ABSTRACT
Purpose: We sought to determine the prevalence of trachomatous inflammation – follicular (TF) in children aged 1–9 years, and trachomatous trichiasis (TT) in those aged ≥15 years, in suspected trachoma-endemic areas of Papua New Guinea (PNG).
Methods: We carried out six population-based prevalence surveys using the protocol developed as part of the Global Trachoma Mapping Project.
Results: A total of 19,013 individuals were sampled for inclusion, with 15,641 (82.3%) consenting to participate. Four evaluation units had prevalences of TF in children ≥10%, above which threshold the World Health Organization (WHO) recommends mass drug administration (MDA) of azithromycin for at least three years; Western Province (South Fly/Daru) 11.2% (95% confidence interval, CI, 6.9–17.0%), Southern Highlands (East) 12.2% (95% CI 9.6–15.0%), Southern Highlands (West) 11.7% (95% CI 8.5–15.3%), and West New Britain 11.4% (95% CI 8.7–13.9%). TF prevalence was 5.0–9.9% in Madang (9.4%, 95% CI 6.1–13.0%) and National Capital District (6.0%. 95% CI 3.2–9.1%) where consideration of a single round of MDA is warranted. Cases of TT were not found outside West New Britain, in which four cases were seen, generating an estimated population-level prevalence of TT in adults of 0.10% (95% CI 0.00–0.40%) for West New Britain, below the WHO elimination threshold of 0.2% of those aged ≥15 years.
Conclusion: Trachoma is a public health issue in PNG. However, other than in West New Britain, there are few data to support the idea that trachoma is a cause of blindness in PNG. Further research is needed to understand the stimulus for the active trachoma phenotype in these populations.
Introduction
Trachoma is an eye disease caused by infection with particular strains of the bacterium Chlamydia trachomatis. It is the leading infectious cause of blindness worldwide, and is found in the most isolated, rural areas of the developing world.Citation1 The bacterium is spread by direct close contact with infected individuals, or indirectly, by contact with fomites or with particular species of flies (Musca sorbens) that passively transfer C. trachomatis on their bodies. The early signs of infection (active trachoma) are most commonly seen in younger children, characterized by inflammatory thickening (trachomatous inflammation – intense, TI) or sub-conjunctival follicles (trachomatous inflammation – follicular, TF) in the area of the upper tarsal conjunctivae. Over many years, recurrent infections may lead to scarring of the conjunctivae (trachomatous scarring), with inward turning of the eyelashes so that they touch the globe, known as trachomatous trichiasis (TT). Persistent rubbing of these lashes can lead to opacification of the cornea, and eventual corneal blindness.Citation2
According to the World Health Organization (WHO), 51 countries are known or suspected to be endemic for blinding trachoma, with the burden of disease concentrated in Sub-Saharan Africa.Citation3 The disease is targeted by WHO for elimination as a public health problem by the year 2020, with control programs implementing the SAFE (Surgery, Antibiotics, Facial cleanliness, Environmental improvement) strategy in endemic areas.Citation4,Citation5
Papua New Guinea (PNG) is a tropical island nation located in the Western Pacific, directly north of Australia. The country has 7.4 million inhabitants in four major regions (Highlands, New Guinea Islands, Momase/Northern, Southern) made up of 22 provinces, and a total of 87 administrative districts (Figure 1).Citation6 Communities are widely scattered and access to services is often difficult, as >80% of the country’s population lives in rural areas, only 3% of the roads are paved, and many villages can only be reached on foot or by air.Citation7 PNG has high rates of visual impairment (29%) and blindness (3.9–8.9%) in older adults; the most recent epidemiological study conducted in urban and rural populations of PNG’s capital (Port Moresby) established that uncorrected refractive error and cataract were the two most common causes of visual impairment in people aged 50 years and older.Citation8,Citation9
