Abstract
Objective: The effect of antioxidant supplements on glucose metabolism and lipid profiles in polycystic ovary syndrome (PCOS) remains controversial. This systematic review and meta-analysis aimed to evaluate whether antioxidant supplements improve glucose metabolism and lipid profiles in women with PCOS to provide optimal nutritional supplement advice in clinical practice. Methods: The search was conducted across multiple medical databases from inception to January 1, 2022 and performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A random effects model was used to calculate the overall effects. Results: Eighteen trials (1113 participants) were included. Antioxidant supplements significantly improved insulin resistance (95% CI, −0.62, −0.30; p < 0.00001; I2 =48%), fasting insulin (95% CI, −0.80, −0.44; p < 0.00001; I2 = 48%), and fasting plasma glucose (95% CI, −0.54, −0.21; p < 0.00001; I2 = 38%) in patients with PCOS. However, antioxidant supplements were found to not improve most indices of lipid profiles in PCOS except triglyceride. Conclusions: Antioxidant supplements are an effective intervention for relieving insulin resistance but do not significantly improve lipid metabolism in women with PCOS.
摘要
目的
抗氧化剂补充剂对多囊卵巢综合征(PCOS)患者糖代谢和血脂的影响仍存在争议。这项系统综述和荟萃分析旨在评估抗氧化剂补充剂是否能改善PCOS患者的糖代谢和血脂状况, 以便在临床实践中提供最佳的营养补充建议。
方法
检索多个医学数据库从创建到2022年1月1日的文献, 并遵循系统综述及Meta分析首选项目报告条目(PRISMA)指南。采用随机效应模型计算总体效应。
结果
共纳入18项试验(1113名参与者)。抗氧化剂可显著改善PCOS患者的胰岛素抵抗(95%CI, −0.62, −0.30;p<0.00001;I2=48%)、空腹胰岛素(95%CI, −0.80, −0.44;p<0.00001;I2=48%)和空腹血糖(95%CI, −0.54, −0.21;p<0.00001;I2=38%)。然而, 抗氧化剂补充剂并不能改善PCOS患者除甘油三酯外的大多数血脂指标。
结论
抗氧化剂补充剂是缓解胰岛素抵抗的有效干预措施, 但不能显著改善PCOS患者的脂代谢。
Introduction
Polycystic ovary syndrome (PCOS) is the most common female reproductive endocrine disorder with a prevalence ranging from 4% to 21% among women of reproductive age [Citation1]. The pathophysiology of PCOS is complex, and current evidence indicates that hyperinsulinemia and insulin resistance play key roles in the etiology, affecting 44%–70% of patients [Citation2]. As a result, PCOS has broad health implications, including abdominal adiposity, insulin resistance, obesity, and cardiovascular risk factors. These are all associated with the risk of long-term metabolic sequelae in patients with PCOS, including cardiovascular disease and diabetes [Citation3,Citation4].
Oxidative stress (OS) refers to the imbalance of the redox system with a disequilibrium between free radicals and antioxidants [Citation5]. A certain amount of reactive oxygen species (ROS) is required for the progression of normal cell functions; however, when free radicals are produced in excess, they can cause an imbalance of the redox system in the body. This may result in pathological states [Citation6], such insulin resistance and lipid metabolic disorders [Citation7,Citation8]. Currently, PCOS is considered an oxidative state associated with decreased antioxidant concentrations [Citation4]. Under common insulin resistance and dyslipidemia, PCOS can cause endoplasmic reticulum stress and lipid peroxidation, which leads to excess ROS [Citation9]. Oxidative stress influences the activation of redox‑sensitive transcription factors; lipid peroxidation may aggravate the pathological symptoms of PCOS and damage glucose and lipid metabolism [Citation10].
Antioxidants are biological and chemical compounds that play a crucial role in both food systems and the human body to reduce oxidative damage [Citation11]. They are a group of organic nutrients that include vitamins, minerals, polyphenols, and polyunsaturated fatty acids [Citation12]. Antioxidants can be classified as enzymatic or non-enzymatic, and most dietary supplements are classified as non-enzymatic antioxidants [Citation13]. Non-enzymatic antioxidants include minerals containing selenium and zinc and phenolic compounds such as vitamin E, curcumin, quercetin, and resveratrol [Citation14], which are ubiquitous in the diet, especially in vegetables and fruits. In addition, antioxidant supplements include those which cannot be classified, such as Omega-3 fatty acids which are known to prevent heart disease and improve diabetic complications, and endogenous antioxidants such as coenzyme Q-10 and melatonin which are used as supplements to prevent or treat specific chronic and degenerative diseases [Citation15].
