ABSTRACT
Purpose: Trachoma is endemic in parts of Nepal; implementation of the surgery, antibiotics, facial cleanliness, environmental improvement (SAFE) strategy started in 2002. Some suspected-endemic districts had not previously been mapped. We aimed to estimate the prevalences of trachomatous inflammation—follicular (TF) and trichiasis in those districts.
Methods: Population-based prevalence surveys were undertaken in 27 districts. In each of those districts, two-stage cluster sampling was used to select a sample of 2000 children aged 1–9 years and 4000 adults aged ≥15 years from a total of 40 wards (clusters), drawn evenly from two subdistricts. Consenting eligible participants were examined for trachoma by Global Trachoma Mapping Project (GTMP)-certified graders, using the World Health Organization simplified grading system. Data were analyzed at district level using GTMP methods.
Results: A total of 43,200 households were surveyed, and 162,094 people were examined for trachoma. District-level TF prevalence in 1–9-year-olds ranged from 0% to 4.3% (95% confidence interval [CI] 2.4–6.2). Among adults aged ≥15 years, trichiasis prevalence ranged from 0% to 0.33% (95% CI 0.08–0.65).
Conclusion: TF was not a public health problem in any of the 27 districts surveyed; thus, antibiotic mass drug administration is not needed. In two districts (Dhanusa and Gorkha), trichiasis prevalence in adults aged ≥15 years was ≥0.2%; thus, further trichiasis surgery interventions at public health level are warranted to achieve elimination. These findings will facilitate planning for elimination of trachoma as a public health problem in Nepal.
Background
Trachoma, a neglected tropical disease, remains a preventable cause of blindness. Its elimination as a public health problem (through the surgery, antibiotics, facial cleanliness, and environmental improvement: “SAFE” strategy) is a global target that was endorsed by the World Health Assembly in 1998.Citation1,Citation2 The World Health Organization (WHO) estimates that by 2016 a total of 190.2 million people worldwide required A, F, and E for trachoma elimination purposes, in 42 countries.Citation3 It has been estimated that 1.9 million people are blind or visually impaired from it; 3.2 million people need surgery to avoid trachomatous blindness.Citation4 Prior to SAFE implementation, baseline surveys of trachoma prevalence are recommended to guide programs to deliver appropriate interventions.Citation5
Three decades ago, trachoma was considered the most widespread potentially blinding disease in Nepal, affecting 6.5% of the population and accounting for 2.4% of bilateral blindness nationally.Citation6 Trachoma was characterized as hyperendemic in the western districts, and there were thought to be over 58,000 people with trichiasis.Citation6 The National Trachoma Program (NTP), in collaboration with partners, started implementation of the SAFE strategy in 2002. The first step involved undertaking trachoma rapid assessments (TRAs).Citation7 In districts where TRA results showed that ≥10% of 1–9 year-olds examined had trachomatous inflammation—follicular (TF), population-based prevalence surveys (PBPSs) were subsequently undertaken.Citation8
The Ministry of Health and Population in Nepal initially set itself the goal to eliminate trachoma as a public health problem by the year 2017. By 2012, Nepal had done PBPSs of trachoma in 27 out of 75 districts, of which 20 districts were under implementation of the SAFE strategy.Citation9 However, eight districts that had not been mapped previously were suspected to be endemic for trachoma. Additionally, districts that previously had TRAs (14) and PBPSs (five) were felt to need further PBPSs because initial findings had indicated the percentage of children having TF in the 5–9.9% range. In the series of surveys described here, we aimed to estimate the prevalence of TF and trichiasis in 27 districts, where trachoma was still suspected to be endemic, and in which trachoma-specific interventions had not previously been undertaken.
Materials and methods
Study settings
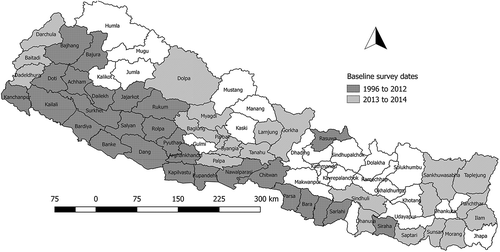
shows the location of the 27 districts where surveys were conducted in 2013 (14 districts) and 2014 (13 districts). Among the 27 districts, 8 districts had not been surveyed, while 19 had previously been evaluated with either a TRA or a PBPS (between 1996 and 2012) and found to have a TF proportion or prevalence of 5–9.9%.