PNG has historically been noted as being trachoma endemic,Citation10 however current data are sparse. A study in Madang Province published in 1982 described the results of a 2-year survey of 6153 children in 30 community schools, in which evidence of trachoma was found in 19.6% of children examined.Citation11 In 1972, 4.6% of children in a village on Manus Island (Manus Province) showed signs of disease.Citation12 A retrospective review of outpatient notes at a Madang ophthalmology clinic found only 18 patients of 30,441 seen between 1980 and 1989 to have had a presentation consistent with trachoma.Citation13 A survey in 1979–1980 in four selected areas (Hanuabada in National Capital District (NCD), Rigo district of Central province, Rabaul township in East New Britain province and Telefomin in Sandaun province) found very low levels (0.3%, n = 3029) of visual impairment or blindness (visual acuity <6/18) associated with trachoma, apart from in those aged 60 years and older in Rabaul and Rigo, where 3.6% and 6.1% of cases, respectively, were thought to be trachoma-related.Citation10
Contemporary ophthalmologists regularly report seeing signs of active trachoma in children in PNG, but rarely report TT. The exception to this is in New Britain province, where corrective trichiasis surgeries have been carried out in recent years (unpublished data, Dr Jambi Garap, Dr Apisai Kerek and Dr David Pahau). A similar pattern has been seen in other Western Pacific countries such as the Solomon Islands, Vanuatu, and Fiji, where recent surveys have found a high prevalence of TF in the children, but with little TT seen in adults (unpublished data, Mr O. Sokana; personal correspondence, Ms F. Taleo).Citation14 However, in neighboring Australia, trachoma is still a significant cause of blindness in remote Indigenous communities. A 2008 population-based survey found trachoma in 60% of such communities, with TT present in 1.4% of Indigenous adults examined.Citation15,Citation16
Until 2014, PNG had few or no current data to guide trachoma control policy. In response to this, the National Prevention of Blindness Committee, on behalf of the Ministry of Health, commissioned trachoma rapid assessments targeting suspected trachoma-endemic sites in six provinces across the country (Central – NCD, Rigo; Madang – Ramu; Morobe – Markham; East New Britain – Rabaul; Southern Highlands – Mendi, Nipa; Western – South Fly, Daru).Citation17 An experienced, Global Trachoma Mapping Project (GTMP) certified trachoma grader trainer (OS) and a PNG ophthalmologist (DP) examined 823 children aged 1–9 years and 600 adults aged 15 years and older in 22 sites, and found 93 cases of TF (11% of children examined).Citation17 In the sites in NCD, Madang, Markham, Mendi, Nipa, and South Fly, >5% of the children examined were found to have TF, and so a recommendation was made for these areas to proceed to population-based prevalence surveys in the current study. West New Britain-Talasea and Kandrian-Gloucester were also included due to a recent history of trichiasis surgeries in these districts.
At the request of the National Prevention of Blindness Committee, and on behalf of the Ministry of Health, we planned to carry out seven cross-sectional trachoma prevalence surveys with the aim in each one of estimating the prevalence of TF in children aged 1–9 years, and the prevalence of TT in adults aged 15 years and older. In addition, we aimed to evaluate the association of water, sanitation and hygiene (WASH) variables with trachoma in these populations.
Materials and methods
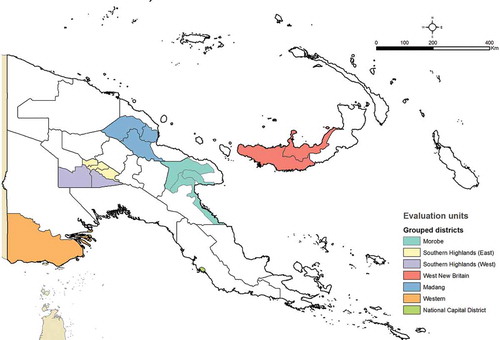
The surveys were carried out in accordance with the methodologies developed for the GTMP,Citation18 with districts grouped into evaluation units (EUs) of no more than 200,000 total population. District grouping was made respecting existing provincial administrative boundaries ().
