ABSTRACT
Background
Late-stage blinding sequalae of trachoma such as trachomatous trichiasis (TT) typically take decades to develop and often do so in the absence of ongoing ocular Chlamydia trachomatis infection. This suggests that most TT risk accumulates in early life; as a result, population-level TT incidence and prevalence can remain high years after C. trachomatis transmission among children has decreased. In Embu and Kitui counties, Kenya, the prevalence of trachomatous inflammation – follicular is low in children. In this survey, we set out to determine the prevalence of TT in ten evaluation units (EUs) in these counties.
Methods
We undertook ten cross-sectional prevalence surveys for TT. In each EU, people aged ≥15 years were selected by a two-stage cluster sampling method and examined for TT. Those with TT were asked questions on whether they had been offered management for it. Prevalence was adjusted to the underlying age and gender structure of the population.
Results
A total of 18,987 people aged ≥15 years were examined. Per EU, the median number of examined participants was 1,656 (range: 1,451 − 3,016) and median response rate was 86% (range: 81 − 95%). The prevalence of TT unknown to the health system in people aged ≥15 years was above the threshold for elimination (≥0.2%) in all ten EUs studied (range: 0.2–0.7%). TT was significantly more common in older than younger individuals and in women than in men.
Discussion
Provision of surgical services should be strengthened in Embu and Kitui counties of Kenya to achieve the World Health Organization threshold for eliminating TT as a public health problem.
Introduction
Trachoma is the leading infectious cause of blindness worldwide and is endemic in >40 countries.Citation1,Citation2 Ocular infection with Chlamydia trachomatis leads to conjunctival inflammation. Recurrent rounds of inflammation over time result in scarring of the upper sub-tarsal conjunctiva which can cause the eyelid to turn inward, resulting in eyelashes rubbing against the eyeball. This painful state is called trachomatous trichiasis (TT) and can damage the cornea, leading to impairment of vision or blindness.Citation3 Because of the number of rounds of infection and inflammation needed for clinically relevant scarring to develop, TT is much more common in older people.Citation4,Citation5
Trachoma has been targetted for elimination as a public health problem,Citation6,Citation7 which is achieved through a package of interventions known as the SAFE strategy (surgery, antibiotics, facial cleanliness and environmental improvement).Citation8 To determine where these interventions need to be implemented, population-based surveys are required to establish the prevalence of trachoma in a given evaluaution unit (EU) or district (defined as an adminstrative division with a population between 100,000–250,0009). One of three key targets for trachoma to be validated as having been eliminated as a public health problem by the World Health Organization (WHO) is that the prevalence of TT unknown to the health system (i.e. individuals with TT who have not previously been offered managementCitation9) must be <0.2% in those aged ≥15 years in all formerly-endemic EUs.Citation7
Trachoma was first confirmed as an issue of public health concern in Kenya in 2004 when the first baseline survey was carried out.Citation10 Subsequent baseline surveys followed in 2007–2012. Of the 47 counties in Kenya, 12 counties, with a combined population of approximately 7 million people, were confirmed to be trachoma-endemic: Turkana, Kajiado, Samburu, Laikipia, Marsabit, Isiolo, Kitui, Embu, Meru, Narok, West Pokot and Baringo. In 2013, the Ministry of Health estimated there to be 41,500 individuals in these 12 counties who had TT and were at risk of progressive blindness, requiring surgery to prevent it. The Kenya Trachoma Elimination Programme (KTEP) was therefore established. Initiated in 2014, the 5-year programme was given the ambitious goal of eliminating trachoma as a public health problem by 2019, using the SAFE strategy.
TT is the sight-threatening stage of trachoma, so timely and appropriate management of individuals with TT is a priority of trachoma elimination programmes. Between its inception and December 2019, KTEP managed 32,598 people with TT. This included 2,094 and 178 surgeries conducted in Kitui and Embu counties, respectively, To allow for proper planning of future work, it became imperative to know the current status of TT prevalence at county level.
