ABSTRACT
Purpose: To determine if topical beta-blocker use is associated with increased cardiovascular mortality, particularly among people with self-reported glaucoma.
Methods: All participants who participated in the first health check (N = 25,639) of the European Prospective Investigation into Cancer (EPIC) Norfolk cohort (1993–2013) were included in this prospective cohort study, with a median follow-up of 17.0 years. We determined use of topical beta-blockers at baseline through a self-reported questionnaire and prescription check at the first clinical visit. Cardiovascular mortality was ascertained through data linkage with the Office for National Statistics mortality database. Hazard ratios (HRs) were estimated using multivariable Cox regression models. Meta-analysis of the present study’s results together with other identified literature was performed using a random effects model.
Results: We did not find an association between the use of topical beta-blockers and cardiovascular mortality (HR 0.93, 95% confidence interval, CI, 0.67–1.30). In the 514 participants with self-reported glaucoma, no association was found between the use of topical beta-blockers and cardiovascular mortality (HR 0.89, 95% CI 0.56–1.40). In the primary meta-analysis of four publications, there was no evidence of an association between the use of topical beta-blockers and cardiovascular mortality (pooled HR estimate 1.10, 95% CI 0.84–1.36).
Conclusion: Topical beta-blockers do not appear to be associated with excess cardiovascular mortality. This evidence does not indicate that a change in current practice is warranted, although clinicians should continue to assess individual patients and their cardiovascular risk prior to commencing topical beta-blockers.
Introduction
Topical beta-blockers remain one of the primary treatments for glaucoma.1 Previous studies have described associations between long-term use of topical beta-blockers and increased cardiovascular mortality.2,3 Topical medications can reach systemic concentrations, and up to 80% of timolol has been shown to be systemically absorbed.4 Furthermore, long-term treatment with timolol has been associated with dyslipidemia,5–7 also indicating systemic effects from topical treatment. Additional cardiovascular mechanisms include dysrhythmias and impaired cardiac output,8 although investigations into the association between topical beta-blocker use and cardiovascular mortality show conflicting results. The Blue Mountains Eye Study2 reported an increase in cardiovascular mortality in glaucoma patients using topical beta-blockers, whereas the Rotterdam Study9 found non-significant associations with long-term exposure. Given the significant global burden of cardiovascular disease, the widespread use of topical beta-blockers10 as a cost-effective treatment, and the availability of off-patent effective alternatives in the form of prostaglandin analogues,11 there is clear justification to explore the question further.
This study aimed to investigate the effects of topical beta-blockers on cardiovascular mortality. First, we examined the association in the European Prospective Investigation into Cancer (EPIC) Norfolk Cohort Study. Second, we conducted a systematic review of the literature and pooled our results with previously published data in a meta-analysis.
Materials and methods
EPIC-Norfolk is a large, prospective, cohort study. All participants were invited to attend via their general practitioner (GP) between March 1993 and December 1997. The participation rate was 33%. As nearly all residents in the United Kingdom are registered with a GP through the National Health Service, general practice lists serve as population registers. Detailed methods have been published elsewhere.12 In brief, all persons aged 40–79 years in databases of the 35 participating general practices in the region were sent invitations (N = 77,360). Consent was received from 30,445 participants, of which 25,639 attended the first health examination.12 The Norfolk Local Research Ethics Committee approved the EPIC-Norfolk study, and all volunteers gave written informed consent.
We ascertained topical beta-blocker use through completion of a detailed health and lifestyle questionnaire and by asking participants to bring all previous prescriptions and medications they were taking at baseline to the health examination, where prescriptions and medicines were checked by trained nurses.12 All EPIC-Norfolk participants were followed up for mortality and flagged for death certification at the Office for National Statistics, with vital status ascertained on the whole cohort up until 30 June 2013.12 Coding of death certificates was performed by trained nosologists according to the International Classification of Diseases (ICD). Cardiovascular mortality was defined as any individual with ICD codes 410–448 (ICD 9th revision) or ICD codes I10–I79 (ICD 10th revision) listed as cause of death.