Figure 2. Evaluation units of grouped districts, Global Trachoma Mapping Project, Papua New Guinea, 2015; Morobe (Markham, Nawae, Huon), Southern Highlands (East; Mendi-Munihu, Imbonggu, Ialibu/Pangia), Southern Highlands (West; Nipa/Kutubu, Kagua/Erave), West New Britain (Talasea, Kandrian-Gloucester), Madang (Madang, Middle Ramu, Usino Bundi), Western Province (South Fly, Daru), National Capital District.
Training
We conducted 5-day training for provincial Papua New Guinean eye care workers. Prospective graders were required to pass a class examination, and a field examination against a GTMP-certified grader trainer (OS). Data recorders were required to pass a class examination, with subsequent direct observation of fieldwork practice by supervising ophthalmologists from the PNG Department of Health. Participants were three ophthalmologists and 13 ophthalmic clinicians, refractionists and community health care workers. All training was carried out in Port Moresby on 5–9 October 2015.
Ethical approval
The study protocol was approved by the Government of PNG, National Department of Health Medical Research Advisory Committee (approval 15.20) with the global project methodologies approved by the London School of Hygiene & Tropical Medicine (approvals 6319 and 8355). Written consent (signature or finger print) was obtained from household heads. Verbal consent to examination, recorded on a custom-coded smartphone application, was considered acceptable in a setting where literacy rates were anticipated to be low. For those younger than 18 years, consent was obtained from a parent or guardian. Participants found to have active trachoma (TF and/or TI in either eye) were provided with two tubes of tetracycline eye ointment to complete a 6-week course. Participants found to have TT were referred to the nearest eye health facility for further assessment and management. Patient confidentiality was maintained at all times, and data were anonymized before analysis.
Field methods
The overall GTMP methodology is outlined elsewhere.Citation18 Briefly, each survey team was made up of one grader, one data recorder, and one driver or logistician. A local guide was also recruited and acted as the interpreter in most cases. The village elder (either a pastor, magistrate or a counsellor) provided permission for the survey team to enter the village, and supplied a village household census list. Every household was allocated a number, from which 30 random numbers were drawn representing the 30 households selected for examination. At each sampled household, the purpose of the survey was explained, with information provided in local languages by the local guide/interpreter. All occupants older than 1 year of age present at the time of the survey were invited to participate. If a household was unoccupied, the most immediate neighboring house was approached. If a sampled village was inaccessible or unsuitable due to bad roads, severe weather, or migration, then the next-nearest village was sampled. Prior to commencement, where possible, public announcements were made on local radio, and written notices highlighting the dates and purpose of the survey were displayed in sampled villages. The ability to provide villages advance notice was limited in some areas of South Fly, Southern Highlands, and West New Britain, as many villages were outside the reach of telecommunication, and access required considerable lengthy travel by road, sea or river.
After informed consent was provided, graders examined the eyelid margins, and conjunctival surface of the upper eyelids under sunlight or good torch light with the aid of a 2.5× magnifying loop, and signs of trachoma were assessed using the WHO simplified trachoma grading system.Citation2 The presence or absence of the clinical signs TT, TF, and TI were called to the data recorder, who immediately recorded findings electronically using a smartphone application (LINKS, Task Force for Global Health, Decatur, GA, USA). Alcohol hand gel was used by the grader between patients to prevent cross-contamination. At each household, Global Positioning System coordinates were collected. Data recorders also recorded data on WASH access by direct observation at the household, and by focused interview with the household head. Full details are published elsewhere.Citation18
Sample size
As described previously,Citation18 each survey was designed to estimate an expected 10% TF prevalence in 1–9-year-olds with an absolute precision of 3% and an estimated non-response rate of 20%. We therefore needed to sample sufficient households in each EU, such that 1222 children aged 1–9 years would be resident in them. Based on the most recent census data,Citation6 it was estimated that a mean of 1.51 children aged 1–9 years would reside in each household, so it was estimated that 45 children aged 1–9 years would be resident in the 30 households sampled in each village. Therefore, to achieve the target sample size of 1222 children in each EU, we estimated that 27 (1222/45 = 27.0) clusters were needed per EU.
