Abstract
Objective
This research evaluated the efficacy of oral nutritional agents including CoQ10, vitamin E, inositols and vitamin D on androgen-associated hormones, glycolipid metabolism and body weight in women with PCOS.
Method
A multi-database search was performed from inception to December 2020. Using multi-variate random effects method, a NMA was conducted by synthesizing data pooled from RCTs. It was registered with PROSPERO (registration number CRD42021230292).
Results
Twenty-three RCTs and 1291 participants were included. Based on NMA, CoQ10, vitamin E, CoQ10 combined with vitamin E, and inositols were successful in decreasing TT as compared with PA; vitamin E was superior to other agents. Vitamin E and inositols were successful in increasing SHBG levels; inositols were stronger than vitamin E. CoQ10 alone or combined with vitamin E, and inositols were successful in decreasing HOMA-IR. Inositols had the best results among included nutraceuticals to ameliorate HOMA-IR, FBG, FINS, TG, TC, and LDL-C and correlated to improvements in BMI. There was no significant difference between the CoQ10 or vitamin E group and the PA group in ameliorating lipid metabolism, and vitamin D had no positive effects in ameliorating hyperandrogenism, BMI, glycolipid metabolism profiles compared with PA.
Conclusion
For women with PCOS, inositols supplementation have some certain advantages in increasing SHBG and improving glycolipid metabolism when compared with nutraceuticals like CoQ10, vitamin E, vitamin D. Besides, vitamin E may be a better option in reducing TT and increasing SHBG. CoQ10 alone or combined with vitamin E can be helpful in decreasing HOMA-IR as well.
辅酶Q10、维生素E、肌醇和维生素D对改善多囊卵巢综合征女性的内分泌和代谢状况的有效性:一项网络荟萃分析 摘要
目的:评价口服营养剂CoQ10、维生素E、肌醇、维生素D对多囊卵巢综合征女性雄激素相关激素、糖脂代谢及体重的影响。
方法:从研究开始至2020年12月进行多数据库检索。采用多变量随机效应方法, 综合随机对照试验的数据进行NMA。在PROSPERO上注册(注册号CRD42021230292)。
结果:纳入23项随机对照研究和1291名参与者。基于NMA, CoQ10、维生素E、CoQ10联合维生素E、肌醇与PA相比成功降低了TT;维生素E优于其他制剂。维生素E和肌醇成功地提高了SHBG水平;CoQ10单独应用或与维生素E联合应用及肌醇均能有效降低HOMA-IR。在改善HOMA-IR、FBG、FINS、TG、TC和LDL-C的营养药物中, 肌醇效果最好, 且与BMI改善相关。CoQ10组和维生素E组在改善脂质代谢方面与PA组无显著差异, 维生素D在改善高雄激素血症、BMI、糖脂代谢方面与PA组无显著差异。
结论:对于多囊卵巢综合征女性患者, 补充肌醇与CoQ10、维生素E、维生素D等营养药物在提高SHBG、改善糖脂代谢方面具有一定优势, 维生素E可能是降低TT、提高SHBG的更好选择。CoQ10单独或与维生素E结合也有助于降低HOMA-IR。
Introduction
Polycystic ovary syndrome (PCOS) is a multifactorial disorder involving both endocrine and reproductive systems of women, affecting 6–10% women of reproductive age [Citation1,Citation2]. Insulin resistance has been recognized as a key contributor to the disease since patients with PCOS are at increased risk for the development of metabolic syndrome, type 2 diabetes, and cardiovascular diseases [Citation3–6]. Currently, certain nutraceuticals such as coenzyme Q10 (CoQ10), vitamin E, vitamin D, inositols, in particular myo-inositol (MI) and D-chiro-inositol (DCI), have received preponderance of the attention owing to their potential benefits in improving insulin resistance and further ameliorating the endocrine and metabolic disorders for PCOS management [Citation7–12]. Moreover, there are few reports available on the adverse effects of these nutraceuticals. Though five meta-analysis [Citation13–17] have been performed on the endocrine and metabolic profiles of patients with PCOS provided with inositols or vitamin D as compared to placebo, since the small sample size and differ inclusion criteria, lessons from the previous studies are not consistent on some important indicators. Hence, we performed a network meta-analysis (NMA) to compare the effects of the nutritional agents involving CoQ10, vitamin E (alone and in combination), inositols, and vitamin D on endocrine and metabolic parameters in women with PCOS and to provide evidence-based outcomes for the treatment of PCOS.
