Abstract
Bowels are the most common site of extrapelvic endometriosis. Still, colonic endometriosis often presents a diagnostic challenge, mimicking a broad spectrum of diseases including primary colonic malignancy. For women of fertile age, the consequences of endometriosis being misdiagnosed as colorectal cancer may include loss of fertility. We hereby present a case of endometriosis mimicking rectosigmoid adenocarcinoma in a young woman, where fertility preservation prior to the start of antineoplastic treatments turned out to be of crucial importance for the woman’s future attempts to achieve a pregnancy and livebirth.
摘要
肠道是盆腔外子宫内膜异位症最常见的部位。尽管如此, 结肠子宫内膜异位症的诊断经常是一个挑战, 因为其临床表现与很多疾病相似, 包括原发性结肠恶性肿瘤。对于育龄期妇女, 子宫内膜异位症被误诊为结直肠癌的后果可能包括丧失生育能力。我们在此提出一例类似直肠乙状结肠腺癌的子宫内膜异位症的年轻妇女, 在抗肿瘤治疗开始前的生育能力保存被证明对于妇女未来的尝试实现怀孕和活产是至关重要的。
The Chinese abstracts are translated by Prof. Dr. Xiangyan Ruan and her team: Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing 100026, China.
Introduction
Endometriosis is defined by the presence of endometrial glands and stroma outside the endometrial cavity. This condition commonly affects women of reproductive age, with an estimated prevalence of ∼10% [Citation1]. It is associated to increased risk of infertility and earlier exhaustion of ovarian reserve [Citation2,Citation3].
Endometriosis can affect almost any anatomical location. Intestinal involvement is rare, but when it happens endometriosis is usually located in the rectosigmoid (50–90%) [Citation4]. Even though endometrium can be found in any layer of the bowel, it most commonly presents in subserosa as superficial serosal implants [Citation5]. Intestinal mucosa usually remains intact [Citation4]. Being well described in the literature, endometriosis with intestinal involvement still often poses a diagnostic challenge for clinicians and pathologists. Clinical symptoms may be very unspecific, depending on the site and the extent of bowel lesions. Radiologic and endoscopic findings can be at times difficult to interpret and confusion with malignancy can occur, especially in patients with mucosal involvement [Citation6,Citation7].
While practice of fertility preservation in young women with cancer is spreading rapidly [Citation8–10], its use for endometriosis in routine clinical practice remains rare and controversial [Citation11]. This article presents a case report of a live birth following fertility preservation in a young woman with iatrogenic infertility due to endometriosis mistaken for rectosigmoid cancer.
Case report
A 30-year-old nulliparous woman presented to the emergency department with a complaint of 2 weeks interval abdominal pain and a 4 days’ history of obstipation. Her past medical history included dysmenorrhea and unsuccessful attempts to achieve pregnancy for the last 2 years. Otherwise, she had been healthy, with no surgical history and no medications. Physical examination revealed a soft abdomen with diffuse tenderness in the left lower quadrant. Blood tests were unremarkable. Abdominal CT scan visualized an obstructive, exophytic mass, ∼5 cm in diameter, protruding into the intestinal lumen at the rectosigmoid junction, with a pre-stenotic dilatation of the transverse colon of up to 7.5 cm. Emergency laparotomy revealed a tumorous stricturing mass in the upper rectum, involving the left ovary. A loop transversostomy was performed to relieve the symptoms of ileus. Investigation on suspicion of colorectal cancer was initiated, with Magnetic Resonance Imaging (MRI) of the pelvis, CT scan of the thorax and abdomen, rectoscopy and colonoscopy.
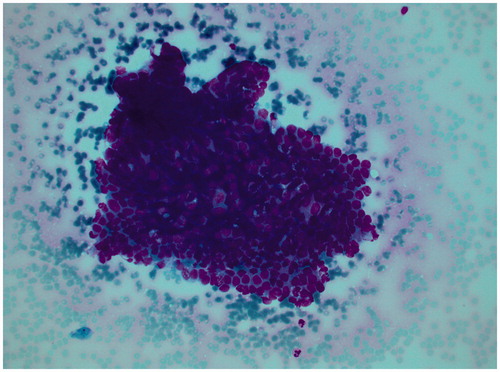
Rectoscopy and colonoscopy from the anus and from the stoma revealed a polypoid lesion at the level of 20 cm, creating a complete stricture 2–3 cm above of its origin. Normal tissue and mesenchymal cells were found in the biopsies. MRI showed a tumor in the upper rectum with overgrowth on the left pelvic sidewall and the left ovary and close proximity to the uterus with no obvious overgrowth (). The MRI and peroperative findings were convincing of an upper rectal cancer. A pre-therapeutic morphologic diagnosis was however mandatory. Repeated colonoscopy failed to reach the suspected tumor due to stenosis in the area and finally an endoscopic ultrasound with cytology was performed (). The pathologist’s conclusion of cytological examination was “Manifestation of adenocarcinoma”. In accordance with the guidelines for locally advanced rectal cancer, the colorectal MultiDisciplinary Team (MDT) conference recommended neoadjuvant chemotherapy with Capecitabine and high-dose pelvic radiotherapy, which would render the woman infertile, and thereafter a surgery.