Data analysis
Descriptive data were produced using Microsoft Excel 2010. Prevalence and risk factor analyses were produced using R 3.0.2 (2013, R Foundation for Statistical Computing, Vienna, Austria). The village was considered the cluster unit for the purposes of analysis. TF prevalence in children aged 1–9 years was adjusted for age in 1-year age-bands using the latest available census data.Citation6 TT prevalence in those aged ≥15 years was adjusted for age and sex in 5-year age-bands. Confidence intervals (CIs) were generated by bootstrapping adjusted mean cluster-level outcome proportions, and taking the 2.5th and 97.5th centiles of 10,000 resampled iterations. Exact binomial CIs were used to produce an upper confidence limit for zero-count prevalence estimates. A multi-level mixed random effects model was used to assess the independent association of determinant factors, accounting for the clustered survey design at both village and household level, where possible. Factors were considered for the full model if statistically significant on univariate analysis (Wald’s test, p < 0.05), trialed with a stepwise inclusion approach and retained if statistically significant (p < 0.05, likelihood ratio test). Sex was included in the final model due to a priori assumptions.
Results
Six teams surveyed six EUs covering 162 villages over 13 districts from October to December 2015. The seventh planned EU, covering Markham, Nawae and Huon districts in Morobe Province, could not be completed in the required timeframe as a result of insecurity following an outbreak of inter-tribal violence. As a result, data from this EU are not available here.
Interviews were conducted with the household heads of 4180 sampled households, and 19,013 individuals were sampled from these households, including 6555 children aged 1–9 years. Overall, 15,641 people (82.3% of residents living in the sampled households) were present and consented to examination. The median age of those examined was 15 years (range 1–97 years). Overall, 3174 household residents (16.6%) were absent at the time of the survey, and 194 (1.0%) were present but did not consent to examination. Of the 194 participants who did not consent to examination, 102 (52.3%), were from the Southern Highlands (East) EU. The breakdown of these findings by EU is shown in .
Table 1. Participation rates and sex distribution of individuals sampled by evaluation unit, Global Trachoma Mapping Project, Papua New Guinea, 2015.
Overall, there were almost equal proportions of males (49.5%) and females (50.5%) sampled. In those aged 15 years and older, 59.4% of those examined were female, and in those aged 1–9 years, 46.7% of those examined were female ().
Table 2. Demographics of individuals aged 1–9 years and ≥15 years, sampled in six evaluation units, Global Trachoma Mapping Project, Papua New Guinea, 2015.
Adjusted TF prevalences in children aged 1–9 years for each EU are shown in . Four EUs had TF prevalences higher than 10%; Western Province (South Fly/Daru) 11.2% (95% CI 6.9–17.0%), Southern Highlands (East) 12.2% (95% CI 9.6–15.0%), Southern Highlands (West) 11.7% (95% CI 8.5–15.3%) and West New Britain 11.4% (95% CI 8.7–13.9%). The remaining two EUs had TF prevalences <10%; Madang 9.4% (95% CI 6.1–13.0%) and NCD 6.0% (95% CI 3.2–9.1%).
Table 3. Adjusted prevalence of trachomatous inflammation – follicular (TF) in children aged 1–9 years and trachomatous trichiasis (TT) in those aged ≥15 years in six evaluation units, Global Trachoma Mapping Project, Papua New Guinea, 2015.
Four cases of TT were identified across all surveys; one male aged 60 years, and three females aged 53, 54 and 70 years. All TT cases identified were found in West New Britain. The details of numbers examined by EU, and overall adjusted TT prevalences in those aged 15 years and older, are shown in .
