ABSTRACT
Purpose: We aimed to estimate the prevalence of trachomatous inflammation—follicular (TF) in children aged 1–9 years, trichiasis in adults aged ≥15 years, and water and sanitation (WASH) indicators in 12 suspected-endemic districts in Uganda.
Methods: Surveys were undertaken in 14 evaluation units (EUs) covering 12 districts. Districts were selected based on a desk review in 2014 (four districts) and trachoma rapid assessments in 2018 (eight districts). We calculated that 1,019 children aged 1–9 years were needed in each EU to estimate TF prevalence with acceptable precision and used three-stage cluster sampling to select 30 households in each of 28 (2014 surveys) or 24 (2018 surveys) villages. Participants living in selected households aged ≥1 year were examined for trachoma; thus enabling estimation of prevalences of TF in 1–9 year-olds and trichiasis in ≥15 year-olds. Household-level WASH access data were also collected.
Results: A total of 11,796 households were surveyed; 22,465 children aged 1–9 years and 24,652 people aged ≥15 years were examined. EU-level prevalence of TF ranged from 0.3% (95% confidence interval [CI] 0.1–0.7) to 3.9% (95% CI 2.1–5.8). EU-level trichiasis prevalence ranged from 0.01% (95% CI 0–0.11) to 0.81% (95% CI 0.35–1.50). Overall proportions of households with improved drinking water source, water source in yard or within 1km, and improved sanitation facilities were 88.1%, 23.0% and 23.9%, respectively.
Conclusion: TF was not a public health problem in any of the 14 EUs surveyed: antibiotic mass drug administration is not required in these districts. However, in four EUs, trichiasis prevalence was ≥ 0.2%, so public health-level trichiasis surgery interventions are warranted. These findings will facilitate planning for elimination of trachoma in Uganda.
Background
Trachoma, a neglected tropical disease, is the most common infectious cause of blindness. Elimination of trachoma as a public health problem (through the surgery, antibiotics, facial cleanliness and environmental improvement: “SAFE” strategy”) is a global initiative that was endorsed by the World Health Assembly in 1998.Citation1,Citation2 The World Health Organization (WHO) estimated that in 2016: 190.2 million people worldwide required the A, F and E components of SAFE for the purposes of trachoma eliminationCitation3; 1.9 million people were blind or visually impaired from the disease; and 3.2 million people needed surgery to avoid trachomatous blindness.Citation4
Trachoma is endemic in Uganda. A review conducted in 1934 reported that tens of thousands of individuals with conjunctivitis and trachoma were presenting to the eye clinic at Mulago Hospital, Kampala.Citation5 Other evidence from the 1960s (Busoga district) and 1970s (Bukedi district) suggested that trachoma was likely to be endemic throughout the country and was the leading cause of blindness there.Citation6–Citation9
Prior to SAFE implementation, baseline surveys of trachoma prevalence are recommended to guide programmes to deliver appropriate interventions.Citation10 To enable planning for implementation of the SAFE strategy, baseline population-based prevalence surveys (PBPS) of trachoma were conducted from 2006–2012 in districts of Uganda suspected to be endemic. Implementation of the SAFE strategy commenced in 2007 with gradual scale-up of interventions to cover, by 2014, all 38 districts confirmed to be endemic.
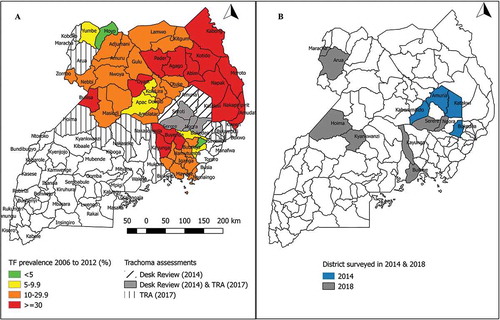
To consolidate SAFE implementation, in 2014 the Ministry of Health and partners launched a Trachoma Action Plan (TAP) that set the national elimination target as 2020,Citation11 in line with the international goal.Citation1 The TAP identified a total of 19 districts bordering known trachoma-endemic districts (based on 2006–2012 PBPS data). Following the criteria for where to map and where not to map used by the Global Trachoma Mapping Project,Citation12 the TAP recommended that additional baseline surveys of these districts be undertaken. This paper describes the methods and results of 14 such surveys undertaken in 12 unmapped districts (four completed in 2014 and eight in 2018). The surveys reported here aimed to estimate the prevalence of TF, trichiasis and key water, sanitation, and hygiene (WASH) indicators.