The design and characteristics of EPIC-Norfolk assessment methods for all covariables of interest have been described previously.12 The following covariables were measured through a self-reported questionnaire: History of angina, cholesterol, diabetes, education, glaucoma, myocardial infarction, stroke, hypertension, smoking and social class. Glaucoma ascertainment was also self-reported through direct questioning on the questionnaire. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded during a health examination as the mean of two measurements taken from the right arm with the participant seated for 5 minutes, using an Accutorr Plus blood pressure monitor (Mindray, Huntingdon, UK).
Differences in characteristics of participants by exposure status were evaluated using Chi-squared and Welch’s unpaired T-tests. All participants with missing covariable data were included in the initial analyses, with a complete case analysis conducted on the final models. Associations between use of topical beta-blockers and cardiovascular mortality were assessed using Cox proportional hazard models. All analyses were adjusted for age and sex. Potential confounders were selected using univariable comparisons of covariables, and variables which were significantly associated with both exposure and outcome were included. We also included covariables based on a priori assumptions of known confounders in the final Cox model. Raised cholesterol was considered to be a potential mediator on the causal pathway5–7 and therefore not included in the final model. A subgroup analysis was performed to look at the association between topical beta-blocker exposure and cardiovascular mortality in only participants with self-reported glaucoma. Multiple sensitivity analyses were carried out to explore the robustness of our findings. The effect of different covariables, glaucoma status, cholesterol as a confounder rather than a mediator, whether having manifest cardiovascular disease might alter exposure patterns and introduce reverse causation were explored. No adjustments were made for change in exposure status over the course of the study period. This allowed direct comparison with methods from the Blue Mountains Eye Study2 and Rotterdam Study9. However, as glaucoma is an age-related disease,13 and given the older age range within the EPIC-Norfolk cohort,12 it is probable that further incident cases of glaucoma and thus topical beta-blocker exposure occurred. Therefore, we used census-derived population data, together with epidemiological models to estimate the potential impact of incident glaucoma during the follow-up period. We also examined the association between topical beta-blocker use and cardiovascular mortality at the second health check (1998–2000) to explore the effect of changing treatment during the follow-up period.
Systematic literature review
A literature-based meta-analysis was performed to look for published evidence on the association between topical beta-blockers and cardiovascular mortality. The search strategy was performed within PubMed (National Center for Biotechnology Information, USA).
The following terms were used in the literature search strategy in July 2014;
(1) topical[All Fields], (2) “adrenergic beta-antagonists” [Pharmacological Action] OR “adrenergic beta-antagonists”[MeSH Terms] OR (“adrenergic” [All Fields] AND “beta-antagonists”[All Fields]) OR “adrenergic beta-antagonists” [All Fields] OR (“beta” [All Fields] AND “blockers” [All Fields]) OR “beta blockers”[All Fields], (3) “glaucoma”[MeSH Terms] OR “glaucoma”[All Fields], (4) “mortality”[Subheading] OR “mortality”[All Fields] OR “mortality”[MeSH Terms], (5) (“cardiovascular system”[MeSH Terms] OR (“cardiovascular”[All Fields] AND “system”[All Fields]) OR “cardiovascular system”[All Fields] OR “cardiovascular”[All Fields]) AND (“mortality”[Subheading] OR “mortality”[All Fields] OR “mortality”[MeSH Terms]), (6), (1) AND (2), (7) (3) OR (6), (8) (4) OR (5). The search (7) AND (8) returned 278 publications.
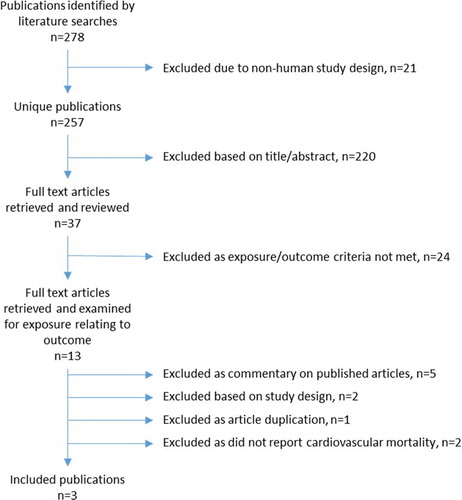
Publications were reviewed if they reported associations between topical beta-blocker use and cardiovascular mortality. No exclusion criteria were applied to language or journal publication date. Publications were excluded if they were non-human studies, in-vitro experiments, ecological studies, case series, case reports, opinion pieces, letters, did not look at topical beta-blockers as an exposure and did not look at cardiovascular mortality as an outcome. Publications were additionally excluded if they were reviews of published associations with no new contributing data.