WASH indicators
Of participating households in the EU in NCD (PNG’s capital city), 99.9% of households used improved drinking water sources (water that is likely to be protected from outside contamination), and 75.6% had improved sanitation (a facility that hygienically separates human excreta from human contact).Citation19 In contrast, the surveys of two EUs in the Southern Highlands, West (Nipa/Kutubu, Kagua/Erave districts), and East (Mendi-Munihu, Imbonggu, and Ialibu/Pangia districts) revealed low prevalences of improved sanitation in households in the Southern Highlands; 1.1% (95% CI 0.4–1.6%) and 0.0% (95% CI 0.0–1.0%), respectively. summarizes key indicators on WASH variables assessed for each EU.
Table 4. Water, sanitation and hygiene (WASH) variables, Global Trachoma Mapping Project, Papua New Guinea, 2015.
Risk factors
The univariable and multivariable analyses for associations of TF in children aged 1–9 years (), showed that the odds of having TF was strongly and independently associated with younger age. Children aged 1–5 years had significantly greater odds of having TF than did those aged 6–9 years (odds ratio, OR, 1.6, p < 0.0001). Other associations were household use of an unimproved washing water source (OR 2.0, p < 0.0001), and household use of an unimproved latrine (OR 1.5, p = 0.03).
Table 5. Multi–level random effects logistic regression of factors against the outcome trachomatous inflammation – follicular (TF) in children aged 1–9 years, Global Trachoma Mapping Project, Papua New Guinea, 2015.
Discussion
Trachoma remains endemic in PNG, with TF prevalences ranging from 6.0 to 12.2% in the six EUs surveyed in the work described here. All six EUs surveyed are eligible for implementation of the A, F and E components of the SAFE strategy, with the four EUs that had TF>10% (Western Province – South Fly/Daru, Southern Highlands – West, Southern Highlands – East, and West New Britain) requiring three mass drug administration (MDA) rounds, and the other two EUs with TF prevalence 5.0–9.9% (NCD and Madang) requiring one round of MDA before impact surveys are undertaken. Population-based TT surgery services are not required since all EUs had TT prevalences in adults below the elimination threshold of 0.2%, although directed case-finding around the identified cases would be recommended. TF prevalence was lowest in NCD (the country’s administrative capital; 6.4%). This is consistent with the generally higher living standards in the capital city, with 99% of households having improved drinking water sources and 75% of households having improved sanitation. WASH access was strikingly lower in all other areas surveyed.
Active trachoma most commonly occurs in children, with the highest prevalence in children younger than 5 years.Citation20–Citation22 In this study, younger children were more likely than their older peers to have active trachoma. This contrasts with findings in nearby Solomon Islands, Fiji, and Vanuatu, where older children (8–9 years) are reportedly more likely to have TF (unpublished data, Mr O. Sokana, Dr C. Macleod; personal correspondence, Ms F. Taleo). Possible reasons for, and the robustness of, this distinction are presently unclear.
