ABSTRACT
Purpose: Following interventions against trachoma in Viet Nam, impact surveys conducted in 2003–2011 suggested that trachoma was no longer a public health problem. In 2014, we undertook surveillance surveys to estimate prevalence of trachomatous inflammation—follicular (TF) and trichiasis.
Methods: A population-based prevalence survey was undertaken in 11 evaluation units (EUs) encompassing 24 districts, using Global Trachoma Mapping Project methods. A two-stage cluster sampling design was used in each EU, whereby 20 clusters and 60 children per cluster were sampled. Consenting eligible participants (children aged 1–9 years and adults aged ≥50 years) were examined for trachoma.
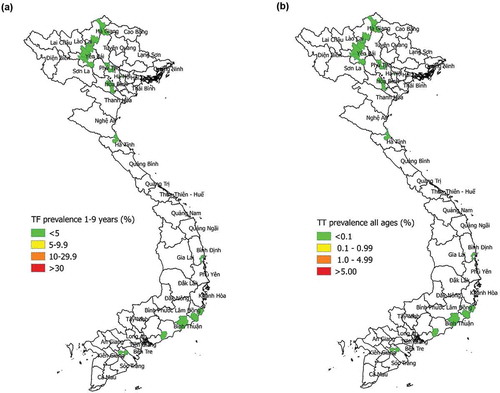
Results: A total of 9391 households were surveyed, and 20,185 participants (98.8% of those enumerated) were examined for trachoma. EU-level TF prevalence in 1–9-year-olds ranged from 0% to 1.6%. In one cluster (in Hà Giang Province), the percentage of children with TF was 10.3%. The overall pattern of cluster-level percentages of children with TF, however, was consistent with an exponential distribution, which would be consistent with trachoma disappearing. Among people aged ≥50 years, prevalence of trichiasis by EU ranged from 0% to 0.75%; these estimates are equivalent to 0–0.13% in all ages. The prevalence of trichiasis unknown to the health system among people aged ≥50 years, by EU, ranged from 0% to 0.17%, which is equivalent to 0–0.03% in all ages.
Conclusion: Findings suggest that trachoma is no longer a public health problem in any of the 11 EUs surveyed. However, given the high proportion of children with TF in one cluster in Hà Giang Province, further investigations will be undertaken.
Introduction
Trachoma is a neglected tropical disease that causes blindness.Citation1 In 2016, it was estimated that worldwide, 200 million people were at risk, and 3.2 million people needed surgery to avoid trachomatous blindness, in 42 countries.Citation2 According to criteria set by the World Health Organization (WHO), trachoma is a public health problem where the prevalence of trachomatous inflammation—follicular (TF) is ≥5% in 1–9-year-olds, and/or the prevalence of trachomatous trichiasis unknown to the health system is ≥0.2% in ≥15-year-olds (approximately equivalent to ≥0.1% in the all-ages population).Citation3 Global political commitment for elimination of trachoma as a public health problem was made manifest by a 1998 World Health Assembly resolution; the target date for achievement of this goal is December 2020.Citation4,Citation5
Viet Nam has been fighting trachoma for more than 60 years. In 1950, the Ophthalmologic Institute of Hanoi (which later became the Viet Nam National Institute of Ophthalmology (VNIO)) estimated that more than 70% of the population on the outskirts of Hanoi suffered from active trachoma at some time during their lives.Citation6 In 1951, more than 200,000 people in North Viet Nam were treated for trachoma by mobile teams.Citation6 Following sustained trachoma control efforts by the government, the national prevalence had declined to 7% in 1995.Citation7 With the 1996 launch of the WHO Alliance for the Global Elimination of Trachoma by 2020 (GET2020), the government of Viet Nam and its partners started implementing the “SAFE strategy” against trachoma, i.e. surgery for trichiasis, antibiotics to clear infection with the causative organism (Chlamydia trachomatis), and facial cleanliness and environmental improvement to reduce transmission.Citation8 In 2000–2001, 67 district-based surveys were undertaken in 27 suspected-endemic provinces to identify local needs for SAFE. After deployment of SAFE interventions, impact surveys were undertaken at four time points: 2003, 2004, 2005, and 2009–2011. These surveys suggested that trachoma was no longer a public health problem, according to WHO criteria. However, WHO’s standard operating procedures for validation of elimination as a public health problemCitation9 had not yet been released. In 2014, given the need to complete a second round of surveys to establish that recrudescence of active trachoma had not occurredCitation10, we undertook a series of pre-validation trachoma surveillance surveys in Viet Nam, aiming to estimate the prevalence of trachomatous inflammation—follicular (TF) in children aged 1–9 years and the prevalence of trichiasis unknown to the health system in adults, in previously trachoma-endemic districts.
