenklinik (A. Thaler, E. Moser), Wien Wilhelminenspital (A. Thaler, K. Schmidt)
Belgium: Brussels (M. Spiritus (CC), M. van den Broeck, S. Vandelannoitte, A. Finck), Edegem (Evens, D. Godts)
France: Lyon (M. Bourron-Madignier (CC), S. Vettard, O. Benhadj)
Germany: Berlin Charité (W) (E-Ch. Schwarz, G. Wunsch), Berlin Steglitz (C. Jandeck, S. Lutt-Freund, D. Jüptner-Johanning), Dresden (E. Sommer, G. Hochmuth), Erlangen (G. Gusek-Schneider, Schürhoff, A. Boss), Frankfurt/M. Universitäts-Augenklinik (A. Zubcov, B. Herrmann), Freiburg Universitäts-Augenklinik (G. Kommerell, B. Lieb), Halle (R. Weidlich, U. Wittenbecher), Hamburg Universitäts-Augenklinik (E. Schulz, B. Schmall), Heidelberg (G. Kolling (CC), B. Stoll), Homburg/Saar (B. Käsmann, E. Grintschuk), Cologne: Köln Universitäts-Augenklinik (A. Kirsch), Munich: München Technische Universität (T. Schmidt, M. Klopfer, C. Ecker), München Universitäts-Augenklinik (K.P. Boergen, O. Ehrt, H.D. Schworm), Regensburg Universitäts-Augenklinik (B. Lorenz, B. Derr)
Great Britain: Dundee (C.J. McEwen), Liverpool (I. Marsh, L. Gannon), London Hospital for Sick Children (C. Timms, D. Taylor), London Moorfields Eye Hospital (P. Fells (CC), J.P. Lee)
Italy: Florence (R. Frosini (CC), L. Campa), Sassari (F. Carta, A. Carta)
Netherlands: Amsterdam (L. Wenniger-Prick (CC), Y. Everhard-Halm), Goes (A.G. Tjiam, M. van Duuren), Rotterdam (H.J. Simonsz, H.M. van Minderhout)
Norway: Aalesund (G. Hanken, A. Angermeier), Bergen (O.H. Haugen (CC)), Førde Sentralsjukehuset (L. Steene Eriksen, B. A. Olsen), Haugesund (E. Dueland, W. Evans Lothe), Lillehammer (T. Bulie), Tønsberg (H.P. Brinck, T. Kalseth)
Sweden: Borås (G. Ladenvall, A.B. Edvinsson), Danderyd (A. Wallin, R. Alvarado, M. Fornander), Eskilstuna (U. Lidén, L. Lindberg, I. Wiklund), Huddinge (Lennerstrand (CC), B. Derouet-Eriksson), Jönköping (B. Sunnqvist, G. Gunnarssen), Linköping (P. Jakobsson, G. Kvarnström), Sundsvall (M. Lindberg, D. Grandell), Umeå (K. Johansson, A.-L. Galin), Växjö (I. Axelsson, B.-M. Petersson)
Switzerland: Lausanne (G. Klainguti (CC), J. Strickler), Zürich (K. Landau, B. Baerlocher)
Turkey: Adana (G. Haciyakupoglu), Ankara Hacettepe Univ. (A. Sefik Sanaç, E. Cumhur Sener), Ankara Saglik Bakanligi (S. Demirci), Ankara University (N. Erkam, Huban Atilla (CC)), Edirne Trakya Univ. (N. Erda), Izmir Eyuel University (A. Tulin Berk)
All final examinations were performed in the presence of one of the following external observers (listed in order of the number of examinations observed: G.H. Kolling, B. Lieb, O. Ehrt, H.J. Simonsz, M. Bourron-Madignier, A. Langmann, G. Klainguti, S. Harrer, M. Koehler, H. Thaller-Antlanger, Schroeder, A. Rydberg, C. Jandeck, Klopfer, H. Gruber-Luka, R. Pelz, B. Derouet-Eriksson, O.H. Haugen, R. Weidlich, M. Spiritus, G. Ladenvall, P. Jakobsson, J.P. Lee, G. Gusek-Schneider, M. Lindberg, Wirsching, A. Chesworth, B. Neikter, S. Priglinger, H. Conrad, J. Esser, T. Ring, M. Andersson, H.D. Schworm, and Spadt.
The committee that decided on the policy for handling dropouts consisted of: U. Abel, G.H. Kolling, G. Kommerell, J.P. Lee, W. Rüßmann, H.J. Simonsz, M. Spiritus, K. Unnebrink.