Figure 1. Sagittal T2-weighted MR-image of the pelvis. The solid lesion invading the recto-sigmoid colon is indicated with yellow arrows.
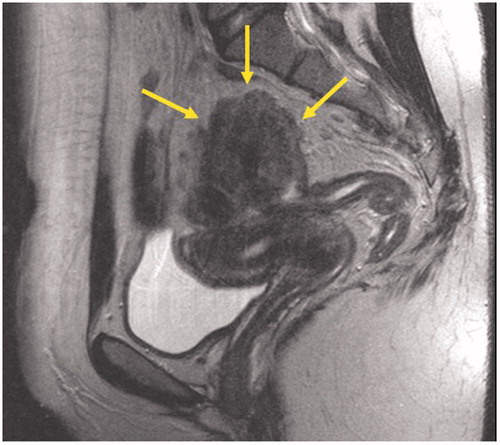
Figure 2. Cytological findings from the suspected tumor with the cell groups interpreted to represent malign epithelial cells.
Before the start of the neoadjuvant treatment, the patient was referred for emergency counseling on fertility preservation to the Reproductive Medicine clinic. Information on available methods for fertility preservation was provided whereafter the woman and her husband decided to attempt fertility preservation by embryo cryopreservation. Controlled ovarian stimulation with gonadotropins using an antagonist protocol starting on day 2 of the cycle was initiated. After 14 days on stimulation, maturation trigger was induced with hCG 10.000 IU and oocyte retrieval planned 37 h later. The treatment resulted in 5 oocytes aspirated through puncture of the right ovary, which was judged as being unaffected by tumor. Conventional In Vitro Fertilization (IVF) was applied and four embryos were cryopreserved, three of them at cleavage stage with development of four-cells and the remaining one at a two-cells stage.
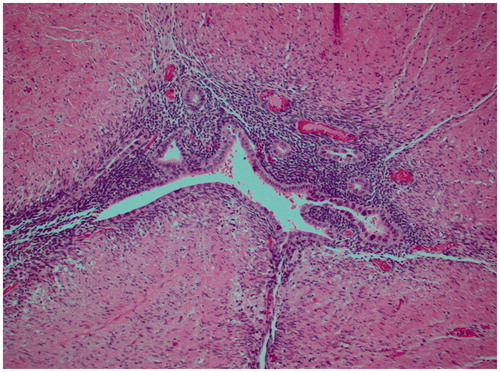
Following neoadjuvant treatment that included radiotherapy of the pelvis (1,8 Gy in fractions up to 50.4 Gy) and chemotherapy (Xeloda), the patient underwent laparotomy with anterior resection of rectum and a bilateral salpingo-oophorectomy. Histopathologic examination of the resected specimen revealed no evidence of malignancy, but clear signs of endometriosis and fibrosis (). The left ovary and tube were adherent to the sigmoid colon and showed deep infiltrating endometriosis. There were no malignant cells in the 19 harvested lymph nodes. Immunohistochemical analysis showed CD10+, ER+, Ck7+, Ck20−, CDX2−, confirming the diagnosis of endometriosis.
Figure 3. Histological image of the resected specimen showing signs of endometriosis and fibrosis (hematoxylin-eosin).
Cytology from the preoperative biopsy was re-evaluated, submitted for second opinion abroad and finally redefined as atypia of undetermined significance. The diagnosis of cancer was withdrawn at the MDT-meeting (surgeons, oncologists, radiologists and pathologists). The possibility that the patient had had a complete response to the neoadjuvant treatment was discussed, but in the end all the clinical, radiological and immunohistological findings were considered to have been caused by endometriosis. The conclusion on whether or not the patient had cancer was of special importance to her, since a cancer diagnosis would prevent the couple from being approved for adoption - a possibility they could consider in case the fertility treatments would not be successful.