Recently, numerous studies have evaluated the efficacy of antioxidants in PCOS; however, the results have been inconsistent [Citation16]. Some studies have shown that antioxidant coenzyme Q10, with or without vitamin E supplementation, can improve serum fasting plasma glucose (FPG) and insulin levels among women with PCOS [Citation17] and zinc as an antioxidative mineral had beneficial impacts on biomarkers of oxidative stress in PCOS [Citation18]. However, resveratrol, a plant-derived antioxidant, was found to have no effect on insulin and lipid levels in women [Citation19]. Overall, the complications of antioxidant supplements on glucose and lipid metabolism in PCOS have not been clearly described. This meta-analysis aimed to integrate the evidence regarding the effectiveness of antioxidant supplements on glucose metabolism and lipid profiles in PCOS.
Materials and methods
This meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [Citation20] and has been registered in the international prospective register of systematic reviews (PROSPERO) under the number CRD42022297265.
Data sources and searches
Databases such as the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Web of Science, EMBASE, Wanfang Data, and China National Knowledge Infrastructure (CNKI) were searched from inception to January 1, 2022. We manually checked the conference proceedings and reference lists to obtain additional relevant data. Titles and abstracts of all potential studies were scanned to eliminate duplicate and ineligible studies [Citation21]. When the information was insufficient to make a decision, we sought further details from the original author. Any discrepancies were resolved by discussion or consensus with the corresponding author [Citation21]. The details of the search strategy in PubMed were as follows: ‘Polycystic Ovary Syndrome’ OR ‘Ovary Syndrome, Polycystic’ OR ‘Syndrome, Polycystic Ovary’ OR ‘Stein-Leventhal Syndrome’ OR ‘Stein Leventhal Syndrome’ OR ‘Syndrome, Stein-Leventhal’ OR ‘Sclerocystic Ovarian Degeneration’ OR ‘Ovarian Degeneration, Sclerocystic’ OR ‘Sclerocystic Ovary Syndrome’ OR ‘Polycystic Ovarian Syndrome’ OR ‘Ovarian Syndrome, Polycystic’ OR ‘Sclerocystic Ovaries’ OR ‘Ovary, Sclerocystic’ OR ‘Sclerocystic Ovary’ AND ‘Antioxidants’ OR ‘Anti-Oxidants’ OR ‘Anti Oxidants’ OR ‘Antioxidant’ OR ‘Anti-Oxidant’ OR ‘Anti Oxidant’ OR ‘Endogenous Antioxidants’ OR ‘Antioxidants, Endogenous’ OR ‘Endogenous Antioxidant’ OR ‘Antioxidant, Endogenous’ OR ‘Antioxidant Activity’ OR ‘Activity, Antioxidant’ OR ‘Antioxidant Effect’ OR ‘Anti-Oxidant Effect’ OR ‘Anti Oxidant Effect’ OR ‘Anti-Oxidant Effects’ OR ‘Anti Oxidant Effects’ OR ‘Antioxidant Effects’ AND ‘randomis(z)ed control trial’, ‘clinical trial’ and ‘RCT’.
Selection criteria
Two review authors performed the study selection independently, following the eligibility and exclusion criteria described previously. Studies that met the following inclusion criteria were used: (1) parallel randomized controlled trial (RCT); (2) evaluation of the effects of antioxidant supplements on PCOS, intervention including an antioxidant or a combination of antioxidants, but not an antioxidant combined with a non-antioxidant; (3) included patients diagnosed with PCOS according to the Rotterdam criteria; (4) medication as a co-intervention in both intervention/control arms; (5) outcomes referred to the following aspects: homeostasis model of insulin resistance (HOMA-IR), fasting insulin (FINS), FPG, triglyceride (TG), total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), sex hormone binding globulin (SHBG) and Testosterone(T); and (6) original article in English or Chinese.