ACKNOWLEDGEMENTS
All orthoptists in the 58 participating clinical centers that treated and documented the study children over an overall period of 10 years deserve the most important thanks. Without their commitment this study would not have been possible. We also thank Theo Stijnen, Hanny Groenewoud, René Eijkemans, Maria Fronius, Larry Tychsen, Redmer van Leeuwen, Hans Vingerling, Carolien Klaver, Sander Schutte and Urs Gessner for their valuable comments.
This study was supported by grant no. Ko 1295 of the DFG (German Research Organization) and by a grant from the Swiss Union for the Prevention of Blindness (1992).
REFERENCES
- Berke R N. Requisites for postoperative third degree fusion. Trans Am Acad Ophthalmol Otolaryngol. 1958; 62: 38–49, [PUBMED], [INFOTRIEVE], [CSA]
- Birch E E, Stager D R, Berry P, Everett M E. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Invest Ophthalmol Vis Sci. 1990; 31: 758–765, [PUBMED], [INFOTRIEVE], [CSA]
- Birch E E, Stager D R, Everett M E. Random dot stereoacuity following surgical correction of infantile esotropia. J Pediatr Ophthalmol Strabismus. 1995; 32: 231–235, [PUBMED], [INFOTRIEVE], [CSA]
- Charles S, Moore A. Results of early surgery for infantile esotropia in normal and neurologically impaired infants. Eye. 1992; 6: 603–606, [PUBMED], [INFOTRIEVE], [CSA]
- Chavasse F B. Worth's Squint—or the binocular reflexes and the treatment of strabismus. 7th ed. Bailliere, Tyndal & Cox, London 1939
- Clarke W N, Noel L P. Vanishing infantile esotropia. Can J Ophthalmol. 1982; 17: 100–102, [PUBMED], [INFOTRIEVE], [CSA]
- Costenbader F D. Infantile esotropia. Trans Am Ophthalmol Soc. 1961; 59: 397–429, [PUBMED], [INFOTRIEVE], [CSA]
- Elliott S, Shafiq A. Interventions for infantile esotropia. The Cochrane Database of Systematic Reviews. 2005, Issue 1, Art. No. CD004917[CSA]
- Horwood A, Williams B. Does neonatal ocular misalignment predict later abnormality?. Eye. 2001; 15: 485–491, [PUBMED], [INFOTRIEVE], [CSA]
- Hubel D H, Wiesel T N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965; 28: 1041–1059, [PUBMED], [INFOTRIEVE], [CSA]
- Ing M R, Costenbader F D, Parks M M, Albert O O. Early surgical treatment for congenital esotropia. Am J Ophthalmol. 1966; 652: 1419–1427, [CSA]
- Ing M R. Early surgical alignment for congenital esotropia. J Pediatr Ophthalmol Strabimus. 1983; 20: 11–18, [CSA]
- Ing M R. Outcome study of surgical alignment before six months of age for congenital esotropia. Ophthalmology. 1995; 102(12)2041–2045, [PUBMED], [INFOTRIEVE], [CSA]
- Kieser M, Reitmeir P, Wassmer G. Test procedures for clinical trials with multiple endpoints. Testing Principles in Clinical and Preclinical Trials. Biometrie in der chemisch-pharmazeutischen Industrie, J. Vollmar. Gustav Fischer Verlag, Stuttgart 1995; vol. 6: 41–60
- Meyer K, Breitschwerdt H, Kolling G H, Simonsz H J. The Early vs. Late Infantile Strabismus Surgery Study: Do sources for bias exist in this non-randomized trial?. Br J Ophthalmol. 1998; 82: 934–938, [PUBMED], [INFOTRIEVE], [CSA]
- Newcombe R G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998; 17: 857–872, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pediatric Eye. Disease Investigator Group. The clinical spectrum of early-onset esotropia: Experience of the congenital esotropia observational study. Am J Ophthalmol. 2002a; 133: 102–108, [CSA], [CROSSREF]
- Pediatric E ye. Disease Investigator Group. Spontaneous resolution of early onset-esotropia: Experience of the congenital esotropia observational study. Am J Ophthalmol. 2002b; 133: 109–118, [CSA], [CROSSREF]
- Von Noorden G K. A reassessment of infantile esotropia. XLIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1988; 105: 1–10, [PUBMED], [INFOTRIEVE], [CSA]
- Wong A M, Foeller P, Bradley D, Burkhalter A, Tychsen L. Early versus delayed repair of infantile strabismus in macaque monkeys: I. ocular motor effects. J AAPOS 2003; 7: 200–209, [PUBMED], [INFOTRIEVE], [CSA]
- Worth C. Squint: its causes, pathology, and treatment. John Bales, Sons and Danielson, London 1903
- Wright K W, Edelman P M, Mc Vey J H, Terry A P, Lin M. High grade stereoacuity after early surgery for congenital esotropia. Arch Ophthalmol l994; 112: 913–919, [CSA]