Materials and methods
The Ministry of Health conducted PBPS using the standardised WHO-recommended methodologyCitation13 with the support of the Global Trachoma Mapping Project (GTMP)Citation14 for the 2014 surveys, and Tropical DataCitation15 (https://www.tropicaldata.org/) for the 2018 surveys. Fieldwork training was conducted using version 1 of the GTMP training system in 2014Citation16 and refresher training undertaken using Tropical Data methods in 2018.Citation17 Unlike the surveys supported by GTMP version 1, those using Tropical Data included examination for trachomatous conjunctival scarring (TS) and questions on previous trichiasis management recommendations from health workers, for all eyes diagnosed to have trichiasis, as recommended by a November 2015 Global Scientific Meeting.Citation18
Study settings
shows the districts in which surveys were undertaken. The four districts surveyed in 2014 were selected following a health facility-based desk-review that covered eight districts of the Teso sub-region. Based on the desk review, districts were prioritised for mapping if: 1) more than 10 cases of trichiasis had been reported per year, for two or more years in the period 2008–2012; 2) more than 40% of the health staff interviewed identified trichiasis as a problem in their catchment area; and 3) the district bordered a district in which the prevalence of TF in 1–9 year-olds was >10%. In 2017, trachoma rapid assessments (TRAs)Citation19 were undertaken in 19 districts. Eight districts in which TRA findings suggested that trachoma might be a public health problem (proportion of children aged 1–9 years with TF≥5% and/or proportion of people aged ≥15 years with trichiasis ≥1%) were prioritised for PBPS. To conform to WHO recommendations,Citation20 two districts (Arua and Hoima) with populations > 500,000 were each split into two evaluation units (EUs), whereas other districts were surveyed as single EUs. This created a total of 14 EUs, four of which were surveyed in 2014, and 10 in 2018.
Sample size estimation
To estimate the district-level prevalence of TF among children aged 1–9 years, the sample size was calculated to allow 95% confidence of estimating an expected prevalence of 10% with absolute precision of ± 3%, using a design effect of 2.65 and inflated by a factor of 1.2 to account for non-response.Citation13 We therefore wanted to sample at least the number of households in which 1,222 children aged 1–9 years would be resident, in each EU, hoping to examine 1,019. For the 2014 surveys, because the mean number of children aged 1–9 years per household was estimated to be 1.5 (from DHS 2011)Citation21 and estimating that a survey team could complete 30 households within a day, a total of 28 clusters (villages) were required for each EU. One of the lessons from the 2014 surveys was that the mean number of children aged 1–9 years per household was higher, at 1.7. This lesson was applied to the 2018 survey and the number of clusters per EU was reduced to 24; the cluster size of 30 households was maintained.
Sample selection
A multi-stage cluster sampling design was used.
Selection of clusters
For each EU, the list of parishes (the second lowest administrative level, mean population 5,000) and their population estimates were obtained from the Uganda Bureau of Statistics. In the first sampling stage, parishes (28 in 2014 and 24 in 2018) were systematically selected with probability proportional to population size. Within selected parishes, the list of villages was obtained from the district authorities and for the second sampling stage, one village was selected randomly using the lottery method.
Selection of households and individuals
A household was defined as persons living together and sharing meals from the same cooking pot. In the third sampling stage, 30 households per village were selected using the compact segment sampling method and all household members aged one year and above were examined for trachoma.