The prevalence of TT was zero in five EUs. In West New Britain, four TT cases were found, giving an EU-level TT prevalence of 0.1% in those aged 15 years and older. This is notable because this was the province in which local ophthalmologists report carrying out trichiasis surgeries in recent years. All EUs surveyed had TT prevalences in adults below the WHO 0.2% threshold for elimination. This is consistent with the prevailing belief that trachoma is endemic in PNG, but not a cause of blindness.Citation10,Citation11
Overall, our data are insufficient to make meaningful generalizations about the significance of the high TF/low TT situation in PNG. Other Western Pacific Island countries have also reported similar findings (unpublished data, Mr O. Sokana, Dr C. Macleod; personal correspondence, Ms F. Taleo).Citation14,Citation23 Interestingly, in Aboriginal communities in nearby Australia, blindness from trachoma is still a public health issue.Citation16 We could hypothesize that different C. trachomatis strains from the ones associated elsewhere with trachoma could be found in the Pacific, perhaps with gene polymorphisms (such as in the matrix metalloproteinases)Citation24 that are important to the scarring process. It could also be hypothesized that the follicles seen in children here are related to stimuli other than C. trachomatis, such as exposure to smoke from cooking fires,Citation25 for example; a common occurrence in PNG. Alternatively, a PNG/Pacific human genotype may exist that confers resistance or limits expression of trachomatous scarring, or protective geoclimatic factors may be present.Citation26
Of note, PNG is highly endemic for yaws,Citation27 a disease of skin, bone, and cartilage targeted by WHO for eradication by the year 2020. Yaws is caused by a spirochaete bacterium, Treponema pallidum pertenue, which is sensitive to a single dose of azithromycin. MDA with azithromycin can effectively treat both trachoma and yaws,Citation28 as well as urogenital chlamydial infections.Citation29,Citation30 This might alter the possible balance of cost and benefits towards implementation of azithromycin mass treatment.
Trachoma has been linked with low levels of sanitationCitation31 and we noted very low levels of sanitation in all EUs other than NCD. Less than 0.1% of households in the Southern Highlands had improved sanitation. The presence of TF in children was strongly linked to household use of an unimproved washing water source, and household use of an unimproved latrine. There is an urgent need for the improvement of sanitation across PNG generally. PNG was recently listed in a global report as having the highest proportion of people without access to safe water (60%).Citation32 The WASH data presented here reflect those of the WHO and United Nations Children’s Fund (UNICEF) Joint Monitoring Programme for Water Supply and Sanitation, showing considerable disparities in access between urban and rural populations, and poor access overall.Citation19 Access to improved sanitation should decrease transmission of a range of pathogens, as well as being a basic human right.
Half of all refusals to participate came from one EU, in Southern Highlands (West). This was reportedly related to fears of visiting teams and a superstition that the team’s smartphones introduced satanic messages. We were also later informed that false rumors had spread among community residents implying that our survey teams were in some way anti-Christian. Whether the individuals examined in this EU were truly representative of the underlying population is unclear, although those who refused only accounted for 2.7% of the total number resident in this EU. Fundamentalist Christian communities are common in PNG, and there is a growing recognition of the importance of understanding the different frameworks people use in explaining their world.Citation33,Citation34 The reluctance to participate that we encountered could conceivably recur in future survey efforts, and in any attempts to deliver community-based trachoma elimination interventions. Spending more time in dialogue with communities will be necessary to appropriately foster trust. As we were unable to collect sufficient data from one EU, no information about the prevalence of trachoma in Morobe was available. Further data will be collected when doing so becomes possible. PNG is a very challenging country in which to conduct research, as internal travel is very difficult and costly, there are high levels of violence, and very low numbers of trained health professionals.Citation35 Additionally, during 2015 much of PNG was affected by a severe drought, which had a major impact on water supply, and forced migration in many parts of the country.Citation36 In all other instances where the survey team was unable to complete data collection from a sampled village (due to villager superstition of the survey team, tribal fighting, inaccessibility due to weather or village migration due to drought), alternative villages were sampled and surveyed.
PNG possesses rich cultural diversity, with hundreds of ethnic groups, marked socio-cultural differences between and within provinces, and more than 800 indigenous languages. There is no internal road structure in the country, and communities are often isolated from their nearest neighbors. Despite this, the epidemiology of trachoma that we documented in this study appeared to be largely uniform, and supports the theory that trachoma is non-blinding here. Based on WHO guidelines, elimination of active trachoma here is recommended through MDA of azithromycin. This approach may also be beneficial in the control of other endemic conditions in PNG. Given the potential cost, and possibly marginal benefit, the decision on whether to implement costly azithromycin MDA rounds is not simple, although almost certainly necessary in West New Britain.
Trachoma is endemic in PNG but appears to not have the blinding effects seen in many other countries. The significance of this is unclear, and more research is urgently needed to guide national and global policy makers.