Survey design and sampling
These surveys were designed prior to the start of the Global Trachoma Mapping Project (GTMP), and their methodology therefore differs from the GTMP templateCitation10 in several ways, notably: (1) the sample for each district was fixed as 2000 children aged 1–9 years and 4000 adults aged ≥15 years, instead of a fixed sample of households; (2) the water and sanitation fields in the household questionnaire were not the GTMP standardized questions (so these data have not been included in this analysis); and (3) data were collected using paper-based questionnaires instead of the GTMP’s Android smartphone-based system.
Surveys were powered to estimate a district-level TF prevalence of 4% in 1–9-year-olds, aiming to have 95% confidence of estimating that prevalence with absolute precision of ± 2% and a (conservative) design effect of 5, which resulted in a sample size estimate of 1844 children aged 1–9 years; we rounded this up to 2000. In addition, the sample size for adults aged ≥15 years was powered to estimate a trichiasis prevalence of 1.0% with an absolute precision of 0.5% and a design effect of 2.6, which resulted in a sample of 3926 (rounded up to 4000). The smallest administrative area (the ward) was defined as a cluster. To obtain the desired sample, each district was stratified into two subdistricts and 40 wards (20 per subdistrict) were systematically selected with probability proportional to population size, using census data from 2011 to generate the sampling frame. In each cluster, households were sampled using the random walk methodCitation5 to obtain a fixed sample of 50 children aged 1–9 years and 100 adults aged ≥15 years.
Trachoma grading
Graders participating in the surveys had obtained a kappa for diagnosing TF of ≥0.7 based on an intergrader agreement test, compared to a GTMP-certified grader trainer as the gold standard.Citation11 The eyelid and tarsal conjunctiva of each eye were examined for the trachoma signs TF and trichiasis, using the definitions of the WHO simplified grading systemCitation12, a 2.5 × magnifying loupe, and torch or sunlight.
Data collection
Survey teams composed of four people (a grader, an enumerator, a community health volunteer, and a helper) undertook the fieldwork, covering, on average, one cluster per day. After obtaining verbal consent, household members were enumerated and examined for trachoma signs.
Data management and analysis
Data collection was done using paper-based questionnaires, after which data were double entered and validated for accuracy. Descriptive statistics were used to examine sample characteristics. Prevalence estimates and 95% confidence intervals for TF and trichiasis were generated using GTMP methods in R (R Foundation for Statistical Computing, Vienna, Austria) and structured query language. For each cluster, the proportion of 1–9-year-olds with TF was adjusted for age, using data from the most recent census. Similarly, for each cluster, the proportion of ≥15-year-olds with trichiasis was adjusted for gender and age in 5-year age bands.Citation10 District-level prevalences of TF and trichiasis were calculated as the means of the adjusted cluster-level proportions, and confidence intervals for each of these prevalence estimates were calculated by bootstrapping, with replacement, over 10,000 replications.
Ethics statement
The survey was carried out by the NTP. Ethical clearance was obtained from the Institutional Review Board of Nepal Netra Jyoti Sangh and Nepal Health Research Council. Verbal informed consent was sought from parents/guardians for children aged 1–9 years, and from each participant aged ≥15 years. Children with TF were treated with 1% tetracycline ointment, with tubes being given to carers to apply twice daily for 6 weeks. Individuals with trichiasis were counseled and referred to hospitals, where trichiasis surgery was offered free of charge.
Results
Characteristic of the sample
shows the demographic characteristics of the sample in 27 districts surveyed. A total of 43,200 households were visited and 162,094 people were examined for trachoma. Examinees included 54,041 children aged 1–9 years and 108,053 adults aged ≥15 years. The proportion of female participants was 47.4% among children aged 1–9 years and 53.2% among those aged ≥15 years. The mean (standard deviation) age was 5.5 (2.6) and 36.2 (16.7) years among children aged 1–9 years and adults aged ≥15 years, respectively.