The exclusion process is detailed in . The resulting three articles were all cohort studies and are summarized in .
Table 1. Summary of three published studies reporting associations between topical beta-blocker use and cardiovascular mortality.
Figure 1. Flowchart of the literature review on the association between topical beta-blockers and cardiovascular mortality illustrating the exclusion process of retrieved publications.
A random effects model was used to pool the results and accommodate between-study heterogeneity. Between-study heterogeneity was analyzed using the I-squared test statistic. All analyses were carried out in Stata 12.1 (StataCorp, College Station, TX, USA).
Results
A total of 25,639 participants with a mean age of 59.2 years (standard deviation, SD, 9.32 years) were included in this analysis. Participants were followed up for a median of 17.0 years and a total of 407,524 person-years. During the follow-up period, 6525 individuals died (25.4%) of which 2158 deaths (33.1%) were attributed to cardiovascular disease. A total of 19,114 individuals were alive at the time of follow-up. Topical beta-blockers were used by 179 individuals (0.7%) of whom 82 (45.8%) had died during follow-up. Of these 36 (20.1%) participants who used topical beta-blockers died of cardiovascular causes.
details the baseline characteristics of the cohort. Those using topical beta-blockers were older at baseline, with a mean difference of 7.4 years (95% confidence interval, CI, 6.2–8.5 years). Just over half (57.8%) of participants using topical beta-blockers were male. Significant differences in the proportions of those with diabetes, angina, myocardial infarction, stroke, self-reported hypertension, raised cholesterol, and raised SBP at baseline were observed, with higher proportions in those using topical beta-blockers. A significantly higher proportion of ever smokers were also seen in topical beta-blocker users. Non-significant differences in social class, education level, and raised DBP were observed between exposure groups. The proportion of participants using topical beta-blockers with self-reported glaucoma was predictably high, with 89.9% of users reporting glaucoma. Of the 514 participants with glaucoma, 353 (68.7%) were not using topical beta blockers compared with 161 (31.3%) who were.
Table 2. Comparison of baseline characteristics between exposure groups in participants in the European Prospective Investigation into Cancer Norfolk Cohort Study (EPIC-Norfolk, 1993–2013).
details the results of the Cox proportional hazards analysis for the complete cohort. Topical beta-blocker use was not associated with cardiovascular mortality (hazard ratio, HR, 1.12, 95% CI 0.80–1.56) when adjusted for age and sex. also presents the maximally adjusted model; no significant association was found between use of topical beta-blockers and cardiovascular mortality (HR 0.93, 95% CI 0.67–1.30).
Table 3. Associations between topical beta-blocker use and cardiovascular mortality in the European Prospective Investigation into Cancer Norfolk Cohort Study (EPIC-Norfolk, 1993–2013).
At baseline, 514 participants (2.0%) had glaucoma of which 229 (44.6%) died during follow-up. Of these 92 participants (17.9%) died of cardiovascular causes. Overall, 285 participants with glaucoma were alive at the end of the study. shows the results of the Cox proportional hazards analysis for glaucoma patients adjusted for age and sex. Comparing participants reporting glaucoma and using topical beta-blockers to those reporting glaucoma not using topical beta-blockers, there was no association with cardiovascular mortality when adjusted for age and sex (HR 0.76, 95% CI 0.49–1.18) or when fully adjusted (HR 0.89, 95% CI 0.56–1.40). Results from the sensitivity analyses did not indicate substantial effects to the final model from additional covariables, inclusion of cholesterol or higher levels of beta-blocker use with follow-up time. Sensitivity analysis to estimate the impact of incident glaucoma did not indicate a substantial effect from incident glaucoma cases. Altogether, 52,986 individuals aged over 40 years currently live in the catchment area of the initial EPIC-Norfolk recruitment. Using the UK National Eye Health Epidemiological Model,14 numbers with incident glaucoma were calculated from this cohort and applied to the aging EPIC-Norfolk cohort. Non-conservative models predicted a 1.56% incident rate of glaucoma. Applied to the baseline population excluding those with self-reported glaucoma, this predicted an additional 392 cases of incident glaucoma over the study period. Trends in glaucoma medication prescribing have changed with time, where prostaglandins are the preferred medication15 and beta-blocker prescription has dropped by a factor of four. Applying an exaggerated 25% prevalence of topical beta-blocker exposure to these incident cases15 would lead to an additional 98 topical beta-blocker exposures in the EPIC-Norfolk cohort. Applying an exaggerated death rate of 50% to those newly diagnosed with glaucoma on topical beta-blockers, would add an additional 49 deaths. Assuming 30% died from cardiovascular causes compared to the 20.1% observed in the present study, along with adjustments in exposure status in baseline users through application of historic trends in prescribing,15 did not yield significant associations of cardiovascular mortality (HR 1.30, 95% CI 0.98–1.70, p = 0.06). This would suggest the findings of this study are robust to changes in exposure status over time.