Acknowledgements
We thank the CEOs of hospitals that released staff for the GTMP survey: Mr Grant Muddle (CEO Port Moresby General Hospital), Dr Joseph Nale (CEO Kimbe General Hospital), Dr Kintwa (CEO Mt Hagen General Hospital), Mr Joseph Turian (CEO Mendi General Hospital), Mr Mark Mauludu (CEO Wewak General Hospital), Sr Opah Tugo (CEO Daru Hospital), Mr Felix Yapai (OIC Gusap Health Centre Ramu) and Mr Aaron Luai (CEO Wabag General Hospital). We also thank staff from the National Department of Health: Health Secretary Mr Pasco Kase, Chief Ophthalmologist Dr Simon Melengas, and the Chairman of the Medical Research Advisory Committee Dr Ururang Kitur. Thanks also to Mr Samuel Koim (PNG Eye Care), Mrs Rose Kehannie (Callan Services Daru), Nick Gan (Madang Fred Hollows Foundation), Professors John Vince and N. Tefuarani (University of Papua New Guinea School of Medicine and Health Sciences), and the Fred Hollows Foundation New Zealand.
The Global Trachoma Mapping Project Participants were: Supervisors Dr Jambi Garap and Dr David Pahau, with Mr Charles Abio, Ms Theresia Gende, Mr Jonah Jerry, Sr Schola Kapou, Dr Apisai Kerek, Dr Robert Ko, Sr Dorcas Kura, Mr Samson Likas, Sr Francisca Mape, Mrs Marychrista Makamoli, Mr Alois Michael, Ms Jennifer Mor, Mr Alphonse Sambai, Mr Alphonse Tagai, Mr Jeffery Tuvi, Dr Waimbe Wahamu, Mr Lawrence Yala, and Mr Francis Yasaking.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the writing and content of this article.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID) through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Welcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
Additional information
Funding
References
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013;1:e339–e349.
- Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 1987;65:477–483.
- World Health Organization. WHO Alliance for the Global Elimination of Blinding Trachoma by the year 2020: Progress report on elimination of trachoma, 2013. Wkly Epidemiol Rec 2014;89(89):421–428. Accessed June 15, 2016 from: http://www.who.int/wer
- Emerson PM, Burton M, Solomon AW, et al. The SAFE strategy for trachoma control: using operational research for policy, planning and implementation. Bull World Health Organ 2006;84:613–619.
- West SK. Blinding trachoma: prevention with the safe strategy. Am J Trop Med Hyg 2003;69(5 Suppl.):18–23.
- National Statistical Office of Papua New Guinea. 2011 National Report. Port Moresby: Author, 2011. http://www.nso.gov.pg/index.php/population-and-social/demographic-indicators
- World Health Organization. Papua New Guinea Country Profile 2011. World Health Organization, Western Pacific Region, 2011. http://www.wpro.who.int/countries/png/en/index.html
- Garap JN, Sheeladevi S, Shamanna BR, et al. Blindness and vision impairment in the elderly of Papua New Guinea. Clin Experiment Ophthalmol 2006;34:335–341. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16764653
- Garap JN, Sheeladevi S, Brian G, et al. Cataract and its surgery in Papua New Guinea. Clin Experiment Ophthalmol 2006;34:880–885. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17181621
- Dethlefs R. The trachoma status and blindness rates of selected areas of Papua New Guinea in 1979-80. Aust J Ophthalmol 1982;10:13–18.
- Parsons GA. An ocular survey of community school children in Madang Province. P N G Med J 1982;25:151–154.
- Heath SA, Heath BH. Trachoma and other eye disease in a New Guinea village. Am J Ophthalmol 1973;75:121–129.
- Parsons GA. A decade of ophthalmic statistics in Papua New Guinea. P N G Med J 1991;34:255–261.