Materials and methods
Literature search
We searched the following electronic databases: PubMed, Web of Science, EMBASE, the Cochrane Library, the PhRMA Clinical Study Results Database (www.clinicaltrials.gov), the Wan Fang database, and the China National Knowledge Infrastructure (CNKI) database, from inception to December 2020. Subject words as well as free words were chosen for retrieval. A second extended search was conducted for improved search results. The main terms, which were based on the Medical Subject Headings (MeSH), were ‘polycystic ovary syndrome’, ‘PCOS’, ‘inositol’, ‘myo-inositol’, ‘D-chiro-inositol’, ‘Coenzyme Q10’, ‘vitamin E’, ‘vitamin D’, and ‘placebo’. The screening of the literature should be strictly followed by the inclusion and exclusion criteria. It was registered in the PROSPER International Prospective Register of Systematic Reviews, registration number CRD 42021230292.
Inclusion and exclusion criteria
The inclusion criteria are as follows: (i) type of studies, randomized clinical trials (RCTs) only, without any limitations regarding the language, race or area constraints; (ii) target population: women of reproductive age (18–49 years old) with PCOS based on the National Institute of Child Health and Human Development (NICHD) standards [Citation18] or the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) standards [Citation19] or the Androgen Excess Society criteria (AES) [Citation20]; (iii) intervention: CoQ10, vitamin E (alone and in combination with CoQ10), vitamin D, or inositols (MI or DCI alone or taken together) for the experimental group and placebo for the control group and other adjunctive agents (excluding folic acids) were maintained consistently between the groups; (iv) target parameter, at least one parameter including total testosterone (TT), free testosterone (FT), sex-hormone binding globulin (SHBG), fasting blood glucose level (FBG), fasting insulin level (FINS), homeostatic model assessment of insulin resistance (HOMA-IR), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), body mass index (BMI), waist-hip-ratio (WHR); (v) duration, at least 8 weeks.
The exclusion criteria are as follows: (i) studies involving women who were pregnant or intended to be pregnant or had other illness such as diabetes, liver disease, or kidney disease during the study period; (ii) duplicated publications; or (iii) studies with partially missing relevant data.
Data extraction
The selection of literature was reviewed and selected independently by two researchers (Z.J.Q. and X.C.). Duplicated articles were removed, then the titles and abstracts were initially screened, and any articles that did not meet the eligibility criteria were excluded. Additionally, two reviewers independently scanned the full-text to further verify their eligibility related to the inclusion and exclusion criteria and extracted data by using same standardized data extraction forms. Study characteristics involving patient information (ethnicity, age, BMI, and outcomes), interventions, and experimental duration were extracted from each full-text article. If a consensus was not reached during the initial meetings, a third investigator (H.B.) would arbitrate.
Assessment of risk of bias
Assessment of risk of bias was performed using the Cochrane Risk of Bias Tool 2.0 for RCTs [Citation21] by two investigators independently (Z.J.Q. and X.C.), and studies were assessed from the following five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selective reporting of results. Following this, each trail would be made as low risk of bias, some concerns and high risk of bias. Any disagreement was resolved by discussions with the third author (H.B.).
Statistical analysis
First, a traditional meta-analysis (TMA) was performed using Revman software (Version 5.3) to directly compared the efficacies of CoQ10, vitamin E, inositols, vitamin D and CoQ10 + vitamin E with placebo. Continuous data were analyzed using mean difference (MD) to express the effect size, and dichotomous data were expressed using the relative risk (RR), with 95% confidence intervals (CIs) and an α error of 0.05. A fixed-effects model (Mantel–Haenszel method) was used if there was no heterogeneity (p > .1 or I2<50%), otherwise, a random-effects model (Mantel–Haenszel method) was utilized. In this analysis, heterogeneity was present; thus, all results were reported using the random-effects model. Funnel plots and Egger’s test were employed for publication bias analysis if the included trials were greater than 10. Stata software (Version 15.1) was used to construct the funnel plots.