The woman was relieved to know that she didn’t have a malignancy but devastated over sterility caused by the treatment. A new consultation with a reproductive medicine specialist was scheduled about 2 months after the surgery. Possible effects of radiation on the uterus were discussed. Radiotherapy of the pelvis has in previous studies been associated to higher rates of miscarriage, premature delivery and low birth weight, in particular if high doses were applied [Citation12,Citation13]. The impact of irradiation on endometrial thickness and responsiveness to exogenous hormonal stimulation was explained to be less well characterized. The patient expressed willingness to proceed with fertility treatment as soon as possible. She had been amenorrhoeic since the start of neoadjuvant therapy and received combined hormone replacement therapy (HRT) with estrogen-progesteron sequentially (Trisekvens). Transvaginal ultrasound exam revealed a remarkably thin endometrium below 2 mm.
A test cycle similar to that prescribed for endometrial preparation of women undergoing egg donor treatment was started using estradiol valerate (Progynon) 6 mg day−1 during 14 days. At the following control the endometrial thickness was of 5.3 mm. The patient continued on estradiol valerate and after addition of medroxiprogesterone acetate (MPA) (Provera) 10 mg day−1 a new checkup revealed premenstrual endometrium of 6 mm. The woman had significant climacteric symptoms and wished to postpone the first attempt of frozen-embryo transfer for several months. She was prescribed sequential combined hormone replacement to alleviate menopausal symptoms with Divina Plus (estradiol valerate 2 mg for 9 days followed by combination of estradiol valerate 2 mg and MPA 10 mg for 12 days).
The first attempt of transfer of a frozen-thawed embryo was performed 2.5 years after surgery. The cycle was programed using 8 mg estradiol valerate per day, resulting in the endometrium of 6 mm on the day of transfer. The embryo was of good quality, being four cells at freezing but having developed to a six-cells embryo to the moment of transfer. This first attempt didn’t result in a pregnancy and the couple came back 18 months later for the second attempt. The cycle was programed on transdermal estrogen due to the suspicion of reduced enteral absorption as consequence of the treatments previously received. Still, even this time no pregnancy occurred. The third time (4.5 years after the operation) the stimulation of endometrium was started with estradiol hemihydrate transdermal patch (Estradot) 150 μg 2 times a week in 12 days and then 300 μg 2 times a week until day 19. The endometrial thickness was optimal of >7 mm. Although both remaining cryopreserved embryos were thawed, only one of them survived and it was transferred at a 4-cellsstadium. Luteal support with Estradot 300 μg and micronized Progesteron 400 μg x3 was prescribed. The pregnancy test was positive and the ultrasound checkup on week 7 revealed a viable intrauterine pregnancy.
At gestational week 28 + 5 the patient presented to obstetric emergency department with symptoms of severe preeclampsia and chorioamnionitis. The treatment with antihypertensive drugs, magnesium sulfate and broad-spectrum antibiotics was started and the next day the decision was taken to deliver the baby by an emergency cesarean. A baby girl was obtained, 46 cm long and weighing 887 gr, with Apgar 3-4-8. The newborn was initially admitted to the neonatal intensive care unit and thereafter remained on neonatal care for about 1 month due to prematurity. Both mother and daughter were discharged home in good health thereafter.
Discussion
In this article we describe a case of erroneous diagnosis of rectosigmoid cancer in a woman with advanced endometriosis, and the resulting challenges of achieving a pregnancy with frozen-thawed embryos in an irradiated uterus after bilateral SOE.
It is noteworthy that the patient herself at an early diagnostic stage has raised a question of endometriosis as a possible reason for the intestinal mass, but a complex preoperative diagnostic workout, including various imaging modalities and tissue biopsy, failed to identify endometriotic origin of the tumor.
This is a reminder of the importance of always questioning and re-assessing clinical, radiological, cytological and histological findings, with each part of multidisciplinary team being aware of the impact of individual assessment.
Correct diagnosis of colorectal endometriosis would have allowed proper treatment, including segmental resection of the obstructed part of the bowel and further medical treatment with low-dose oral contraceptive or progestins [Citation14]. Treatment-induced sterility could be avoided. Even though endometriosis itself is associated with a risk of reduced fertility, and women with advanced stages of this disease are known to have lower success rates with in vitro fertilization (IVF) [Citation15], the consequences of pelvic radiotherapy and bilateral salpingoophorectomy (SOE) on female fertility are still much more detrimental.