Methods
We implemented population-based prevalence surveys (PBPSs) using the systems and methods of the WHO-recommended Global Trachoma Mapping Project (GTMP), including version 2 of the training system,Citation11,Citation12 with five exceptions: (1) graders were certified to participate in fieldwork if they passed a slide-based test, rather than an inter-grader agreement exercise using live subjects; (2) we did not collect data on access to water and sanitation; (3) examination was limited to 1–9-year-olds and ≥50-year-olds, unlike other GTMP projects where all consenting individuals aged ≥1 year living in sampled households were examined; (4) a fixed number of children (rather than a fixed number of households) was recruited in each sampled cluster; and (5) for individuals diagnosed with trichiasis, we asked questions about previous management of trichiasis through surgery or epilation.
Individual-level demographic and examination data were entered into the GTMP-LINKS application on Android smartphones, transmitted to the Cloud, then processed, and reviewed by VNIO (the agency designated by the Ministry of Health for oversight of ophthalmic issues in Viet Nam), as described elsewhere.Citation12 Our principal outcome measures were the evaluation unit (EU)-level TF prevalence in 1–9-year-olds, and the EU-level prevalence of trichiasis unknown to the health system in ≥50-year-olds.
We surveyed 11 EUs (covering 24 districts) selected in consultation with programme partners. The full list of EUs and the rationale for conducting a survey in each one form .
Table 1. Characteristics of survey population by evaluation unit, Global Trachoma Mapping Project, Viet Nam, 2014.
Sample size calculations
We powered each survey to estimate the EU-level TF prevalence in 1–9-year-olds, using an expected prevalence of 10%, and aiming to have 95% confidence of estimating that prevalence with absolute precision of ±3%. Our design effect, based on previous surveys, was 2.65, which resulted in a sample size estimate of 1019 children.Citation12 We additionally wished to estimate trichiasis prevalence in adults, but our sample size was calculated only using parameters relating to TF in children.
Sample selection
Selection of clusters
We used two-stage cluster sampling, categorizing villages (thon) as our first-stage clusters. A list of thon in each EU was obtained from the district authorities. We systematically selected 20 thon with probability proportional to population size, using a computer-generated random starting point.
Selection of households and participants
In selected thon, we used compact segment sampling to choose one segment (xom) at random. Within that xom, a household was visited if at least one child aged 1–9 years, or at least one adult aged ≥50 years, lived there (i.e., had slept there for the past month). If individuals in relevant age ranges were away from home but were expected to be available later the same day, an attempt was made to revisit the household. Once 60 children had been enrolled, the cluster was considered complete. If 60 children could not be enrolled in a single xom, the next-nearest xom was also visited.
Trachoma grading
Graders participating in the surveys had obtained a kappa for diagnosing TF of ≥0.9 in an inter-grader agreement test based on 50 conjunctival photographs of children with and without active trachoma.Citation11 Examination was undertaken according to the criteria set out in the WHO simplified trachoma grading scheme.Citation13 Graders used binocular loupes with 2.5× magnification, and the sun (or if necessary, a torch) for illumination. When trichiasis was diagnosed in an eye, the subject was asked if health workers had previously recommended surgery or epilation for that eye.