Second, a NMA was performed, using Stata software (Version 15.1), to simultaneously compare the curative effect of various treatment options for the management of PCOS. Thus, NMA could provide more comprehensive information compared to TMA through combining results from both direct and indirect evidence. A network plot was portrayed to present the comparison network of interventions and controls. In the plot, circular nodes represented the size of included sample, and yellow lines represented trails using blinding while green lines represented trails without using blinding. Before performing data synthesizing, transitivity assumption and consistency assumption was assessed by us to check for the presence of inconsistency. Transitivity assumption could be tested by investigating the inclusion and exclusion criteria of all studies included to determine whether patients, study characteristics, and outcomes are similar enough that could be modify relative treatment effects. Consistency assumption could be assessed by using the loop-specific and the node-splitting method to find significant incongruity between direct and indirect comparisons. The random-effects model was used to pool all relevant parameters. Continuous data were summarized as MD with 95% credibility intervals (Crls), and dichotomous data as RR [Citation22]. Surface under the cumulative ranking curve analysis (SUCRA) is used to estimate the likelihood of each rank order using scores that range from 0% to 100%. It can reveal intervention with high values, indicating a high preference for those interventions.
Results
Literature identification
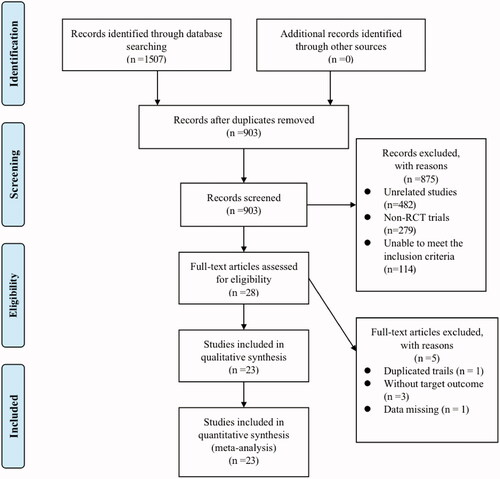
Of the 1507 publications retrieved via literature search, 903 records left after removing duplicates. Then, 28 of the remaining records were included after screening title and abstract and excluding 875 records that were irrelevant (n = 482), non-RCT (n = 279), or unable to meet the inclusion criteria (n = 114). After screening the full-texts, 5 records that involved duplicated trails (n = 1), or target outcomes with partially missing data (n = 4) were excluded. Finally, a total of 23 studies [Citation23–45] and 1291 PCOS patients were included, with 754 in the experimental group and 537 in the control group.
The inclusion and exclusion criteria were strictly followed in the process of literature screening, and the flow of this screening process is presented in a PRISMA flow diagram in . Details of the characteristics of included studies can be seen in .
Table 1. The characteristics of the included studies.
Quality assessment of the included studies
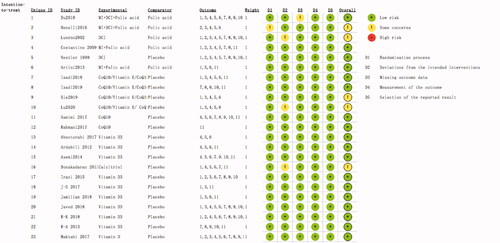
The results of quality assessment were shown in . The revised Cochrane Risk-of-bias Tool for RCTs (RoB 2.0) was used to assess the quality of 23 included RCTs from five domains. The summary of results are as follows: 22 (95.6%) RCTs with low risk of bias arising from the randomization process, 20 (86.9%) RCTs with low risk of bias due to deviations from intended interventions and 22 (95.6%) RCTs with low risk of bias due to missing outcome data; all of the including RCTs had low risk of bias in measurement of the outcome and selection of the reported results. The overall risk of bias in five studies [Citation24,Citation27,Citation32,Citation33,Citation37] were categorized as some concerns, other included studies were categorized as low risk, none of the study was found to be high risk.