Training of survey teams
Initial training in 2014 was undertaken over 4 days using the GTMP training system.Citation16 The same grader/recorder teams participated in 2018 surveys; prior to that work, a specifically designed two-day refresher team training course was undertaken using Tropical Data methods.Citation17 Trainings were facilitated by GTMP/Tropical Data-certified grader and recorder trainers. For graders, inter-grader agreement tests were conducted using sets of 50 subjects. Only graders achieving a kappa of at least 0.7 for TF, compared to a certified grader trainer, were eligible to participate in the surveys. Survey recorders were trained on household interviews and electronic capture of survey data using the GTMP (2014) and Tropical Data (2018) systems. Graders and recorders had to pass their respective tests at each training point to qualify to contribute to the following surveys. Once grader and recorder training was completed, participants who qualified were paired into teams for further team training.
Household interviews
Household interviews on WASH were undertaken by recorders using a standard questionnaire.Citation13 Heads of households (or another adult, if the head of household was absent or unable to respond for any reason) were interviewed about types of water sources, distance to water source and type of sanitation facilities used by the household; when the family reported using a latrine, the type of latrine was verified through observation.
Trachoma grading
The eyelid and tarsal conjunctiva of each eye were examined using a 2.5× magnifying loupe and torch or daylight, looking for signs of trichiasis and TF. Following successful piloting in Viet NamCitation22 and Pakistan,Citation23 for the Uganda surveys conducted in 2018 (but not in 2014), the tarsal conjunctiva of eyes with trichiasis was examined for TS, and individuals were asked about previous trichiasis management recommendations from health workers.
Data management and analysis
Data were collected electronically using Android smartphones loaded with purpose-built apps developed for the GTMP (2014) or Tropical Data (2018). Descriptive statistics were generated to examine sample characteristics and EU-level proportions of households with key WASH indicators. WASH data were categorised based on WHO/UNICEF Joint Monitoring Program (JMP) definitions for improved and unimproved water sources and sanitation facilities (https://www.wssinfo.org/definitions-methods/watsan-categories/). Prevalence estimates and 95% confidence intervals (CIs) for TF and trichiasis were generated using GTMP methods in R (R Foundation for Statistical Computing, Vienna, Austria) and Structured Query Language. For each cluster, the proportion of 1–9 year-olds with TF was adjusted for age in one-year age bands, using data from the most recent (2014) census.Citation24 Similarly, for each cluster, the proportion of ≥15 year-olds with trichiasis was adjusted for gender and age in five-year age bands.Citation13 EU-level prevalences of TF and trichiasis were calculated as the means of the adjusted cluster-level proportions, and CIs for each were calculated by bootstrapping, with replacement, sets of n observations (where n was the number of clusters in the EU) over 10,000 replications and taking the 2.5th and 97.5th centiles of ordered results as the lower and upper CI bounds.
Ethical considerations
Ethical approval was granted by the ethics committee of the London School of Hygiene & Tropical Medicine, London, UK (reference numbers 6319 and 8355) and the Biomedical Ethics Board of Uganda. We obtained informed verbal consent for examination from each participant or (for children) from their parent or guardian. Individuals with conjunctivitis, whether meeting the definition of active trachoma (TF and/or trachomatous inflammation—intense [TI]) or not, were provided with two tubes of 1% tetracycline eye ointment, while individuals with trichiasis were referred to a district hospital surgeon.
Results
Characteristics of survey population
summarises the characteristics of the population by EU. A total of 47,117 participants aged 1–9 years and ≥15 years (93.8% of those enumerated) were examined. The proportions of male participants among children aged 1–9 years and people aged ≥15 years were 50.4% and 38.8%, respectively. The mean ages (standard deviations) of examinees aged 1–9 years and ≥15 years were 4.8 years (2.5) and 35.7 years (16.8), respectively.
Table 1. Characteristics of survey population by evaluation unit, trachoma prevalence surveys, Uganda, 2014 and 2018.