Data analysis
We conducted analyses in R (R Foundation for Statistical Computing, Vienna, Austria) and Structured Query Language. Cluster (xom)-level data on TF in1–9-year-olds were adjusted for the age of those examined, using 1-year age bands and the data from the most recent Viet Nam census as the reference dataset.Citation14 Similarly, cluster-level data on trichiasis were adjusted for the age and gender of those examined, using 5-year age bands and the most recent census data.Citation12 The EU-level prevalence of each sign was calculated as the mean of the adjusted cluster-level proportions. We calculated confidence intervals by bootstrapping, with replacement, over 10,000 replications. Prevalence estimates for trichiasis in those aged ≥50 years were used to calculate estimates for the all-ages population by multiplying the prevalence in ≥50-year-olds by 0.173: the proportion of the national population aged ≥50 years, based on the 2009 national census.Citation14
Although we powered our surveys to estimate TF prevalence at EU level, our compact segment sampling approach (involving examination of 60 consenting 1–9-year-olds in a block of adjacent households in one or two xom) means that the proportion of children with TF in a cluster estimates the true prevalence for that group of households. To visualize the distribution of TF by cluster across the entire surveyed area, the means of the northings and eastings for all households within a cluster (obtained by GPS) were calculated to provide a single set of coordinates; results were plotted using QGIS (http://www.qgis.org/en/site/). Using Mathematica 11.1 (Wolfram Research, Champaign, IL, USA), we fit the cluster-level TF prevalence data to a zero-inflated negative binomial, parametrized so the mean of the distribution scaled with the sample size in that community.
Ethical considerations
Ethical approval was granted by the ethics committees of the London School of Hygiene & Tropical Medicine, London, UK (reference 6319) and the Biomedical Ethics Board of National Institute of Ophthalmology, Hanoi, Viet Nam. We obtained informed verbal consent for examination from each participant or (for children) from their parent or guardian. Individuals with conjunctivitis, whether meeting the definition of active trachoma or not, were provided with two tubes of 1% tetracycline eye ointment; individuals with trichiasis were referred to a local surgeon.
Results
Fieldwork was undertaken from May to August, 2014. In 9391 households, survey teams examined a total of 20,185 residents (98.8% of those enumerated), composed of 13,028 1–9-year-olds and 7,157 ≥ 50-year-olds. summarizes the characteristics of the population sampled by EU. Overall, 52% of 1–9-year-olds examined and 42% of ≥50-year-olds examined were male. The mean ages of those examined were 4.8 years (standard deviation 2.6) for the 1–9-year-old group, and 63.4 years (10.4) for the ≥50-year-old group.
Table 2. Characteristics of survey population by evaluation unit, Global Trachoma Mapping Project, Viet Nam, 2014.
Prevalence of trachoma
The prevalence of TF in 1–9-year-olds was <5% in all 11 EUs and ranged from 0% to 1.6% (, ). Among people aged ≥50 years, prevalence of any trichiasis by EU ranged from 0% to 0.75%; these estimates are equivalent to prevalences of any trichiasis in all ages of 0–0.13%. Of 136 people identified as having trichiasis, 125 (92%) were known to the health system, meaning that management for eyes with trichiasis had previously been (1) offered but refused; (2) accepted and a surgical date set; or (3) received, but disease had subsequently recurred. Accounting for previous management of trichiasis, the prevalence of trichiasis unknown to the health system among people aged ≥50 years, by EU, ranged from 0% to 0.17%, which was equivalent to 0–0.03% in all ages (, ).
Table 3. Prevalence of trachoma by evaluation unit, Global Trachoma Mapping Project, Viet Nam, 2014.