Typically, surveys to estimate trachoma prevalence enroll people aged ≥1 year and assess both TF prevalence in 1–9-year-olds and TT prevalence in ≥15-year olds at the same time. However, in certain contexts, surveys that include examination of both children and adults are unnecessary, for example, where the TF prevalence in 1–9-year-olds is known to be <5% but TT may still constitute a public health problem.Citation11 Since most conjunctival C. trachomatis infection occurs in children, individuals probably accumulate most of the risk of developing TT in early life but do not manifest it until many years later: the presence of low TF prevalence but moderate-to-high TT prevalence may reflect recent decreases in C. trachomatis transmission intensity and does not constitute an epidemiological paradox like the high-TF, low-TT situation seen in Melanesia.Citation12
In 2012, the baseline prevalence of TF in 1–9-year-olds was <5% (range: 0.0–4.8%) in all 9 surveyed EUs of Kitui and Embu counties, whereas the prevalence of TT was ≥0.2% in those aged ≥15 years in 7/9 EUs (). Although Mwingi West and Mwingi Central each had TT prevalences of <0.2% at baseline, it was noted post-survey that there was manifest demand for TT surgical services in local residents. The aim of the current round of surveys was, therefore, to assess TT prevalence in those aged ≥15 years in EUs across these two counties to determine proximity to the WHO-recommended elimination threshold for TT.
Table 1. Summary of survey demographics of ten evaluation units surveyed for trachomatous trichiasis in Kenya (July 2018–April 2019).
Methods
Study ethics and participant consent
The surveys were approved by the Kenya Medical Research Institute. Ethical approval for Tropical Data to support these surveys was provided by the London School of Hygiene & Tropical Medicine Ethics Committee (reference: 16105). Consent forms were translated into Kamba, Embu and Swahili and verbally explained to all participants in their local language. Written informed consent to take part in the survey was provided by those aged ≥18 years, or by a parent or guardian for those aged 15–17 years. Illiterate individuals were permitted to provide verbal consent to take part. Consent for examination was also recorded in the Tropical Data app used for data collection. Individuals identified as having TT or other significant ocular pathology (e.g. cataract) were referred to local ophthalmic services for appropriate management.
Study design and participant selection
This study followed a validated methodology for population-based prevalence surveys for TT, referred to as a “TT-only” survey.Citation11 For the purposes of this survey, each EU was planned to cover an administrative unit of 100,000 − 250,000, which was equivalent to a constituency in Kenya.Citation9 The EUs selected to be surveyed were, for the most part, EUs in which 2012 baseline surveys had found the TF prevalence to be <5% but the TT prevalence to be ≥0.2%. There were two exceptions (Mwingi West and Mwingi Central) for which the 2012 TF prevalence was <5% and the 2012 TT prevalence was <0.2% but where subsequent outreach services had continued to identify TT cases, as noted above; TT-only surveys were also planned here.
We determined that a sampling frame incorporating 3,382 individuals aged ≥15 years would be required in each EU to estimate, with 95% confidence, a TT prevalence in those aged ≥15 years of 0.2 ± 0.2%. That target accounted for disease clustering by incorporating a design effect of 1.47 and for non-response by incoporating an inflation factor of 1.2; the target sample size of ≥15-year-olds was 2,818.Citation11
Individuals were selected using a two-stage cluster selection approach. The primary sampling unit, referred to hereafter as the cluster, was the village; the secondary sampling unit was the household. In the first stage, clusters were systematically selected from each EU with selection probability proportional to village population size. In the second stage, households were selected using compact segment sampling. For these surveys, a household was defined as a group living together and sharing meals. Consenting individuals aged ≥15 years in selected households were eligible for inclusion.
The number of households per cluster was pre-determined as 30, the number a team could routinely visit in one day. The number of clusters was determined as the smallest number needed to yield the sample size, assuming 30 households would be surveyed per cluster. Based on the most recent census data, a mean of 3.8 individuals aged ≥15 years were expected to be resident per household; therefore, 30 clusters needed to be surveyed to achieve the target sample size; this is the maximum number of clusters recommended by WHO for TT-only surveys.Citation11 To maximise enrolment, field teams notified clusters of their visit in advance of their arrival and also made one return visit to households where one or more adults were absent at the first visit.