Prevalence of trachoma
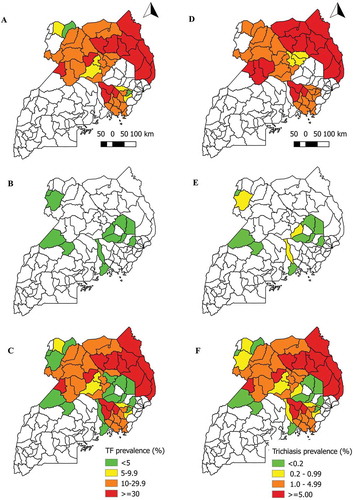
A total of 22,465 children aged 1–9 years and 24,652 people aged ≥15 years were examined for TF and trichiasis, respectively. Prevalence of TF by EU ranged from 0.3% (95% CI 0.1–0.7) in Buikwe to 3.9% (95% CI 2.1–5.8) in Katakwi. Among people aged ≥ 15 years, prevalence of any trichiasis by EU ranged from 0.04% (95% CI 0.0–0.11) in Katakwi to 0.81% (95% CI 0.35–1.50) in Arua 2 ( and ). For 2018 surveys, in addition to calculating the prevalence of any trichiasis for each EU, we were able to determine EU-level prevalence estimates of trichiasis in which TS was also present in the same eye (trichiasis+TS) and the existence of that state was unknown to the health system.Citation25 Of the 10 EUs with these data, the prevalence of trichiasis+TS unknown to the health system was ≥0.2% in three ().
Table 2. Prevalence of trachoma by evaluation unit, Uganda, 2014 and 2018.
Figure 2. Prevalence of trachomatous inflammation—follicular (TF) in children aged 1–9 years, and any trichiasis in people aged ≥15 years, Uganda, 2006–2018.
A) TF prevalence in districts mapped in 2006–2012; B) TF prevalence in districts mapped in 2014 or 2018; C) TF prevalence combining all data sources from 2006–2018D) Trichiasis prevalence in districts mapped in 2006–2012; E) Trichiasis prevalence in districts mapped in 2014 or 2018; F) Trichiasis prevalence combining all data sources from 2006–2018
Prevalence of access to water and sanitation
summarises key indicators of access to WASH. The overall proportion of households that reported: using an improved drinking water source was 88% (range by EU 76–99); having a drinking water source in the household yard or within 1km of it was 23% (range by EU 4–41); and having access to improved sanitation facilities was 24% (range by EU 10–61).
Table 3. Proportion of households with key water, sanitation and hygiene indicators by evaluation unit, trachoma prevalence surveys, Uganda, 2014 and 2018.
Discussion
Over the last decade, Uganda has made tremendous progress towards trachoma elimination, with 34 of 38 eligible endemic districts having stopped azithromycin mass drug administration (MDA) by December 2017. With the national 2020 elimination targetCitation11 fast approaching, data from baseline surveys in suspected endemic districts are critical for planning SAFE interventions. The data presented here show that TF was below <5% in all 14 EUs surveyed, indicating that antibiotic MDA will not be required. However, four EUs had trichiasis prevalences of ≥0.2%, suggesting a requirement for public health-level trichiasis surgery programmes. Further trichiasis-only surveysCitation26 will ultimately be needed in those EUs to document attainment of elimination.Citation27
Our surveys estimated key indicators of access to WASH, based on WHO/UNICEF JMP definitions, and this enables comparison with Demographic and Health Survey (DHS) findings. The 2016 Uganda DHS reported that of surveyed rural households, 74% used an improved source of drinking water; 45% had a drinking water source in the yard or within 1km distance; and 16% had access to an improved sanitation facilityCitation28; those national-level estimates can be compared to our more granular, EU-level ones ().
Our surveys were epidemiologically robustCitation29 because they used PBPS methodsCitation10 recommended by WHOCitation30 for sampling of populations and examination for trachoma, and incorporated a suite of quality assurance and quality control measures.Citation12 Unlike the GTMP version 1 template, the Tropical Data system involved examination of individuals with trichiasis for TS and asking them about previous trichiasis management recommendations from health workers. These data help the country determine whether EUs had reached the elimination threshold for TT, which is a prevalence of TT “unknown to the health system” – i.e., the prevalence of TT counting only those individuals with the condition who have not previously been offered an operation or epilation for it – of < 0.2%.Citation20 However, disproportionately low participation by adult males posed a potential limitation to the precision of our trichiasis estimates. Based on the 2014 population census, we expected roughly equal numbers of men and women aged ≥15 years to be enumerated during the surveys.Citation24 However, the number of males aged 15 years and above that our teams examined was low compared to the number of females in the same age group. We observed similar absences of men in trachoma surveys in neighbouring TanzaniaCitation31,Citation32, and Democratic Republic of Congo.Citation33 This might be expected to lead to overestimation of TT prevalence (given that TT is generally more common in women than men).Citation34 Standardisation of TT prevalence estimates for age and gender will have partially corrected this bias, without, of course, eliminating it.