The exclusion criteria were as follows: (1) cohort or case-control studies, quasi-randomized trials, case reports, meta-analyses, reviews, animal or cell experiments; (2) women with other causes of hyperandrogenism and abnormal ovulation, taking any medication with consequent metabolic or hormonal changes, or any serious medical, psychiatric, or neurological problems; (3) studies with unreported target outcomes or unavailable data; and (4) studies not published in English or Chinese.
Assessment of risk bias in included studies
The study characteristics, risk of bias, and outcome data were manually extracted independently by two authors (R-Y Wang and Y Zhao) and entered into data extraction forms adapted from the Cochrane Collaboration for systematic critical appraisal [Citation21]. Differences in opinion were resolved by discussion and consensus with a third reviewer (Y. Chen).
Statistical analysis
The analysis was conducted using Review Manager (RevMan) 5.4. To standardize the numerical values from all RCTs, the mean and standard deviations were calculated when feasible. Random-effect models were used as statistical methods of analysis to obtain estimates of standardized mean differences (SMD) or mean differences (MD) and 95% confidence intervals (CIs). p < 0.05 was considered statistically significant.
Heterogeneity between studies was investigated using the I2 statistic (I2 > 50% was considered indicative of heterogeneity), the P value from the χ2 test and visual inspection of the forest plots. For significant heterogeneity, subgroup and sensitivity analyses were used to identify the source of heterogeneity. If the distributions were skewed and the results were reported as the median and range with non-parametric tests of significance, the results were excluded from the meta-analysis.
Results
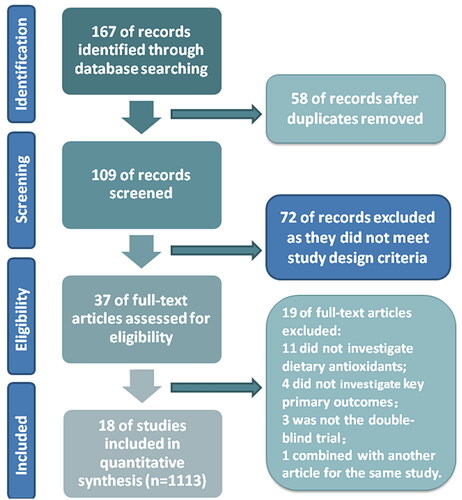
In total, 167 studies were identified in the preliminary search, and 109 records were evaluated by screening titles and abstracts after removing 58 duplicate studies. Seventy-two items were excluded for obvious ineligibility, such as meta-analyses, reviews, case reports, and animal or cellular experiments. Among them, 37 articles were selected for full-text revision, and 19 of these were excluded: 11 studies did not investigate dietary antioxidants, 4 studies did not investigate key primary outcomes, 3 studies were not double-blind trials, and 1 article was combined with another article for the same study. Finally, 18 RCTs (1113 participants) were eligible for meta-analysis. Details of the selection process are shown in the PRISMA flow diagram ().
Figure 1. PRISMA flow diagram of studies included in the meta-analysis.
Abbreviations: n = Number of total participants in all randomized controlled trials; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Included studies
Design
The general characteristics of the included studies are summarized in . The sample sizes ranged from 30 [Citation22] to 128 [Citation17,Citation23]. All studies were parallel-design and single-center RCTs conducted at university hospitals or clinical research centers in the USA [Citation24], Iran [Citation17,Citation19,Citation23,Citation25–38], and Poland [Citation22] between 2012 and 2021.
Table 1. Characteristics of trials included in the meta-analysis.
Participants
A total of 1113 women with a definite diagnosis of PCOS were included in the analysis. Five trials assessed overweight and obese participants (BMI≥ 25 kg/m2) [Citation17,Citation23,Citation31,Citation33,Citation35,Citation38], whereas the other 12 did not consider obesity as an inclusion criterion [Citation19,Citation22,Citation24–26,Citation28–30,Citation32,Citation34,Citation36,Citation37]. One trial included participants who were ‘matched one-by-one according to BMI (<25 and ≥25 kg/m2), age (<30 and ≥ 30 y), and phenotypes A and D of PCOS and then randomly divided into two groups’ [Citation27]
Interventions
Nine antioxidant supplements were investigated (). The treatment durations ranged from 6 weeks [Citation38] to 12 weeks, with most treatment durations (17 studies) consisting of 8–12 weeks (). Omega-3 fatty acids plus vitamin E and Omega-3 fatty acids alone were investigated in five studies. Curcumin, melatonin, quercetin, resveratrol, coenzyme Q10, and selenium were each involved in two studies. One study investigated vitamin E and one study investigated mineral zinc. Four studies used oral metformin as the primary treatment for all patients [Citation17,Citation23,Citation32,Citation34,Citation36].