- Mathew AA, Keeffe JE, Le Mesurier RT, et al. Trachoma in the Pacific Islands: evidence from Trachoma Rapid Assessment. Br J Ophthalmol 2009;93:866–870.
- Taylor HR, The National Indigenous Eye Health Survey Team. National Indigenous Eye Health Survey: Minum Barreng (Tracking Eyes). Melbourne: The University of Melbourne, Centre for Eye Research Australia, Vision CRC, 2009.
- Taylor HR, Fox SS, Xie J, et al. The prevalence of trachoma in Australia: the National Indigenous Eye Health Survey. Med J Aust 2010;192:248–253.
- Sokana O, Pahau D. Report on the Trachoma Preliminary Exploratory Survey in Papua New Guinea, 2014.
- Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol 2015;22:214–225.
- World Health Organization, UNICEF. Joint Monitoring Programme (JMP) for water supply and sanitation. 2013. http://www.wssinfo.org/definitions-methods/
- Taylor HR, Burton MJ, Haddad D, et al. Trachoma. Lancet 2014;384(9960):2142–2152.
- West SK, Munoz B, Turner VM, et al. The epidemiology of trachoma in Central Tanzania. Int J Epidemiol 1991;20:1088–1092.
- Dolin PJ, Faal H, Johnson GJ, et al. Trachoma in the Gambia. Br J Ophthalmol 1998;82:930–933.
- International Agency for the Prevention of Blindness. Trachoma Mapping in the Pacific. Melbourne: IAPB, 2013.
- Natividad A, Cooke G, Holland MJ, et al. A coding polymorphism in matrix metalloproteinase 9 reduces risk of scarring sequelae of ocular Chlamydia trachomatis infection. BMC Med Genet 2006;7.
- Zambrano AI, Muñoz BE, Mkocha H, et al. Exposure to an indoor cooking fire and risk of trachoma in children of Kongwa, Tanzania. PLoS Negl Trop Dis 2015;9(6).
- Schémann JF, Laffly D, Sacko D, et al. Trichiasis and geoclimatic factors in Mali. Trans R Soc Trop Med Hyg 2007;101:996–1003.
- Solomon AW, Marks M, Martin DL, et al. Trachoma and yaws: common ground? PLoS Negl Trop Dis 2015;9:e0004071.
- Marks M, Vahi V, Sokana O, et al. Impact of community mass treatment with azithromycin for trachoma elimination on the prevalence of yaws. PLoS Negl Trop Dis 2015;9(8).
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med 2015;373:2512–2521.
- Marks M, Bottomley C, Tome H, et al. Mass drug administration of azithromycin for trachoma reduces the prevalence of genital Chlamydia trachomatis infection in the Solomon Islands. Sex Transm Infect 2016;92:261–265. doi:10.1136/sextrans-2015-052439
- Stocks ME, Ogden S, Haddad D, et al. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med 2014;11:e1001605.
- Burgess T. Water: at what cost? The State of the World’s Water 2016. London: WaterAid, 2016.
- Eves R. Resisting global AIDS knowledges: born-again christian narratives of the epidemic from Papua New Guinea. Med Anthropol 2012;31:61–76.
- Kelly-Hanku A, Aggleton P, Shih P. “We call it a virus but I want to say it’s the devil inside”: redemption, moral reform and relationships with God among people living with HIV in Papua New Guinea. Soc Sci Med 2014;119:106–113. doi:http://dx.doi.org/10.1016/j.socscimed.2014.08.020
- World Health Organization, National Department of Health Papua New Guinea. Health Service Delivery Profile: Papua New Guinea. Geneva: WHO, 2012. http://www.wpro.who.int/countries/png/en/index.html
- Bourke M. As Papua New Guinea faces worsening drought, a past disaster could save lives. The Conversation. 2015. Accessed April 6, 2015 from: https://theconversation.com/as-papua-new-guinea-faces-worsening-drought-a-past-disaster-could-save-lives-46390
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.