Clinical examinations
Grader trainees were trained to identify the presence of TT according to the simplified trachoma grading systemCitation13 and undertook an objective structured clinical examination (OSCE) for TT grading under the assessment of an experienced Tropical Data-certified grader trainer. The OSCE involved stations on practical examination methods (loupe use, hand cleanliness, patient placement, upper and lower eyelid margin examination), grading of TT photo sets and eversion of upper eyelids to examine the tarsal conjunctivae for trachomatous scarring (TS).Citation13 To ensure grader quality, graders needed to complete all training components and pass the OSCE to be certified to participate in the field survey.Citation14 Ten field teams were supported by two supervisory teams.
All consenting survey participants were examined for evidence of TT using a 2.5× magnifying loupe. The eyelid of origin of trichiatic eyelashes was recorded. Those identified to have TT were subsequently examined for TS through eversion of the upper eyelid. Where an individual had TT but the eyelid could not be everted, they were considered to have TS. Individuals found to have TT were asked if they had previously been offered surgery or epilation.
Definitions
A recommendation to change the definition of TT was made at the 4th Global Scientific Meeting on Trachoma in November 2018.Citation15,Citation16 The survey series presented here was planned and commenced before that meeting. Therefore, definitions in this manuscript are as follows: first, TT refers to any eyelash touching the eyeball or evidence of removal of inturned eyelashes, regardless of whether that eyelash originates from the upper or lower eyelid, as described in the WHO simplified grading system as published in 1987.Citation13 Where eyelashes from the upper eyelid touched the eyeball, this has been specified in the results. Individuals with TT who had not been offered surgery, or who could not remember whether they had been offered surgery, were classified as “unknown to the health system”, in accordance with WHO guidance.Citation11,Citation17 The presence or absence of TS is disregarded in the general definition of TT in this manuscript.
Data recording, storage and analysis
Field data were collected into Android devices using the Secure Data Kit-based Tropical Data app. Data were encrypted and uploaded through a secure connection to the Tropical Data server (www.tropicaldata.org). The proportion of TT-affected individuals in each five-year age band up to the age of 79 years, and for those aged ≥80 years, was determined at the cluster level. (Five-year age bands were unavailable in census data for the population aged ≥80 years.) To account for non-random differences in participant availability, these estimates were weighted at cluster level according to population composition, in the same age and gender groups, from the most recent (2009) public census data.Citation18 The EU-level prevalence was then determined as the mean of the age- and gender-adjusted cluster-level prevalences. The 95% confidence interval around each EU-level prevalence estimate was calculated by resampling, with replacement, the cluster-level adjusted prevalence estimates 10,000 times.Citation19 Association between TT, age and gender was assessed using a mixed-effects multivariable binomial logistic regression model (lme4::glmer function in R). EU, cluster and household were tested as random-effect variables. The relative contribution of each variable was assessed by comparing models with and without each variable using likelihood ratio tests.
Results
Study population and recruitment
Fieldwork took place between July 2018 and April 2019. Of the 21,820 people aged ≥15 years enumerated in selected households across all 10 EUs, 18,987 were examined. Of those who were enumerated but not examined, the main reason for non-participation (reported for 97% of non-respondents) was absence at the time of survey teams’ visits. The median number of participants examined per EU was 1,656 (range: 1,451 − 3,016). The median response rate per EU was 86% (range: 81 − 95%). contains an EU-level summary of the surveyed populations.
Clinical findings
A total of 262 TT cases were identified across all EUs. 198 (76%) of those cases were bilateral. 245 (94%) had at least one trichiatic eyelash coming from the upper eyelid. 256 (98%) of TT cases reported not having been offered surgery for their TT. shows the age- and gender-adjusted prevalence of TT unknown to the health system in each EU. Also presented are the number of TT cases affecting the upper eyelid, the number of TT cases with TS and the number who had previously reported being operated on. The prevalence of TT unknown to the health system in those aged ≥15 years is displayed in .