Table 4. Associations between topical beta-blocker use and cardiovascular mortality in 514 participants with self-reported glaucoma in the European Prospective Investigation into Cancer Norfolk Cohort Study (EPIC-Norfolk, 1993–2013).
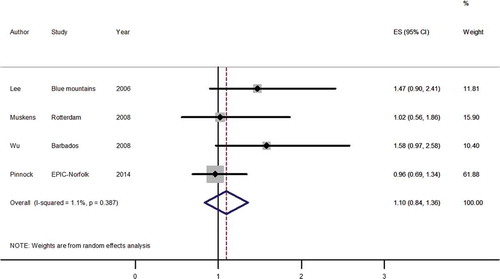
A total of three studies,2,3,9 that looked at topical beta-blockers and cardiovascular mortality were identified from the systematic literature search. presents results from the meta-analysis for the effect of topical beta-blockers on cardiovascular mortality, regardless of glaucoma status. Between-study heterogeneity using the I2 statistic was 1.1% (p = 0.387). Topical beta-blocker use was not associated with cardiovascular mortality (pooled HR estimate 1.10, 95% CI 0.84–1.36).
Figure 2. Forest plot of random effects meta-analysis examining effect of topical beta-blocker use on cardiovascular mortality.
ES, effect size; CI, confidence interval; Blue Mountains, Blue Mountains Eye Study; Rotterdam, Rotterdam Study; Barbados, Barbados Eye Studies; EPIC-Norfolk, European Prospective Investigation into Cancer Norfolk Cohort Study.
Discussion
In this prospective cohort study of 25,639 participants, topical beta-blocker use was not associated with increased cardiovascular mortality. Furthermore, in the 514 participants within the cohort who had self-reported glaucoma, we did not detect an association between the use of topical beta-blockers and a higher risk of cardiovascular mortality. There was also no evidence of an association between the use of topical beta-blockers and cardiovascular mortality. These results do not support higher cardiovascular mortality from topical beta-blocker use. However, the current study is based on self-reported glaucoma, which is a key limitation.
Results from this EPIC-Norfolk study are in agreement with the Rotterdam Study9 but differ from the Blue Mountains Eye Study6 and Barbados Eye Studies.3 In the Blue Mountains Eye Study, the association between topical beta-blockers and cardiovascular mortality was only seen in participants using timolol with glaucoma, compared to those not using topical timolol without glaucoma. The effect on cardiovascular mortality was not shown in baseline users of timolol before stratification by glaucoma status (relative risk, RR, 1.47, 95% CI 0.90–2.41). In baseline timolol users in the Barbados Eye Studies, there was also no association shown between timolol use and cardiovascular mortality (RR 1.58, 95% CI 0.97–2.58). The association was only seen when compared within users diagnosed with open-angle glaucoma. The primary meta-analysis used these summary statistics in order to compare like with like and did not show an association between topical beta-blocker use and cardiovascular mortality. Subgroup analysis of participants with self-reported glaucoma in the present study found no association between exposure to topical beta-blockers and cardiovascular mortality (HR 0.89, 95% CI 0.56–1.40).