The findings from these surveys suggest that TF was not a public health problem in any of the 14 EUs that were previously suspected to be endemic, so antibiotic MDA is not required for trachoma elimination. However, four EUs (Kayunga, Kaberamaido, Arua 1 and Arua 2) need to undertake public-health-level trichiasis surgery interventions and trichiasis-only surveys thereafter. These findings will facilitate planning for elimination of trachoma in Uganda.
Conflict of interest
None of the following authors have any proprietary interests or conflicts of interest related to this submission:
Gilbert Baayenda, Francis Mugume, Patrick Turyaguma, Edridah M. Tukahebwa, Ben Binagwa, Ambrose Onapa, Stella Agunyo, Martin K Osilo, Michael D. French, Wangeci Thuo, Lisa Rotondo, Kristen Renneker, Rebecca Willis, Ana Bakhtiari, Emma M. Harding-Esch, Anthony W. Solomon, Jeremiah M. Ngondi
Acknowledgments
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
1. Advisory Committee, 2. Information Technology, Geographical Information Systems, and Data Processing, 3. Epidemiological Support, 4. Ethiopia Pilot Team, 5. Master Grader Trainers, 6. Methodologies Working Group, 7. Prioritisation Working Group, 8. Proposal Development, Finances and Logistics, 9. Statistics and Data Analysis, 10. Tools Working Group, 11. Training Working Group
Additional information
Funding
References
- World Health Organization. Future Approaches to Trachoma Control : Report of a Global Scientific Meeting, Geneva, 17-20 June 1996. Geneva: World Health Organization; 1997. http://apps.who.int/iris/bitstream/10665/63413/1/WHO_PBL_96.56.pdf. Accessed January 10, 2018.
- World Health Assembly. Global elimination of blinding trachoma. In: 51st World Health Assembly. May 16, 1998; Geneva, Resolution WHA51.11: 1998. http://www.who.int/blindness/causes/WHA51.11/en/. Accessed December 14, 2015.
- World Health Organization. WHO Alliance for the Global Elimination of Trachoma by 2020: progress report on elimination of trachoma, 2014–2016. Index. 2017;92(1–26). http://apps.who.int/iris/bitstream/10665/255778/1/WER9226.pdf.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Eliminating Trachoma: accelerating Towards 2020. 2016. http://www.who.int/trachoma/news/News_Trachoma_Towards_2020/en/. Accessed September 12, 2016.
- MacCallan AF. Trachoma in the British colonial empire-its relation to blindness; the existing means of relief; means of prophylaxis. Br J Ophthalmol. 1934;18:625–645.
- Iverson HA. Survey of eye diseases in Uganda. East Af. Med J. 1964;41:289–294.
- Bruce S. Busoga eye project. Nurs Times. 1967;63:1281–1282.
- Emiru VP, Dechet G. Trachoma survey in Bukedi District, Uganda. East Afr Med J. 1970;47:30–37.
- Davanger M. [Age and sex of trachoma patients in Uganda]. Rev Int Trach. 1973;50:87–100.
- Ngondi J, Reacher M, Matthews F, Brayne C, Emerson P. Trachoma survey methods: a literature review. Bull World Health Organ. 2009;87(2):143–151. doi:10.2471/BLT.07.046326.
- Ministry of Health, Neglected Tropical Disease Programme. Uganda Trachoma Action Plan: 2014-2018. Kampala, Uganda: Ministry of Health; 2014.
- Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the global trachoma mapping project. Am J Trop Med Hyg. 2018:99(4):858–863. doi:10.4269/ajtmh.18-0082.
- Solomon AW, Pavluck AL, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Solomon AW, Kurylo E. The global trachoma mapping project. Community Eye Health. 2014;27:18.
- Hooper P, Millar T, Rotondo LA, Solomon A. Tropical Data: a new service for generating high quality epidemiological data. Community Eye Health. 2016;29:38.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: Training for Mapping of Trachoma. Version 1. London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/resources/global-trachoma-mapping-project-training-mapping-trachoma. Accessed December 15, 2015.