Outcomes
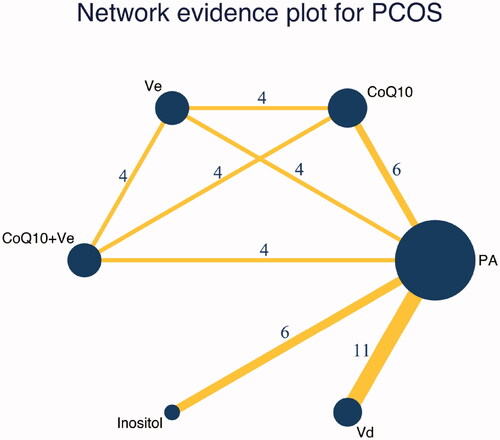
Transitivity assumption was conducted by us before data synthesizing, after confirming there was no violations of transitivity assumption, the consistency was examined. Among the included studies, six interventions were involved: CoQ10, vitamin E, CoQ10 + vitamin E, inositol, vitamin D and PA. A pairwise meta-analysis and a network meta-analysis were performed, and a network evidence graph was illustrated in , which shows the direct and indirect associations between the six interventions investigated. A league table is presented to summarize all pairwise comparisons () while the summary of the traditional meta-analysis statistical results is shown in Supplementary Table S2. The SUCRA was shown in of all relevant parameters.
Table 2. Results of network meta-analysis for PCOS.
Table 3. SUCRA values and ranks of efficacy outcomes.
Improvement of hyperandrogenemia
TT was measured in 13 studies [Citation25–29,Citation32,Citation33,Citation38–40,Citation42,Citation43,Citation45]. TMA showed that except for vitamin D, the following nutritional agents were significantly different compared with PA: CoQ10 (MD −0.55, 95%CI −0.68 to −0.43, p < .00001, I2 = 62),vitamin E (MD −0.61, 95%CI −0.70 to −0.53, p < .0001, I2 = 0), CoQ10 + vitamin E (MD −0.60, 95%CI −0.79 to −0.42, p < .00001, I2 = 74), inositols (MD −0.42, 95%CI −0.76 to −0.07, p = .02, I2 = 100). Similarly, NMA showed significant difference in CoQ10 (MD −0.56, 95%CrI −0.82 to −0.30), vitamin E (MD −0.61, 95%CrI −0.87 to −0.35), CoQ10 + vitamin E (MD −0.60, 95%CrI −0.87 to −0.33), inositols (MD −0.42, 95%CrI −0.63 to −0.20). SUCRA showed that vitamin E (SUCRA 81.0) may be the best intervention for lowering TT.
SHBG was measured in 12 studies [Citation23,Citation26–29,Citation31–33,Citation35–38,Citation41–43]. TMA showed that a significant difference was observed between PA and the following agents: vitamin E (MD 11.88, 95%CI 6.32 to 17.44, p < .00001, I2=0), CoQ10 + vitamin E (MD 10.53, 95%CI 2.47 to 18.59, p = .01, I2=0), and inositols (MD 19.78, 95%CI 10.01 to 29.55, p = .0003, I2=81). NMA revealed a significant difference was noted between PA and the following agents: vitamin E (MD 10.69, 95%CrI 0.80 to 20.58) and inositols (MD 18.42, 95%CrI 10.35 to 26.48). SUCRA showed that inositols (SUCRA 94.5) may be the best intervention for increasing SHBG.
Improvement of glucose and lipid metabolism
FBG was recorded in 15 studies [Citation23,Citation26–29,Citation31–33,Citation35–38,Citation41–43]. TMA revealed that only inositols were significantly differently compared with PA (MD −5.34, 95%CI −6.52 to −4.17, p < .0001, I2=0). Similarly, NMA also revealed that inositols were more effective than PA (MD −5.88, 95%CrI −10.01 to −1.75). In addition to inositols, CoQ10 might be more effective than PA (MD −5.06, 95%CrI −9.56 to −0.56) based on NMA. However, SUCRA showed that among the agents investigated, inositols (SUCRA 80.3) may be the best intervention for decreasing FBG.
FINS was reported in 17 studies [Citation23–29,Citation31–33,Citation35–38,Citation41–43]. TMA revealed that a significant difference was observed between PA and the following agents: CoQ10 (MD −2.31, 95%CI −4.09 to −0.53, p = .01, I2=56) and inositols (MD −5.34, 95%CI −6.62 to −4.05, p = .0001, I2=80). However, NMA revealed only inositols were effective than PA among these oral agents.