Distribution of trichiasis by age, gender
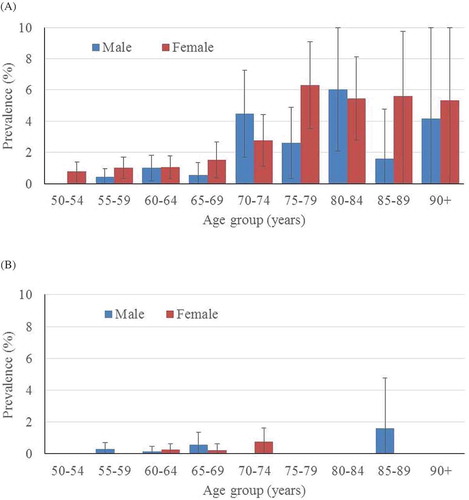
The age- and gender-specific distribution of trichiasis (in all EUs pooled together) is shown in . The age/gender-specific prevalence of trichiasis known to the health system increased with age; however, there were no statistically significant differences among males compared to females (). There were very few cases of trichiasis unknown to the health system ().
Distribution of TF at cluster level
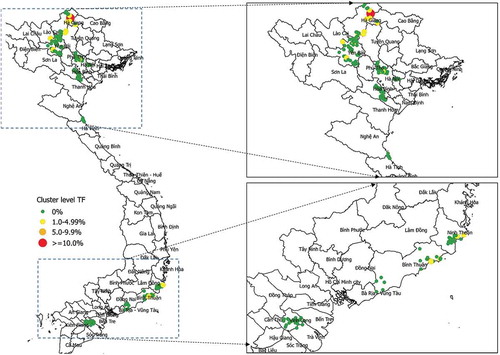
Of the 220 clusters surveyed, the proportion of 1–9-year-olds with TF was 0% in 200 clusters; 1.6 to 3.5% in 19 clusters, and 10.3% in one cluster in Yên Minh district of Hà Giang Province (). Using maximum likelihood estimation, we calculated a shape parameter for the negative binomial distribution of 0.17 (95% CI 0.07–388, bootstrap percentile, n = 999), the very wide confidence intervals commensurate with the fact that only 20 clusters contained cases of TF.
Discussion
This series of pre-validation trachoma surveillance surveys showed that all 11 of the formerly endemic EUs studied now have TF prevalence estimates below the 5% elimination threshold. While in two of 11 EUs, our data suggest that the prevalence of trichiasis in all ages was > 0.1% in 2014, most people with trichiasis appear to be “known to the health system”. The prevalence of trichiasis unknown to the health system in all ages was below the elimination threshold in each EU.
These surveys were epidemiologically robust. Whereas previous trachoma surveys in Viet Nam used the assurance sampling trachoma rapid assessment (ASTRA) methodology,Citation15 we used PBPS methods recommended by WHO.Citation12 Unlike ASTRA, PBPSs provide point prevalence estimates for TF and trichiasis,Citation16 which are needed to determine if elimination thresholds have been reached.Citation9 A second point of difference was the fact that individuals with trichiasis were asked about previous management recommendations from health workers: the first GTMP-supported survey in which this was done. These questions were added in order to help the country determine whether EUs had reached elimination thresholds for trichiasis, which are couched in terms of the prevalence of trichiasis “unknown to the health system” – that is, the prevalence of trichiasis, counting only those individuals with the condition who have not previously been offered an operation or epilation for it.Citation17 Following successful implementation of the questions in Viet Nam, their use was adopted throughout the GTMP.
There were some weaknesses in our work in Viet Nam that should be acknowledged. Although we took our methodological template from previous WHO guidanceCitation18 and worked within the framework of the GTMP,Citation12 we did not examine individuals aged 10–49 years, in order to try to save time for our fieldworkers, who went house-to-house to recruit survey subjects in steeply mountainous terrain. The relatively small numbers of ≥50-year-olds examined in each EU resulted in relatively wide confidence intervals around the trichiasis prevalence estimates (). The lack of examination of adults aged <50 years potentially limits the comparison of our findings to elimination targets, since in many trachoma-endemic settings, trichiasis can be seen in people less than 50 years old. We have assumed that the prevalence of trichiasis in people aged <50 years was negligible. To a certain extent, this assumption is supported by the age distribution of trichiasis cases that the teams identified in the field (), which underpinned our transformation of the trichiasis prevalence estimates in ≥50-year-olds to all-ages estimates. Our assessment of the trichiasis situation is further limited by the fact that we did not record the presence or absence of scars in the tarsal conjunctivae of eyes noted to have trichiasis, because the recommendation to do so was released after our surveys were complete.Citation19 However, this point may be moot for Viet Nam, because the data at hand, allowing for the limitations noted above, suggest that elimination threshold prevalences for trichiasis have been reached.