- Courtright P, MacArthur C, Gass K, et al. Tropical Data: Training System for Trachoma Prevalence Surveys. London: International Coalition for Trachoma Control; 2016. http://www.who.int/trachoma/news/News_Trachoma_Tropical_Data_launch/en/. Accessed December 15, 2016.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016. http://www.who.int/trachoma/resources/who_htm_ntd_2016.5/en/.
- Negrel A, Taylor H, West S Guidelines for rapid assessment for blinding trachoma (WHO/PBD/GET/00.8). 2001. http://www.who.int/blindness/TRA-ENGLISH.pdf. Accessed January 1, 2016.
- World Health Organization. Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010. Geneva: World Health Organization; 2010. http://www.who.int/blindness/publications/3RDGLOBALSCIENTIFICMEETINGONTRACHOMA.pdf.
- Uganda Bureau of Statistcs (UBOS) and ICF. Uganda Demographic and Health Survey 2011. Kampala, Uganda and Rockville, Maryland, USA: UBOS and ICF; 2012.
- Hiep N, Ngondi J, Anh V, et al. Trachoma in Viet Nam: results of 11 surveillance surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2018;25(Sup 1):93–102. doi: 10.1080/09286586.2018.1477964.
- Khan A, Florea V, Hussain A, et al. Prevalence of Trachoma in Pakistan: results of 42 population-based prevalence surveys from the global trachoma mapping project. Ophthalmic Epidemiol. SUBMITTED.
- Uganda Bureau of Statistics. The National Population and Housing Census 2014 – Main Report. Kampal, Uganda: UBOS; 2016.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis: Cape Town, 4-6 November, 2015. WHO/HTM/NTD/2016.5. Geneva: World Health Organization; 2016. http://apps.who.int/iris/bitstream/10665/250571/1/WHO-HTM-NTD-2016.5-eng.pdf. Accessed October 1, 2017.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Design and Validation of a Trachomatous Trichiasis-Only Survey ; 2017. http://apps.who.int/iris/bitstream/10665/259815/1/WHO-HTM-NTD-PCT-2017.08-eng.pdf. Accessed. Geneva: World Health Organization. (WHO/HTM/NTD/PCT/2017.08); 2018.
- World Health Organization. Validation of elimination of trachoma as a public health problem. 2016. http://apps.who.int/iris/bitstream/10665/208901/1/WHO-HTM-NTD-2016.8-eng.pdf. Accessed January 5, 2017.
- Uganda Bureau of Statistics (UBOS) and ICF. Uganda Demographic and Health Survey 2016. Kampala, Uganda and Rockville, Maryland, USA: UBOS and ICF; 2018.
- Engels D. The global trachoma mapping project: a catalyst for progress against neglected tropical diseases. Ophthalmic Epidemiol. 2016;23(sup1):1–2. doi:10.1080/09286586.2016.1257139.
- Solomon A, Zondervan M, Kuper H, Buchan J, Mabey D, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva, Switzerland: WHO; 2006.
- Omar FJ, Kabona G, Abdalla KM, et al. Baseline trachoma surveys in Kaskazini A and Micheweni Districts of Zanzibar: results of two population-based prevalence surveys conducted with the global Trachoma mapping project. Ophthalmic Epidemiol. 2016;23(6):412–417. doi:10.1080/09286586.2016.1235206.
- Mwingira UJ, Kabona G, Kamugisha M, et al. Progress of trachoma mapping in mainland Tanzania: results of baseline surveys from 2012–2014. Ophthalmic Epidemiol. 2016;23(6):373–380. doi:10.1080/09286586.2016.1236974.
- Kilangalanga J, Ndjemba JM, Uvon PA, et al. Trachoma in the democratic republic of the congo: results of 46 baseline prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2017 August 1-9. doi: 10.1080/09286586.2017.1306869.
- Cromwell EA, Courtright P, King JD, Rotondo LA, Ngondi J, Emerson PM. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(10):985–992. doi:10.1016/j.trstmh.2009.03.012.