Table 1. Characteristics of survey population, population-based trachoma surveys, Nepal, 2013–2014.
Prevalence of trachoma signs
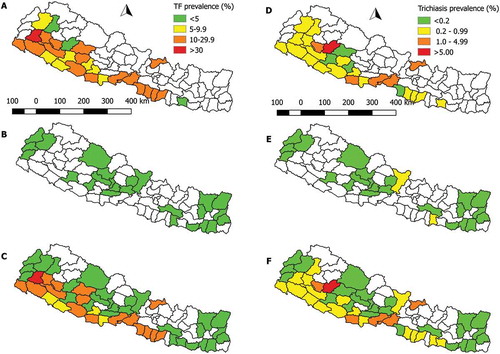
summarizes the prevalence of TF and trichiasis by district. Prevalence of TF in children aged 1–9 years ranged (by district) from 0% to 4.3% (95% confidence interval [CI] 2.4–6.2), while trichiasis prevalence in adults aged ≥15 years ranged from 0% to 0.33% (95% CI 0.08–0.65). Trichiasis prevalence was ≥0.2% in two districts. Prevalence categories are shown in , alongside those generated at baseline in districts of Nepal that were surveyed prior to this tranche of work.
Table 2. Prevalence of TF among children aged 1–9 years and trichiasis among adults aged ≥15 years, Nepal, 2013–2014.
Figure 2. Baseline prevalence of TF in children aged 1–9 years and trichiasis in adults aged ≥15 years, Nepal, 1996–2014. (a) TF prevalence from districts mapped in 1996–2012; (b) TF prevalence from districts mapped in 2013–2014; (c) TF prevalence combining data from all surveys conducted in 1996–2014; (d) Trichiasis prevalence from districts mapped in 1996–2012; (e) Trichiasis prevalence from districts mapped in 2013–2014; (f) Trichiasis prevalence combining data from all data surveys conducted in 1996–2014.
Discussion
Baseline trachoma surveys are essential for planning implementation of the SAFE strategy and setting targets for elimination. This set of surveys completes baseline mapping of suspected-endemic districts in Nepal, and showed that trachoma was not a public health problem in 25 of 27 districts for which we generated data. The prevalence of TF among children aged 1–9 years was < 5% in all districts, and only two districts (Dhanusha and Gorkha) had trichiasis prevalence of ≥0.2% in adults aged ≥15 years, indicating a requirement for public health-level trichiasis surgery programs to achieve the trichiasis elimination target.Citation13 Following intervention, further prevalence surveys of trichiasis will be needed to document attainment of elimination.Citation14
Our survey design’s major strength was the over-sampling compared to the most recent sample sizes recommended by the WHO for both TF (1019 children aged 1–9 years)Citation10 and trichiasis (2818 adults aged ≥15 years)Citation15, which thus provided power for very precise prevalence estimates at district level. However, there are a number of potential limitations. First, the use of a fixed sample of 50 children and 100 adults per cluster is a potential source of (albeit minor) bias, since examinees were recruited by selecting households, not individuals, and it is unlikely that the group of households chosen in every cluster yielded exactly the desired number of examinees in each age group. Second, sampling of households was done using the random walk method; this is not optimally rigorous from an epidemiological perspective. Third, we did not check to see whether cases of trichiasis were associated with trachomatous scar of the conjunctiva, which was a recommendation made in 2015,Citation16 well after these surveys were completed. Finally, the water and sanitation data that we collected were not translatable to WHO/UNICEF Joint Monitoring Program definitions for improved and unimproved water sources and sanitation facilities (https://www.wssinfo.org/definitions-methods/watsan-categories/), and we have therefore not presented them here. We do not believe that this weakens our conclusions: any comparison of TF and access to water and sanitation would be moot, given that TF prevalence was < 5% in each district.
The findings from these surveys suggest that TF was not a public health problem in any of 27 districts, so antibiotic mass drug administration will not be needed for trachoma elimination. However, two districts need to undertake public-health-level trichiasis surgery interventions to attain elimination of trachoma. The data suggest that with intensive effort and a small number of further prevalence surveys, Nepal may soon be able to eliminate trachoma and demonstrate that this has been achieved.