Outcomes
Eleven trials reported on the primary outcomes, glycemic metabolic index (HOMA-IR) and lipid metabolism index (TG, TC) [Citation17,Citation19,Citation23,Citation25,Citation26,Citation28–30,Citation32,Citation36–38], and further included the secondary outcomes glycemic metabolic index (FINS, FPG) and lipid metabolism index (HDL-C, LDL-C). Four trials only reported the glycemic metabolic index [Citation27,Citation31,Citation33,Citation35] and two trials only reported the lipid metabolic index [Citation24,Citation34].
Risk of bias assessment
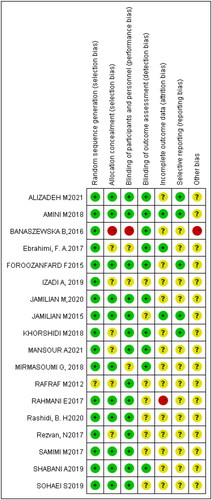
All 18 studies were assessed as having a risk of bias (). Seventeen studies reported randomization methods in detail [Citation17,Citation22,Citation23,Citation26–28,Citation30–32,Citation34–38] with seven explaining allocation concealment [Citation24,Citation26,Citation28,Citation30,Citation32,Citation37,Citation38]. Fifteen trials performed blinding using objective figures calculated by machines [Citation17,Citation23–32,Citation34–38] and therefore, had outcomes less prone to bias. Fifteen studies did not report the outcomes of attrited patients [Citation17,Citation19,Citation22–25,Citation28,Citation29,Citation31–38]. Fifteen trials that mentioned trial registration [Citation17,Citation19,Citation22,Citation23,Citation25,Citation26,Citation28–31,Citation33–38] were considered to have a low risk of reporting bias ().
Effects of interventions
Eighteen studies with 1113 participants compared antioxidant supplements with placebo or standard treatments (metformin) [Citation17,Citation19,Citation22–38]. A random-effects model was used for statistical analysis due to clinical heterogeneity. Subgroup analysis was performed based on the type of antioxidant.
Primary outcomes
HOMA-IR
Fifteen trials (949 participants) reported the effects of antioxidant treatment on HOMA-IR [Citation17,Citation19,Citation23,Citation25–33,Citation35–38]. The pooled data indicated a significant decrease in HOMA-IR in participants receiving antioxidant interventions (MD = −0.46; 95% CI, −0.62, −0.30; p < 0.00001; I2 =48%) (). Subgroup analysis showed that antioxidant minerals provided more benefits compared with phenolic compound antioxidants and combination antioxidants ().
Figure 3. Meta-analysis antioxidant versus placebo for HOMA-IR in women with PCOS.
HOMA-IR = Homeostasis model of insulin resistance; PCOS = Polycystic ovary syndrome
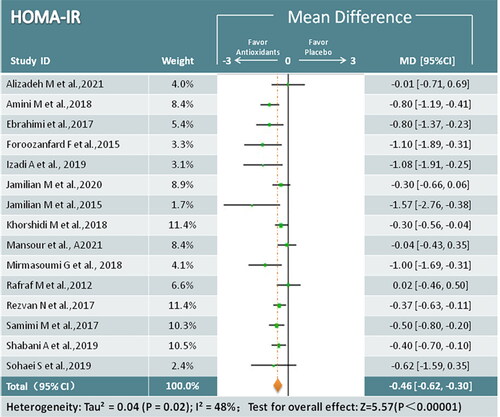
Table 2. Effect estimates and heterogeneity of subgroup analysis for outcomes.