Figure 1. Trachomatous trichiasis (TT) prevalence unknown to the health system in people aged ≥15 years in ten evaluation units of Kenya (July 2018–April 2019). The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
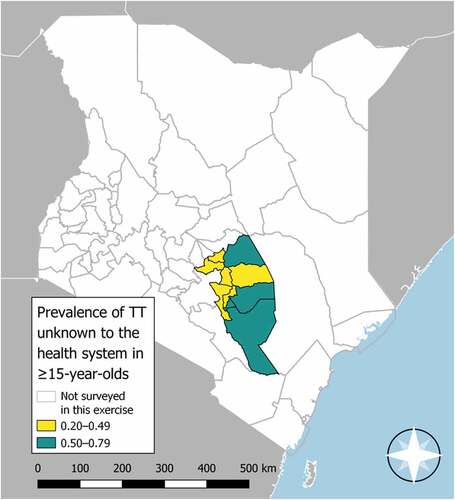
Table 2. Trachomatous trichiasis prevalence in ten evaluation units surveyed in Kenya (July 2018–April 2019).
Association between trachomatous trichiasis, age and gender
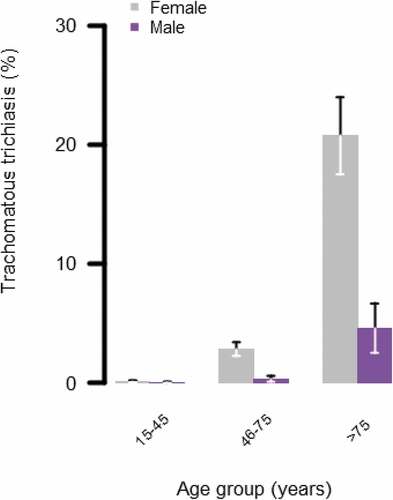
Optimal mixed-effect model fit was achieved with EU and cluster as random-effect variables. TT was signficantly more common in females than in males (adjusted odds ratio [aOR]: 6.1, 95% confidence interval [CI]: 4.1–9.1, p < .001) and significantly more common in those aged 46 − 75 years and those aged >75 years than those aged 15 − 45 years (reference level: 15 − 45 years; aOR for 46 − 75 years: 18.9 [95% CI: 10.6 − 33.8]; aOR for those aged >75 years: 194.2 [95% CI: 108.6 − 347.3]). The majority of TT cases were found in people aged >75 years (172/245, 70%). The distribution of TT cases among males and females of different ages is shown in .
Figure 2. Percentage of men and women with trachomatous trichiasis (management status not specified) separated by age group. Whiskers represent 95% confidence intervals around age group-specific percentage estimates. Data collected during population-based prevalance surveys of ten evalution units in Kitui and Embu counties, Kenya (July 2018–April 2019).
Discussion
The survey data presented here suggest the prevalence of TT unknown to the health system was above the threshold for elimination as a public health problem (<0.2% in people aged ≥15 yearsCitation7) in all ten surveyed EUs of Embu and Kitui counties. This suggests that an ongoing public health response to TT is warranted. The response could include, for example, active case finding and management, and improvement of awareness of surgical services. Planning such services can be supported by rough estimation of case numbers. If we take the combined estimated population of each of these EUs in 2018 and assume 58% of that population is ≥15 years (as indicated by 2009 census dataCitation18) we estimate that there are ~3,900 individuals living in these EUs with TT who have yet to be identified and offered management by the health system, although the inaccuracy in such estimates is well described.Citation20 It is also notable that, of the 2,272 people offered trichiasis surgery in Embu and Kitui counties by the KTEP up to 2019, there were 64 individuals who refused. It is important to understand the reasons behind these refusals to support programme staff to maximise uptake. It should also be noted that Kitui has roughly double the population of Embu, but has carried out more than ten-times the number of trichiasis operations. Embu has one of the lowest numbers of surgeries conducted of any county. This highlights the necessity to ensure programmes are delivered equitably.