Conflict of interest
None of the following authors have any proprietary interests or conflicts of interest related to this submission:
Shekhar Sharma, Jeremiah M. Ngondi, Sailesh Mishra, Raman D. Prasad, Kathryn Crowley, Delali Bonuedi, Lisa A. Rotondo, Lionel Nizigama, Aryc Mosher, Rob Henry, Rebecca Willis, and Anthony W. Solomon.
Additional information
Funding
References
- World Health Organization. Future Approaches to Trachoma Control: Report of a Global Scientific Meeting, Geneva, 17-20 June 1996; 1997. http://www.who.int/iris/handle/10665/63413. Accessed September 12, 2016.
- World Health Assembly. Global elimination of blinding trachoma. In: 51st World Health Assembly. Geneva, 16 May 1998, Resolution WHA51.11; 1998. http://www.who.int/blindness/causes/WHA51.11/en/. Accessed December 14, 2015.
- World Health Organization. WHO Alliance for the Global Elimination of Trachoma by 2020: progress report on elimination of trachoma, 2014–2016. Index, Wkly Epidemiol Rec. 2017;92(26):359–368. http://apps.who.int/iris/bitstream/10665/255778/1/WER9226.pdf.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Eliminating Trachoma: Accelerating Towards 2020; 2016. http://www.who.int/trachoma/news/News_Trachoma_Towards_2020/en/. Accessed September 12, 2016.
- Ngondi J, Reacher M, Matthews F, Brayne C, Emerson P. Trachoma survey methods: A literature review. Bull World Health Organ. 2009;87:143–151.
- Brilliant LB, Pokhrel RP, Grasset NC, et al. Epidemiology of blindness in Nepal. Bull World Health Organ. 1985;63(2):375–386.
- Negrel A, Taylor H, West S Guidelines for Rapid Assessment for Blinding Trachoma (WHO/PBD/GET/00.8); 2001. http://www.who.int/blindness/TRA-ENGLISH.pdf. Accessed January 1, 2016.
- RTI International. Nepal National Trachoma Program: Review and Recommendations. Washington DC: RTI International; 2011. http://pdf.usaid.gov/pdf_docs/pnaeb721.pdf. Accessed September 29, 2016.
- Smith JL, Haddad D, Polack S, et al. Mapping the Global Distribution of Trachoma: Why an Updated Atlas Is Needed. PLoS Negl Trop Dis. 2011;5(6):e973. doi:10.1371/journal.pntd.0000973.
- Solomon AW, Pavluck AL, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-Country Population-Based Study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: Training for Mapping of Trachoma. Version 1. London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/resources/global-trachoma-mapping-project-training-mapping-trachoma. Accessed December 15, 2015.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- World Health Organization. Validation of elimination of trachoma as a public health problem; 2016. http://apps.who.int/iris/bitstream/10665/208901/1/WHO-HTM-NTD-2016.8-eng.pdf. Accessed January 5, 2017.
- Courtright P, Flueckiger RM, Harding-Esch EM, Lewallen S, Solomon AW. Tropical data: Trichiasis surveys – training for mapping of trachomatous trichiasis. London: International Coalition for Trachoma Control; 2017.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Design and Validation of a Trachomatous Trichiasis-Only Survey. Geneva: World Health Organization. (WHO/HTM/NTD/PCT/2017.08); 2017. http://apps.who.int/iris/bitstream/10665/259815/1/WHO-HTM-NTD-PCT-2017.08-eng.pdf. Accessed January 30, 2018.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis: Cape Town, 4–6 November, 2015. WHO/HTM/NTD/2016.5. Geneva: World Health Organization; 2016. http://apps.who.int/iris/bitstream/10665/250571/1/WHO-HTM-NTD-2016.5-eng.pdf. Accessed October 1, 2017.
Appendix
The GTMP investigators are as follows: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (2,9), Berhanu Bero 245 (4), Sarah Bovill (8), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad 250 (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), 255 Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4), Sheila K. 260 West (1,10,11), and Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.