We also sought to trim fieldwork time by not collecting household-level data on access to water and sanitation, which was routinely integrated within trachoma surveys in other GTMP sub-projects.Citation20–Citation26 Due to a paucity of cases of active trachoma, our graders did not undertake live subject inter-grader agreement exercises to qualify for survey deployment, but were instead assessed using a standard set of 50 conjunctival photographs. During the GTMP, Lao PDRCitation27 and CambodiaCitation28 circumvented a similar lack of local cases by sending trainee graders to Ethiopia for field training and certification, but this was not possible for Viet Nam. It is possible that this lack of live-subject testing makes our data less accurate. Finally, in most EUs, males were relatively under-represented in the sample of ≥50-year-olds examined (). This is a source of potential bias that would tend to lead to overestimation of trichiasis prevalence (because trichiasis occurs more frequently in women than in men)Citation29; age- and gender-based standardization will have partially, but not completely, compensated. In all other respects, the usual quality assurance and quality control measures of the GTMPCitation30 were deployed, and within the limitations we have identified, we are confident of the strength of the data we present here.Citation31
Notwithstanding the wide confidence intervals for the distribution shape parameter, the cluster-level distribution of TF cases was consistent with a geometric distribution. We expect to see this pattern when an infectious disease is disappearingCitation32,Citation33; it was not consistent with a Poisson distribution. Occasional higher prevalence communities are expected with a geometric distribution. These do not necessarily represent persistent outliers. Selecting a community in the tail of the geometric distribution with a high prevalence is a predictable event. These outliers do not necessarily have more transmission potential in the future, and may regress towards the mean on future visits. While a single cluster in Hà Giang Province had more than one in 10 children with TF, it may not necessarily be a focus of disease of public health significance. Nonetheless, further investigations will be undertaken in Hà Giang.
In the last few decades, Viet Nam has made huge progress against trachoma. Momentum was accelerated following national adoption of the SAFE strategy in the late 1990s. From 2000 to 2008, 83,830 surgeries for trichiasis were performed, more than 2.1 million azithromycin (Zithromax®, Pfizer, New York, NY) treatments were distributed to people in 850 endemic communes of 21 northern and central coastal provinces.Citation34 The F and E components of SAFE were also implemented. Evaluations completed in 2004 and 2005 concluded that (1) health promotion activities were excellent and implementation of water and sanitation improvement activities were underwayCitation35 and (2) that F & E had significantly contributed to reductions in the prevalence of active trachoma.Citation36 National-level estimates for 2015 suggested that 91% of households had access to drinking water within a 30 minute round-trip, while 81% of households had access to improved sanitation.Citation37
The data presented here suggest that Viet Nam is on track to meet GET2020 targets, and we recommend that the country now starts populating a dossier on trachoma’s elimination as a public health problem.Citation9 This dossier could serve as a valuable information repository whilst further work is undertaken to investigate (and, if necessary, manage) trachoma in Hà Giang and its surrounds. Members of the WHO Alliance for GET2020 are ready and willing to support.