This study has some limitations. The target sample size of 2818 examined adults was not met in nine of the 10 surveyed EUs (). The lower-than-expected precision with which TT prevalence has been estimated in these EUs is corresondingly lower () than we aimed to achieve in our method statement. However, TT-only survey guidelines are explicit in their advice to limit the number of clusters to be visited to 30 where >30 clusters are expected to be needed to acheive the sample size target. This recommendation is supported by simulation studies of over-sampled datasets, which suggest that surveying >30 clusters does not yield a useful improvement in precisionCitation11; in our surveys, 30 clusters were sampled in all EUs. Second, TT was graded according to WHO’s original simplified grading system. The system has recently been amended so that trichiasis is only regarded as “trachomatous” when the inturned eyelashes come from the upper eyelid.Citation16 The proportion of TT cases with upper eyelid involvement in this series of surveys was 94% (), but as questions on management were not specifically disaggregated by eyelid, it is not possible to determine the prevalence of upper-eyelid TT unknown to the health system. This difference in grading should be considered when comparing these estimates to future estimates. Finally, two constituencies of Embu county (Manyatta and Runyenjes) were not surveyed in this exercise. However, these constituencies are in a different climatic zone to the other surveyed constituencies, have better access to a major water tower at Mount Kenya and do not regularly report cases of trachoma. They are, therefore, considered to be at low risk of high trachoma prevalence.
It is difficult to use these prevalence estimates to make inferences about the success of any interventions which have taken place between the 2012 and 2018 survey rounds, for a number of reasons. The limitations of the current exercise have been described in the paragraph above, but additional factors also make comparison problematic. First, assuming a constant incidence of TT and a constant mortality rate in the general population, it would be reasonable to assume that prevlance of TT would remain stable. However, in reality, both TT incidence and population mortality are likely to vary with time based on a range of factors. A systematic review evaluating the incidence of TT in trachoma-enemic communities produced estimates of between 0.06–3.0% per year, depending on location, age, gender and conjunctival scarring status,Citation21 though data that inform this range come from only three countries.Citation22–26 Estimates from the World Bank suggest mean life expectancy is increasing in Kenya (data.worldbank.org), which could lead to an expansion in the population of older age groups at highest risk of TT. Second, neither the 2012 nor 2018 surveys were designed to estimate prevalence with sufficiently high precision to enable comparisons if differences were small. Third, given the low number of cases, small differences in survey design (such as the method for undertaking adjustment for age and gender) between 2012 and 2018 could have a disproportionaly large effect on the estimated prevalence. Face-value comparisons between these two survey estimates are therefore unlikely to be informative. It is, however, notable that 98% of individuals with TT reported not previously having been offered management for their TT, despite the KTEP conducting >32,000 TT surgeries nationwide between 2014 and 2018.
These data highlight the need for implementation of robust systems to identify and manage incident cases of TT. Investment in such systems now would also help the programme get a head-start on the third criterion for trachoma’s elimination as a public health problem.Citation7
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated, USAID or the United States Government.
Acknowledgments
We thank the leadership of the Ministry of Health in Kenya, the County Health management teams and the provincial administration in the respective counties for their support and facilitation. The Environmental Health Officers and the Community Health infrastructre played a major role in mobilizing the communities before and during the surveys. We are grateful for the diligence of the graders and recorders who collected data and the survey supervisors who ensured that fieldwork was conducted according to agreed standards. Kenya’s National Bureau of Statistics provided advice on cluster sampling. We thank Peter Otinda and Elizabeth Owuor-Oyugi (Sightsavers) and all other staff from the implementing partners and Tropical Data who supported this work.
Disclosure statement
AB and RW are employed by the International Trachoma Initiative at The Task Force for Global Health, which receives an operating budget and research funds from Pfizer Inc., the manufacturers of Zithromax® (azithromycin). EMHE receives salary support from the International Trachoma Initiative. The other authors declare no conflicts of interest.
Additional information
Funding
References
- Bourne RA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–49. doi:10.1016/S2214-109X(13)70113-X.
- World Health Organization. Global alliance for the elimination of trachoma by 2020. WHO alliance for the global elimination of trachoma by 2020: progress report, 2019. Wkly Epidemiol Rec. 2020;30(95):349–360.
- Taylor HR. Trachoma: A Blinding Scourge from the Bronze Age to the Twenty-First Century. East Melbourne: Centre for Eye Research, Australia; 2008.
- Burton MJ, Bowman RJC, Faal H, et al. The long-term natural history of trachomatous trichiasis in the Gambia. Invest Ophthalmol Vis Sci. 2006;47(3):847–852.doi:10.1167/iovs.05-0714.
- Gambhir M, M-g B, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462.doi:10.1371/journal.pntd.0000462.