Fifteen studies mentioned the HOMA-IR [Citation23–25,Citation29,Citation31–33,Citation35–41,Citation43]. TMA showed that CoQ10 (MD −0.61, 95%CI −0.98 to −0.24, p = .001, I2=57), CoQ10 + vitamin E (MD −1.03, 95%CI −1.70 to −0.35, p = .003, I2=74), and inostiols (MD −1.65, 95%CI −2.82 to −0.48, p < .00001, I2=98) were significantly different compared with PA. Similarly, NMA revealed that CoQ10 (MD −0.62, 95%CrI −1.14 to −0.10), CoQ10 + vitamin E (MD −0.95, 95%CrI −1.55 to −0.36), and inostiols (MD −1.76, 95%CrI −2.35 to −1.17) were more effective than PA. SUCRA showed that inositols (SUCRA 99.0) may be the best intervention for decreasing HOMA-IR.
TG was reported in 12 studies [Citation23,Citation26–28,Citation30,Citation31,Citation38,Citation40–44].TMA revealed that a significant difference was observed between PA and the following agents: vitamin E (MD −6.91, 95%CI −12.10 to −1.72, p < .00001), CoQ10 + vitamin E (MD −16.85, 95%CI −21.48 to −12.22, p < .00001), and inostiols (MD −40.33, 95%CI −57.75 to −22.92, p = .001, I2=81). NMA revealed that a significant difference only between inositols (MD −40.27, 95%CrI −58.19 to −22.34) and PA. SUCRA showed that inositols (SUCRA 99.3) may be the best intervention for lowering TG.
TC was recorded in 12 studies [Citation23,Citation26–28,Citation30,Citation31,Citation38,Citation40–44]. TMA showed that significant difference was observed between inositols (MD −26.31, 95%CI −36.12 to −16.50, p < .00001, I2=81) and PA. Likewise, NMA showed a significant difference between inositols (MD −26.32, 95%CrI −36.15 to −16.49) and PA.
HDL-C was reported in 10 studies [Citation23,Citation28,Citation30,Citation31,Citation38,Citation40–44]. Based on both TMA and NMA, there was no significant difference between all agents and PA.
LDL-C was reported in 10 studies [Citation23,Citation28,Citation30,Citation31,Citation38,Citation40–44]. None of agents were significantly different compared with placebo based on TMA; however, inositols (MD −16.27, 95%CrI −31.09 to −1.44) were significantly different compared with PA based on NMA. SUCRA also showed that inositols (SUCRA 90.9) may be the best intervention for lowering LDL-C.
Improvement of body weight
BMI was reported in 14 studies [Citation25–29,Citation31,Citation34,Citation36,Citation38–41,Citation43–45]. TMA showed that a significant difference was observed between PA and inositols (MD 0.55, 95%CI 0.34 to 0.77, p < .00001, I2=62). Similarly, NMA revealed that only inositols (MD 0.54, 95%CrI 0.32 to 0.77) were significantly different compared with PA.
Discussion
To examine the impact of the five treatments (CoQ10, vitamin E, CoQ10 combined with vitamin E, vitamin D, inositols) on endocrine and metabolism profiles in women with PCOS, we conducted a NMA of 23 studies involving 1291 patients. Our findings indicated that the all interventions employed, except for vitamin D, showed a significantly higher efficacy than PA in terms of at least one of the following parameters: TT, SHBG, FBG, FINS, HOMA-IR, TG, TC, and LDL-C. CoQ10, vitamin E, CoQ10 combined with vitamin E and inositols seemed significantly more efficacious than PA in lowering TT; vitamin E might be the most successful agent among these treatments. Vitamin E and inositols were associated with positive effects on SHBG based on NMA and TMA, moreover, inositols was superior to vitamin E. CoQ10 and inositols appeared to be more successful in decreasing FBG than PA in NMA, inositols was found superior to CoQ10. CoQ10 and inositols were found to have positive effects on FINS compared with PA based on TMA, but only inositols was found to be effective based on NMA. CoQ10, CoQ10 combined with vitamin E, and inositols were found to have statistically significant advantages in decreasing HOMA-IR relative to PA based on both TMA and NMA; inositols were the most successful agent among these drugs. Only inositols were found to be statistically beneficial in decreasing TG, TC and LDL-C compared with PA based on TMA and NMA.