Conflict of interest
None of the following authors have any proprietary interests or conflicts of interest related to this submission: Nguyen Xuan Hiep, Jeremiah M. Ngondi, Vu Tuan Anh, Tran Minh Dat, Cung Hong Son, Tran Van An, Nguyen Chi Dung, Nguyen Duy Thang, Brian K. Chu, Rebecca Willis, Ana Bakhtiari, Alexandre L. Pavluck, James Johnson, Joshua Sidwell, Molly Brady, Rob Henry, Aryc Mosher, Travis C. Porco, Thomas M. Lietman, Lisa Rotondo, Susan Lewallen, Paul Courtright, Anthony W. Solomon.
Additional information
Funding
References
- World Health Organization. Neglected Tropical Diseases: Hidden Successes, Emerging Opportunities. Geneva: World Health Organization; 2006. http://apps.who.int/iris/bitstream/10665/69367/1/WHO_CDS_NTD_2006.2_eng.pdf.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Eliminating trachoma: accelerating towards 2020. 2016. http://www.who.int/trachoma/news/News_Trachoma_Towards_2020/en/. Accessed September 12, 2016.
- World Health Organization. Report of the 2nd Global Scientific Meeting on Trachoma, Geneva, 25–27 August, 2003 (WHO/PBD/GET 03.1). Geneva: World Health Organization; 2003.
- World Health Organization. Future Approaches to Trachoma Control: Report of a Global Scientific Meeting, Geneva, 17-20 June 1996; 1997. http://www.who.int/iris/handle/10665/63413. Accessed September 12, 2016.
- World Health Assembly. Global elimination of blinding trachoma. 51st World Health Assembly. Geneva, 16 May 1998, Resolution WHA51.11: 1998. http://www.who.int/blindness/causes/WHA51.11/en/. Accessed December 14, 2015.
- Braff E, Winklestein W, Winkelstein W. Field treatment of trachoma in North Vietnam. Public Health Rep 1896-1970. 1952;67(12):1233. doi:10.2307/4588340.
- Hoffman PR. ITI Expands Trachoma Control Program in Vietnam, Launches New Program in Nepal. http://www.hoffmanpr.com/press-release/iti-expands-trachoma-control-program-in-vietnam-launches-new-program-in-nepal/. Accessed May 8, 2017.
- Mabey DC, Solomon AW, Foster A. Trachoma. Lancet. 2003; 362:223–229.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem. Geneva, Switzerland: WHO; 2016. http://www.who.int/neglected_diseases/resources/who_htm_ntd_2016.8/en/. Accessed May 9, 2017.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical Consultation on Trachoma Surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva, Switzerland: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/174085/1/WHO_HTM_NTD_2015.02_en.pdf.
- Courtright P, Gass K, Lewallen S, et al. Global trachoma mapping project: training for mapping of trachoma (version 2). London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/node/357.
- Solomon AW, Pavluck AL, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225.doi:10.3109/09286586.2015.1037401.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- General Statistics Office of Viet Nam. The 2009 Vietnam Population and Housing Census: Completed Results. Ha Noi, Viet Nam; 2010. http://www.gso.gov.vn/default_en.aspx?tabid=515&idmid=5&ItemID=10799.
- Myatt M, Mai NP, Quynh NQ, et al. Using lot quality-assurance sampling and area sampling to identify priority areas for trachoma control: Viet Nam. Bull World Health Organ. 2005;83(10):756–763.
- Ngondi J, Reacher M, Matthews F, Brayne C, Emerson P. Trachoma survey methods: a literature review. Bull World Health Organ. 2009;87(2):143–151. doi:10.2471/BLT.07.046326.
- World Health Organization. Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010. Geneva: World Health Organization; 2010. http://www.who.int/blindness/publications/3RDGLOBALSCIENTIFICMEETINGONTRACHOMA.pdf.
- Solomon A, Zondervan M, Kuper H, Buchan J, Mabey D, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva, Switzerland: WHO; 2006.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis, Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016. http://www.who.int/trachoma/resources/who_htm_ntd_2016.5/en/.
- Freeman MC, Ogden S, Jacobson J, et al. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis. 2013;7(9):e2439.doi:10.1371/journal.pntd.0002439.