There is well-established literature on the protective effects of oral beta-blockers. Multiple randomized controlled trials and systematic reviews have found that use of systemic beta-blockers decreases mortality, improves survival, and is cardio-protective.16–20 However, the majority of these studies have shown the protective effect only in participants who are post myocardial infarction or with established cardiovascular disease. These observed benefits may not translate to people with no pre-existing cardiovascular disease. The main cardiovascular effects of beta-blockers include reduction of heart rate, myocardial contractile force, cardiac output and peripheral vasoconstriction. Unwanted side effects include bradycardia and exacerbation of heart failure in susceptible patients, and these complications form a potential mechanism for increased risk of cardiovascular mortality from topical beta-blocker use, although there is little observed evidence of this beyond case reports.8 Randomized controlled trials have shown that topical beta-blockers may have an adverse effect on lipid profiles. A review of timolol, the most common topical beta-blocker linked to adverse lipid profiles, found that when given systemically, timolol reduced mortality after myocardial infarction21 indicating that any deleterious effects on serum lipids did not attenuate its protective effect on the heart.
The strengths of this study lie in its study design; EPIC-Norfolk is a long-term prospective cohort study. Other strengths include the long period of follow-up, the number of participants, and the high number of incident cardiovascular mortality events, enabling high statistical power. The large number of covariables measured at baseline allowed for adjustment of multiple confounders in the analysis and the assessment of the robustness of the findings across different analytical variations. A validation study22 has confirmed accuracy of cardiovascular mortality in EPIC-Norfolk, with 38 of 39 cases confirmed as correct. Last, the linking of outcome data through the Office for National Statistics death register allowed for complete ascertainment of outcome status in this study, regardless of whether the participant was lost to follow-up. This eliminated attrition bias.
There were several key limitations of the study. There was limited power within the subgroup analysis of glaucoma users whose baseline cohort was small (n = 514). This was further reduced after excluding deaths not from cardiovascular causes (n = 377) and may have allowed type II error to occur. There may be a degree of healthy-volunteer bias occurring. This is suggested by the considerably lower proportion of current and ex-smokers, and a higher proportion of never smokers compared to the rest of England. This may also have contributed to the relatively low proportions of cardiovascular deaths in this older population. The study only assigned participants to the exposed category if topical beta-blockers were used before or at baseline and no adjustments were made for changes in exposure status with time. Additionally, it was not possible to ascertain compliance of individuals exposed to topical beta-blockers, who may have filled prescriptions they were not taking. This may have resulted in an underestimate of effect. Our methods are in line with the previous studies examined in the meta-analysis, and allowed for direct comparisons. We did, however, examine the impact of incident glaucoma and did not detect a significant effect. Publication bias was possible in the meta-analyses, but the use of a funnel plot was of little value due to the number of studies identified. It is plausible that there are smaller studies that have unpublished non-significant results that would strengthen the absence of an association between topical beta-blocker use and cardiovascular mortality and favor the null hypothesis.
We did not examine the relationship between glaucoma diagnosis and mortality as it was beyond the scope of this study. It is possible that topical beta-blocker use may mask heterogeneous pathophysiological processes that underpin both glaucoma and ocular hypertension. Both conditions are treated with topical beta-blockers and display different associations with hypertension and diabetes.23–26 This is particularly relevant to this study as we used self-reported glaucoma, where participants could report a glaucoma diagnosis even if they had ocular hypertension. Furthermore, up to 50% of glaucoma in the community can be undiagnosed.27,28 These considerations would lead to a measurement error in exposure ascertainment, and although likely to contribute to a null finding, the exact impact is unknown.
In conclusion, no association was observed between topical beta-blocker use and cardiovascular mortality in all participants or in participants with self-reported glaucoma. A meta-analysis with other large population-based studies showed no association in the pooled estimates between topical beta-blockers and cardiovascular mortality, both for all participants and for only participants with glaucoma. However, further studies are required that allow for adjustment for changes in exposure to topical beta-blockers. We have found no further evidence to suggest that patients using topical beta-blockers experience excess cardiovascular mortality, and no indications that current clinical practice requires revision. Clinicians should continue to exercise caution when assessing individual glaucoma patients and their cardiovascular risk prior to commencing topical beta-blockers.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the writing and content of this article.
Acknowledgments
We would like to thank Mr. Pak S. Lee for the training of research clinic nursing staff and equipment maintenance, Ms. Marlene Lentjes for analytical support, Nichola Dalzell for running the health examination clinic and all the EPIC-Norfolk participants for their time and support
Funding
EPIC-Norfolk infrastructure and core functions are supported by grants from the Medical Research Council (G1000143) and Cancer Research UK (C864/A14136). The clinic for the third health examination was funded by Age UK Research into Ageing (262). Mr. Khawaja is a Wellcome Trust Clinical Research Fellow. Mr. Foster has received additional support from the Richard Desmond Charitable Trust (via Fight for Sight) and the Department for Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital and the UCL Institute of Ophthalmology for a specialist Biomedical Research Centre for Ophthalmology. None of the funding organizations had a role in the design or conduct of the research.