In the context of androgen-associated hormones, the results on TT lowering are compatible with those of Pundir’s [Citation13] but discordant with those of Zeng’s [Citation14], possibly owing to the large sample size in the analysis. However, considering the results on SHBG increase, this meta-analysis is the first one to show that vitamin E supplementation at the dose of 400 U/days for 8 weeks is helpful in improving TT and SHBG levels in PCOS. The accurate mechanism through which vitamin E intake might influence androgen-associated hormones in PCOS is still unknown. By contrast, inositols have been discovered to reduce androgen concentrations in PCOS by balancing the MI-to-DCI ratio [Citation46]. The ratio disturbances of MI and DCI in ovaries lead to an androgen-excess environment and insulin resistance, thereby inhibiting the hepatic production of SHBG and increasing FT, which might be a more reliable indicator of androgen activity than TT [Citation47,Citation48]. Unfortunately, studies on CoQ10 and vitamin E did not investigate the FT level, therefore, an in-depth discussion on FT was not possible in this analysis.
In the context of glucose metabolism, our findings showing the beneficial effects of inositols on FBG, FINS, and HOMA-IR, are consistent with those of Pundir’s and Liu’s [Citation13,Citation14]. However, this is the first meta-analysis to report that CoQ10, given for a minimum treatment period of 8 weeks, is beneficial in improving glucose metabolism in PCOS women. The underlying mechanism of the effect of CoQ10 on glucose metabolism remains unclear; however, it might be associated with the block of interleukin-1 beta (IL-1β), which allows glucose-stimulated insulin from pancreas islet cells [Citation49]. Based on this NMA, vitamin D was not significantly different compared to PA, contradicting Karolina’s and Vasilios’ TMA findings [Citation15,Citation16]. The result may be accounted for the expanded sample size and the differ inclusion criteria.
In the context of lipid metabolism, it is the first time to show that inositols are capable of reducing blood lipid levels, particularly TG, TC, and LDL-C. In the context of body weight, though we found that inositols can significantly improve BMI based on both TMA and NMA, since the study populations were nor differentiated in terms of lean and obese patients, significant difference could not be acquired by synthesizing data after removing two studies with patients of normal-weight [Citation26,Citation27].
There are multiple explanations for the source of high heterogeneity. First, differences in ethnicity, age and basic BMI of the study populations might contribute to the apparent heterogeneity. Second, the adaptable duration of the treatment, the difference between inositol isoforms administered and the difference between vitamin D and its active forms, such as vitamin D3 and calcitriol, the most appropriate dosages/ratio and the safety profile of agents still lack unified standards, the vitamin D and its other forms are absorbed by the human body at different rates might have also caused the heterogeneity. hence, more high-quality prospective cohort studies and randomized controlled trials are needed to obtain more convincing evidences for clinical medication. Despite the high heterogeneity, the funnel plots of study had marginal asymmetry or no publication bias.
Regarding to the included studies, several possible risks of bias and limitations need to be considered. First, although all the included studies are RCTs, the quality of each study was limited by the small sample size and the short duration of the study period, potentially affecting the outcome of NMA. Second, that the studies did not clearly describe blinding might have introduced bias to the included studies. Third, the process of selecting and extracting data was limited by the fact that not all outcomes of interest, such as reproductive parameters, were available.
From a practical point of view, the oral nutraceuticals investigated here may have therapeutic effects on PCOS, with no significant adverse effects. The potential application of these agents in the treatment of PCOS merits further exploration through comprehensive and in-depth clinical trials. Other traditional drugs such as metformin can also be investigated in comparison to the nutritional agents to provide further knowledge on the differences among these agents. Additionally, refining the selection of the study populations, for instance, by making a distinction between lean and obese patients is necessary. Moreover, the underlying mechanism for improving insulin resistance and hyperandrogenemia following nutraceuticals administration must be explored in the future.