- Kalua K, Chisambi A, Chinyanya D, et al. Completion of baseline trachoma mapping in malawi: results of eight population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(sup1):32–38.doi:10.1080/09286586.2016.1230224.
- Mwingira UJ, Kabona G, Kamugisha M, et al. Progress of trachoma mapping in mainland Tanzania: results of baseline surveys from 2012 to 2014. Ophthalmic Epidemiol. 2016;23(6):373–380.doi:10.1080/09286586.2016.1236974.
- Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur States and Khartoum State, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–391.doi:10.1080/09286586.2016.1243718.
- Ko R, Macleod C, Pahau D, et al. Population-based trachoma mapping in six evaluation units of Papua New Guinea. Ophthalmic Epidemiol. 2016;23(sup1):22–31.doi:10.1080/09286586.2016.1235715.
- Adera TH, Macleod C, Endriyas M, et al. Prevalence of and risk factors for trachoma in Southern Nations, Nationalities, and Peoples’ Region, Ethiopia: results of 40 population-based prevalence surveys carried out with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(sup1):84–93.doi:10.1080/09286586.2016.1247876.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in Kano State, Nigeria: results of 44 local government area-level surveys. Ophthalmic Epidemiol. 2017;24(3):195–203.doi:10.1080/09286586.2016.1265657.
- Southisombath K, Sisalermsak S, Chansan P, et al. National trachoma assessment in the Lao People’s Democratic Republic in 2013–2014. Ophthalmic Epidemiol. 2016;23(sup1):8–14.doi:10.1080/09286586.2016.1236973.
- Meng N, Seiha D, Thorn P, et al. Assessment of trachoma in Cambodia: trachoma is not a public health problem. Ophthalmic Epidemiol. 2016;23(sup1):3–7.doi:10.1080/09286586.2016.1230223.
- Cromwell EA, Courtright P, King JD, Rotondo LA, Ngondi J, Emerson PM. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(10):985–992. doi:10.1016/j.trstmh.2009.03.012.
- Solomon A, Willis R, Pavluck AL, Alemayehu W Quality Assurance and Quality Control in the Global Trachoma Mapping Project. Am J Trop Med Hyg. 2018; 99(4):858–863. doi:10.4269/ajtmh.18-0082.
- Engels D. The global trachoma mapping project: a catalyst for progress against neglected tropical diseases. Ophthalmic Epidemiol. 2016;23(sup1):1–2. doi:10.1080/09286586.2016.1257139.
- Lietman TM, Gebre T, Abdou A. et al. The distribution of the prevalence of ocular chlamydial infection in communities where trachoma is disappearing. Epidemics. 2015;11:85–91. doi:10.1016/j.epidem.2015.03.003.
- Rahman SA, West SK, Mkocha H, et al. The distribution of ocular chlamydia prevalence across tanzanian communities where trachoma is declining. PLoS Negl Trop Dis. 2015;9(3):e0003682. doi:10.1371/journal.pntd.0003682.
- International Trachoma Initiative. Bring on the light: the coming defeat of blinding trachoma. International Trachoma Initiative 10th Anniversary Report. 2010. http://www.pfizer.com/sites/default/files/responsibility/ITI_10th_anniversary_report.pdf. Accessed May 10, 2017.
- Kuper H, Solomon AW, Buchan JC, Zondervan M, Mabey D, Foster A. Participatory evaluations of trachoma control programmes in eight countries. Trop Med Int Health. 2005;10(8):764–772. doi:10.1111/j.1365-3156.2005.01451.x.
- Khandekar R, Ton TKT, Do Thi P. Impact of face washing and environmental improvement on reduction of active trachoma in Vietnam-a public health intervention study. Ophthalmic Epidemiol. 2006;13(1):43–52. doi:10.1080/09286580500477507.
- WHO and UNICEF JMP for WASH. Estimates on the use of water, sanitation and hygiene in Viet Nam. 2017. https://washdata.org/data.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (2,9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9). Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.