Additional information
Funding
References
- National Institute for Health and Care Excellence (NICE). CG85: Glaucoma: Diagnosis and management of chronic open angle glaucoma and ocular hypertension. London, UK: NICE, 2009.
- Lee AJ, Wang JJ, Kifley A, et al. Open-angle glaucoma and cardiovascular mortality: the Blue Mountains Eye Study. Ophthalmology 2006;113:1069–1076.
- Wu SY, Nemesure B, Hennis A, et al. Open-angle glaucoma and mortality: the Barbados Eye Studies. Arch Ophthalmol 2008;126:365–370.
- Nieminen T, Lehtimaki T, Maenpaa J, et al. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest 2007;67:237–245.
- Yamamoto T, Kitazawa Y, Noma A, et al. The effects of the beta-adrenergic-blocking agents, timolol and carteolol, on plasma lipids and lipoproteins in Japanese glaucoma patients. J Glaucoma 1996;5:252–257.
- Mitchell P, Wang JJ, Cumming RG, et al. Long-term topical timolol and blood lipids: the Blue Mountains Eye Study. J Glaucoma 2000;9:174–178.
- Freedman SF, Freedman NJ, Shields MB, et al. Effects of ocular carteolol and timolol on plasma high-density lipoprotein cholesterol level. Am J Ophthalmol 1993;116:600–611.
- Stewart WC, Garrison PM. Beta-blocker-induced complications and the patient with glaucoma. Newer treatments to help reduce systemic adverse events. Arch Int Med 1998;158:221–226.
- Muskens RP, Wolfs RC, Witteman JC, et al. Topical beta-blockers and mortality. Ophthalmology 2008;115:2037–2043.
- Connor AJ, Fraser SG. Glaucoma prescribing trends in England 2000 to 2012. Eye 2014;28:863–869.
- Perry CM, McGavin JK, Culy CR, et al. Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging 2003;20:597–630.
- Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 1999;80(Suppl. 1):95–103.
- Shields B. Text book of glaucoma, 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins, 2010.
- NEHEM. National Eye Health Epidemiological Model. 2014. Available from: www.eyehealthmodel.org
- Connor AJ, Fraser SG. Glaucoma prescribing trends in England 2000 to 2012. Eye (London, England) 2014;28:863–869.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Int Med 2001;134:550–560.
- Quint JK, Herrett E, Bhaskaran K, et al. Effect of beta blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare records. BMJ 2013;347:f6650.
- Lee DS, Markwardt S, McAvay GJ, et al. Effect of beta-blockers on cardiac and pulmonary events and death in older adults with cardiovascular disease and chronic obstructive pulmonary disease. Medical Care 2014;52(Suppl. 3):S45–51.
- Al-Gobari M, El Khatib C, Pillon F, et al. beta-Blockers for the prevention of sudden cardiac death in heart failure patients: a meta-analysis of randomized controlled trials. BMC Cardiovascular Disorders 2013;13:52.
- Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005;353:349–361.
- Gundersen T, Kjekshus J, Stokke O, et al. Timolol maleate and HDL cholesterol after myocardial infarction. Eur Heart J 1985;6:840–844.
- Boekholdt SM, Peters RJ, Day NE, et al. Macrophage migration inhibitory factor and the risk of myocardial infarction or death due to coronary artery disease in adults without prior myocardial infarction or stroke: the EPIC-Norfolk Prospective Population study. Am J Med 2004;117:390–397.
- Leske MC, Wu SY, Nemesure B, et al. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol 2002;120:954–959.
- Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology 1995;102:54–60.
- Mitchell P, Smith W, Chey T, et al. Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology 1997;104:712–718.
- Tielsch JM, Katz J, Quigley HA, et al. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology 1995;102:48–53.
- Tielsch JM, Katz J, Singh K, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol 1991;134:1102–1110.
- Mitchell P, Smith W, Attebo K, et al. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:1661–1669.