Conclusion
For women with PCOS, inositols supplementation have some certain advantages in increasing SHBG and improving glycolipid metabolism when compared with nutraceuticals like CoQ10, vitamin E, vitamin D. Besides, vitamin E may be a better option in reducing TT and increasing SHBG. CoQ10 alone or combined with vitamin E can be helpful in decreasing HOMA-IR as well. These results may guide clinical physicians in choosing an appropriate therapeutic option among nutraceuticals according to patient’s individual differences. Further studies in larger-scale randomized trails, with a longer follow-up are warranted.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contributions
Zhang Jiaqi designed the research, collected data, analyzed data, and wrote the manuscript. Zhang Jiaqi and Xing Chuan screened and evaluated the literature. Zhao Han collected materials. He Bing reviewed and edited the manuscript. All the authors have read and approved the final manuscript.
Figure_S1.jpg
Download JPEG Image (26.5 KB)Table_S2.docx
Download MS Word (25.8 KB)Table_S1.docx
Download MS Word (20.3 KB)Acknowledgments
We gratefully acknowledge the statistical guidance and assistance of provided by Professor Wu Qijun from the department of Medical Statistics.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pradas I, Rovira-Llopis S, Naudí A, et al. Metformin induces lipid changes on sphingolipid species and oxidized lipids in polycystic ovary syndrome women. Sci Rep. 2019;9(1):16033.
- Manzoor S, Ganie MA, Amin S, et al. Oral contraceptive use increases risk of inflammatory and coagulatory disorders in women with Polycystic Ovarian Syndrome: an observational study. Sci Rep. 2019;9(1):10182.
- Christianson MS, Wu H, Zhao Y, et al. Metformin use in patients undergoing in vitro fertilization treatment: results of a worldwide web-based survey. J Assist Reprod Genet. 2015;32(3):401–406.
- Glintborg D, Altinok ML, Ravn P, et al. Adrenal activity and metabolic risk during randomized escitalopram or placebo treatment in PCOS. Endocr Connect. 2018;7(3):479–489.
- Kokosar M, Benrick A, Perfilyev A, et al. A single bout of electroacupuncture remodels epigenetic and transcriptional changes in adipose tissue in polycystic ovary syndrome. Sci Rep. 2018;8(1):1878.
- Haidari F, Banaei-Jahromi N, Zakerkish M, et al. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr J. 2020;19(1):8.
- Banshoya K, Nakamura T, Tanaka T, et al. Coenzyme Q10-polyethylene glycol monostearate nanoparticles: an injectable water-soluble formulation. Antioxidants (Basel, Switzerland). 2020;9(1):86.
- Farboud ES, Nasrollahi SA, Tabbakhi Z. Novel formulation and evaluation of a Q10-loaded solid lipid nanoparticle cream: in vitro and in vivo studies. Int J Nanomed. 2011;6:611–617.
- Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130(Pt 8):2037–2044.
- Wong SK, Chin K-Y, Suhaimi FH, et al. Vitamin E as a potential interventional treatment for metabolic syndrome: evidence from animal and human studies. Front Pharmacol. 2017;8:444.
- Ostadmohammadi V, Jamilian M, Bahmani F, et al. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J Ovarian Res. 2019;12(1):5.
- Laganà AS, Garzon S, Casarin J, et al. Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab. 2018;29(11):768–780.
- Pundir J, Psaroudakis D, Savnur P, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG. 2018;125(3):299–308.
- Zeng L, Yang K. Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;59(1):30–38.
- Pergialiotis V, Karampetsou N, Panagopoulos P, et al. The effect of Vitamin D supplementation on hormonal and glycaemic profile of patients with PCOS: a meta-analysis of randomised trials. Int J Clin Pract. 2017;71(6):e12957.
- Łagowska K, Bajerska J, Jamka M. The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(11):1637.
- Azadi-Yazdi M, Nadjarzadeh A, Khosravi-Boroujeni H, et al. The effect of vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2017;49(3):174–179.
- Zawadri JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine F, editors. Polycystic ovary syndrome: current issues in endocrinology and metabolism. Boston (MA): Black-well Scientific; 1992.
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488.
- Higgins J, Sterne J, Savović J, et al. A revised tool for assessing risk of bias in randomized trails. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Database of Systematic Reviews. 2016;10(Suppl 1):29–31.
- Zhang L, Zhang M, Zhang Y, et al. Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review. Sci Rep. 2016;6:18904.
- Du J, Wu R, Lin X, et al. Clinical effect of myo-inositol combined with D-chiro-inositol on insulin resistant polycystic ovary syndrome. China Med. 2018;13(9):1389–1393.