TG
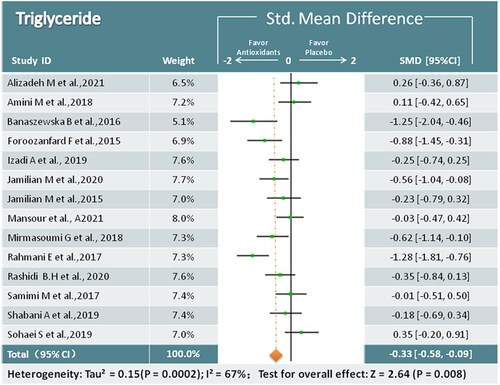
In total, 14 studies (824 participants) mentioned changes in TG [Citation17,Citation19,Citation22–26,Citation28–30,Citation32,Citation34,Citation36–38], and antioxidant treatment was found to reduce TG levels (SMD = −0.33; 95% CI, −0.58, −0.09; p = 0.008; I2 = 67%) (). Based on the subgroup analysis, a significant reduction in TG levels was observed for antioxidant mineral treatment ().
TC
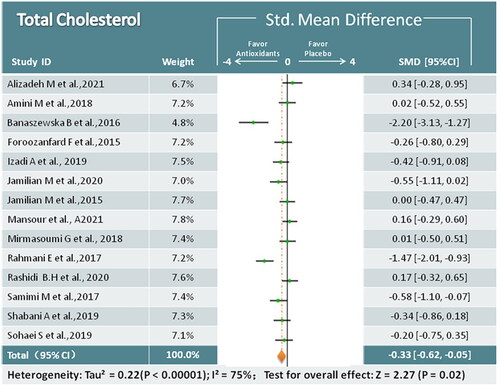
Analysis of 14 studies (824 participants) indicated that antioxidant interventions had reduced TC (SMD = −0.33; 95% CI, −0.62, −0.05; p = 0.02; I2 = 75%) [Citation17,Citation19,Citation22–26,Citation28–30,Citation32,Citation34,Citation36–38] (). In subgroup analysis, coenzyme Q10 was significantly associated with decreased TC, whereas other antioxidants were not significantly different from placebo.
Secondary outcomes
FINS
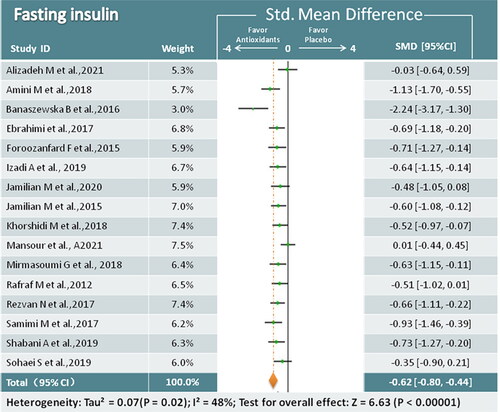
Sixteen trials (979 participants) analyzing antioxidant treatment and FINS were identified [Citation17,Citation19,Citation22,Citation23,Citation25–33,Citation35–38]. Overall analysis revealed that antioxidant supplements were superior in reducing FINS compared to placebo (SMD = −0.62; 95% CI, −0.80, −0.44; p < 0.00001; I2 = 48%) (). In subgroup analysis, we found that almost every type of antioxidant supplementation, except melatonin, was associated with a decrease in FINS ().
FPG
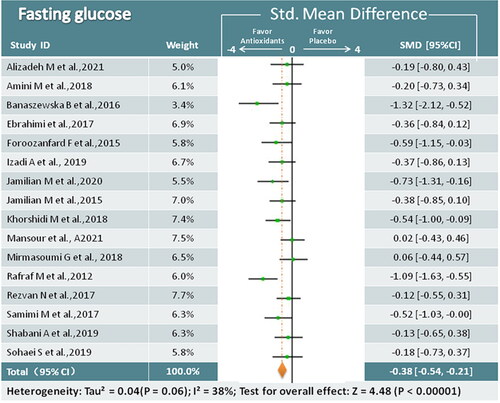
Sixteen trials (979 participants) examined the relationship between antioxidant supplements and FPG [Citation17,Citation19,Citation22,Citation23,Citation25–33,Citation35–38]. Meta-analysis showed that antioxidant interventions led to a greater decrease than placebo (SMD = −0.38; 95% CI, −0.54, −0.21; p < 0.00001; I2 = 38%) (). The results of the subgroup analysis revealed that the phenolic compound antioxidants and antioxidant minerals could significantly affect FPG, whereas the effects of the other antioxidant supplements were uncertain ().