In this case, embryo-banking prior to the start of radio- and chemotherapy has years later finally resulted in a successful embryo transfer and a live birth. Previous reports have indicated that in women who survive cancer, reproductive outcomes after radiotherapy seem to be worse than outcomes after chemotherapy or surgery [Citation12,Citation16,Citation17]. Situation in this particular case was additionally complicated by the aberrant effects of endometriosis and previous bilateral SOE on the milieu during implantation. Also, the 50.4 Gy given to the pelvis, entails risk that the uterus cannot support the growth of a pregnancy until full-term, and even a risk for uterine rupture which necessitates thorough surveillance during the pregnancy. Pregnancy success under these circumstances supports the practice of fertility preservation in women with cancer who face multimodal treatments with surgery, radio- and chemotherapy [Citation18], and also in women with chronic conditions impending their fertility, such as advanced stages of endometriosis [Citation19].
Conclusion
Endometriosis with intestinal involvement has an amazing mimicking capacity and should be considered as a differential diagnosis in complicated diagnostic work-outs of diverse gastrointestinal symptoms in women. Practice of fertility preservation counseling in young women of fertile age facing treatments with potential risk of subsequent sterility should be supported and promoted.
Patient consent
Patient consent has been given for writing and publishing of this case report.
Authors contributions
KARW conceived the idea of this article. KARW is clinical responsible of program for fertility preservation at Karolinska University Hospital, she counseled the patient on fertility preservation, coordinated IVF cycles and surveillance for this patient. AS was involved in oncological surgery and postoperative surveillance for this patient. AM collected data and prepared the first version of manuscript. LB and JC provided illustrative radiologic and histo-pathologic images. All the authors participated in study design, execution, analysis, manuscript drafting and critical discussion.
Acknowledgements
The authors thank the staff of Section of Reproductive Medicine at Karolinska University hospital and the Colorectal Team at the Karolinska University Hospital.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–1769.
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738.
- Vercellini P, Vigano P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275.
- Pisanu A, Deplano D, Angioni S, et al. Rectal perforation from endometriosis in pregnancy: case report and literature review. World J Gastroenterol. 2010;16:648–651.
- Yantiss RK, Clement PB, Young RH. Endometriosis of the intestinal tract: a study of 44 cases of a disease that may cause diverse challenges in clinical and pathologic evaluation. Am J Surg Pathol. 2001;25:445–454.
- Remorgida V, Ferrero S, Fulcheri E, et al. Bowel endometriosis: presentation, diagnosis, and treatment. Obstet Gynecol Survey. 2007;62:461–470.
- Dimoulios P, Koutroubakis IE, Tzardi M, et al. A case of sigmoid endometriosis difficult to differentiate from colon cancer. BMC Gastroenterol. 2003;3:18.
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. JCO. 2013;31:2500–2510.
- Peccatori FA, Azim HA, Jr., Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi160–70.
- Lambertini M, Del Mastro L, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1.
- Somigliana E, Vigano P, Filippi F, et al. Fertility preservation in women with endometriosis: for all, for some, for none? Hum Reprod. 2015;30:1280–1286.
- Urbano MT, Tait DM. Can the irradiated uterus sustain a pregnancy? A literature review. Clin Oncol (R Coll Radiol). 2004;16:24–28.
- Critchley HO, Bath LE, Wallace WH. Radiation damage to the uterus – review of the effects of treatment of childhood cancer. Hum Fertil (Camb). 2002;5:61–66.
- Vercellini P, Frattaruolo MP, Rosati R, et al. Medical treatment or surgery for colorectal endometriosis? Results of a shared decision-making approach. Hum Reprod. 2018;33:202–211.
- Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017;96:659–667.
- Munoz E, Fernandez I, Martinez M, et al. Oocyte donation outcome after oncological treatment in cancer survivors. Fertil Steril. 2015;103:205–213.
- Marklund A, Nasiell J, Berger AS, et al. Pregnancy achieved using donor eggs in cancer survivors with treatment-induced ovarian failure: obstetric and perinatal outcome. J Womens Health (Larchmt). 2018;27:939–945.
- Rodriguez-Wallberg, K A, Karlström, P-O, Rezapour, M, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94:324–328.
- Rodriguez‐wallberg, K A, Marklund, A, Lundberg, F, et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non‐oncologic indications in Sweden–Trends in patients’ choices and benefit of the chosen methods after long‐term follow up. Acta Obstet Gynecol Scand. 2019;98:604–615.