- Benelli E, Del Ghianda S, Di Cosmo C, et al. A combined therapy with myo-inositol and D-chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int J Endocrinol. 2016;2016:3204083.
- Artini PG, Di Berardino OM, Papini F, et al. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol Endocrinol off J Int Soc Gynecol Endocrinol. 2013;29(4):375–379.
- Costantino D, Minozzi G, Minozzi E, et al. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: a double-blind trial. Eur Rev Med Pharmacol Sci. 2009;13(2):105–110.
- Iuorno MJ, Jakubowicz DJ, Baillargeon J-P, et al. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8(6):417–423.
- Nestler JE, Jakubowicz DJ, Reamer P, et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340(17):1314–1320.
- Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(2):319–327.
- Izadi A, Shirazi S, Taghizadeh S, et al. Independent and additive effects of coenzyme Q10 and vitamin E on cardiometabolic outcomes and visceral adiposity in women with polycystic ovary syndrome. Arch Med Res. 2019;50(2):1–10.
- Samimi M, Zarezade Mehrizi M, Foroozanfard F, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2017;86(4):560–566.
- Ying XC. Effect of coenzyme Q10 combined with vitamin E on glucose metabolism indexes and levels of sex hormones in patients with polycystic ovary syndrome. Matern Child Heal Care China. 2019;34(20):4747–4750.
- Tao LU, Jianmin H, Juan Q, et al. Effect of coenzyme Q10 combined with vitamin E on metabolism and hormone in patients with polycystic ovary syndrome. China Med Eng. 2020;28(01):16–20.
- Rahmani E, Jamilian M, Samimi M, et al. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol Endocrinol off J Int Soc Gynecol Endocrinol. 2018;34(3):217–222.
- Seyyed Abootorabi M, Ayremlou P, Behroozi-Lak T, et al. The effect of vitamin D supplementation on insulin resistance, visceral fat and adiponectin in vitamin D deficient women with polycystic ovary syndrome: a randomized placebo-controlled trial. Gynecol Endocrinol off J Int Soc Gynecol Endocrinol. 2018;34(6):489–494.
- Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32(3):195–201.
- Bonakdaran S, Mazloom Khorasani Z, Davachi B, et al. J. The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: a randomized placebo- controlled clinical trial. Iran J Reprod Med. 2012;10(5):465–472.
- Raja-Khan N, Shah J, Stetter CM, et al. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril. 2014;101(6):1740–1746.
- Jamilian M, Foroozanfard F, Rahmani E, et al. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients. 2017;9(12):1280.
- Irani M, Seifer DB, Grazi RV, et al. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2015;100(11):4307–4314.
- Asemi Z, Foroozanfard F, Hashemi T, et al. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015;34(4):586–592.
- Maktabi M, Chamani M, Asemi Z. The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res. 2017;49(07):493–498.
- Javed Z, Papageorgiou M, Deshmukh H, et al. A randomized, controlled trial of vitamin D supplementation on cardiovascular risk factors, hormones, and liver markers in women with polycystic ovary syndrome. Nutrients. 2019;11(1):188.
- Rahimi-Ardabili H, Pourghassem Gargari B, Farzadi L. Effects of vitamin D on cardiovascular disease risk factors in polycystic ovary syndrome women with vitamin D deficiency. J Endocrinol Invest. 2013;36(1):28–32.
- Jafari-Sfidvajani S, Ahangari R, Hozoori M, et al. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Endocrinol Invest. 2018;41(5):597–607.
- Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61(2):111–117.
- Unfer V, Carlomagno G, Papaleo E, et al. Hyperinsulinemia alters myoinositol to d-chiroinositol ratio in the follicular fluid of patients with PCOS. Reprod Sci. 2014;21(7):854–858.
- Wojciechowska A, Osowski A, Jóźwik M, et al. Inositols’ importance in the improvement of the endocrine-metabolic profile in PCOS. IJMS. 2019;20(22):5787.
- Schroeder MM, Belloto RJJ, Hudson RA, et al. Effects of antioxidants coenzyme Q10 and lipoic acid on interleukin-1 beta-mediated inhibition of glucose-stimulated insulin release from cultured mouse pancreatic islets. Immunopharmacol Immunotoxicol. 2005;27(1):109–122.