- World Health Organization. Report of the First Meeting of the WHO Alliance for the Elimination of Trachoma (WHO/PBL/GET/97.1). 30 June - 1 July. Geneva, Switzerland; 1997.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva: Switzerland; 2016.
- Francis V, Turner V. Acheiving Community Support for Trachoma Control: A Guide for District Health Work (WHO/PBL/93.36). Geneva, Switzerland: World Health Organization; 1995.
- World Health Organization. Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010 (WHO/PBD/2.10). Geneva, Switzerland; 2010.
- Karimurio J, Gichangi M, Ilako DR, Adala HS, Kilima P. Prevalence of trachoma in six districts of Kenya. East Afr Med J. 2006;83(4):63–68. Accessed September 11, 2013. doi:10.4314/eamj.v83i4.9417.
- World Health Organization Strategic and Technical Advisory Group for Neglected Tropical Diseases., Working Group on Monitoring and Evaluation. Design and Validation of a Trachomatous Trichiasis-Only Survey (WHO/HTM/NTD/PCT/2017.08). Geneva: Switzerland; 2017.
- Butcher R, Tagabasoe J, Manemaka J, et al. Conjunctival scarring, corneal pannus and Herbert’s pits in adolescent children in trachoma-endemic populations of the Solomon Islands and Vanuatu. Clin Infect Dis. 2020:ciaa1151. doi:10.1093/cid/ciaa1151.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- Courtright P, Flueckiger R, Harding-Esch E, Lewallen S, Solomon A. Tropical Data: Training Manual for Trachomatous Trichiasis Population-Based Prevalence Surveys (Version 1). 1st. London: International Coalition for Trachoma Control; 2017.
- World Health Organization. Report of the 4th Global Scientific Meeting on Trachoma (WHO/HTM/NTD/PCT/2019.03). Geneva: Switzerland; 2018.
- Solomon AW, Kello AB, Bangert M, et al. The simplified trachoma grading system, amended. Bull World Health Organ. 2020;98(10):645–724. doi:10.2471/BLT.19.248708.
- Organization WH. Second Global Scientific Meeting On Trachomatous Trichiasis. Cape Town: South Africa; 2015 November) (4-6.
- Kenya National Bureau of Statistics. The 2009 Kenya Population and Housing Census. Nairobi: Kenya; 2010.
- Solomon AW, Pavluck AL, Courtright P, et al. The global trachoma mapping project: Methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Solomon AW, Bella AL, Negussu N, Willis R, Taylor H. How much trachomatous trichiasis is there? A guide to calculating district-level estimates. Community Eye Health. 2019;31:S5–S8.
- Ramadhani AM, Derrick T, Holland MJ, Burton MJ. Blinding trachoma: Systematic review of rates and risk factors for progressive disease. PLoS Negl Trop Dis. 2016;10:(8). e0004859. doi:10.1371/journal.pntd.0004859.
- Munoz B, Bobo L, Mkocha H, Lynch M, Hsieh YH, West S. Incidence of trichiasis in a cohort of women with and without scarring. Int J Epidemiol. 1999;28(6):1167–1171. doi:10.1093/ije/28.6.1167.
- Dawson C, Marx R, Daghfous T, Juster R, Schachter J. What clinical signs are critical in evaluating the intervention in trachoma? In: Bowie W, ed. Chlamydial Infections. Cambridge, UK: Cambridge University Press; 1990:271–278.
- Munoz B, Aron J, Turner V, West S. Incidence estimates of late stages of trachoma among women in a hyperendemic area of central Tanzania. Trop Med Int Heal. 1997;2(11):1030–1038. doi:10.1046/j.1365-3156.1997.d01-186.x.
- Bowman RJC, Jatta B, Cham B, et al. Natural history of trachomatous scarring in the gambia: Results of a 12-year longitudinal follow-up. Ophthalmology. 2001;108(12):2219–2224.doi:10.1016/S0161-6420(01)00645-5.
- Burton MJ, Holland MJ, Makalo P, et al. Profound and sustained reduction in Chlamydia trachomatis in the gambia: a five-year longitudinal study of trachoma endemic communities. PLoS Negl Trop Dis. 2010;4:(10). 10. doi:10.1371/journal.pntd.0000835.