HDL-C
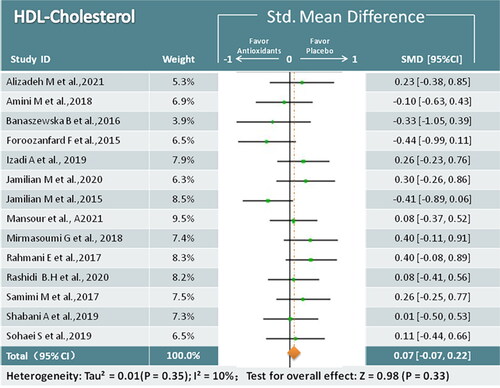
Fourteen studies (824 participants) reported on HDL-C after antioxidant treatment [Citation17,Citation19,Citation22–26,Citation28–30,Citation32,Citation34,Citation36–38]. Compared to the control groups, the antioxidant groups showed no significant improvement in HDL-C levels (SMD = 0.07; 95% CI, −0.07, 0.C22; p = 0.33; I2 = 10%) ().
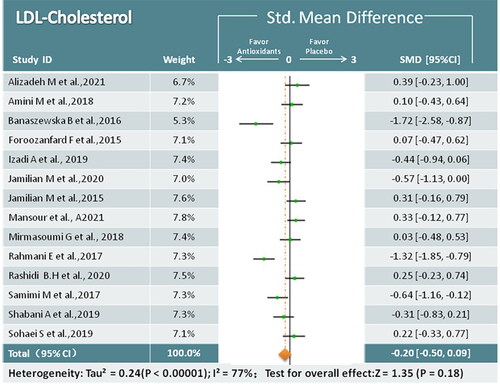
LDL-C
Fourteen studies mentioned LDL-C changes in 824 women assigned randomly to antioxidant treatment (n = 48) and placebo (n = 49) groups. Based on the pooled data, no significant reduction in LDL-C was observed in the antioxidant group (SMD = −0.20; 95% CI, −0.50, 0.09; p = 0.18; I2 = 77%) (). In the subgroup analysis, coenzyme Q10 was found to be beneficial in reducing LDL-C.
Figure 9. Meta-analysis of antioxidant versus placebo for LDL-C in women with PCOS.
LDL-C = Low-density lipoprotein cholesterol
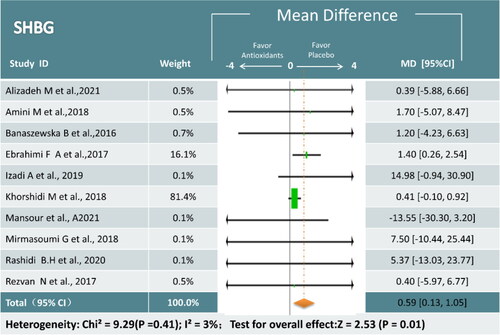
SHBG Ten trials (643 participants) examined the relationship between antioxidant supplements and SHBG [Citation17,Citation19,Citation22,Citation24–27,Citation31,Citation32,Citation35]. Meta-analysis showed that antioxidant interventions led to a greater increase than placebo (MD = 0.59; 95% CI,0.13, 1.05; p = 0.01; I2 = 3%) (). The results of the subgroup analysis revealed that the omega-3 and combined antioxidants could significantly affect SHBG, whereas the effects of the other antioxidant supplements were uncertain ().
Figure 10. Meta-analysis of antioxidant versus placebo for SHBG in women with PCOS.
SHBG = Sex hormone binding globulin
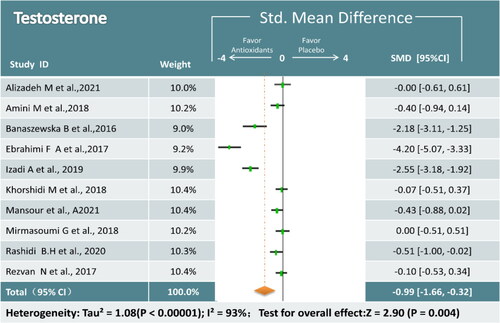
T Ten trials (643 participants) analyzing antioxidant treatment and T were identified [Citation17,Citation19,Citation22,Citation24–27,Citation31,Citation32,Citation35]. Overall analysis revealed that antioxidant supplements were superior in reducing T compared to placebo (SMD = −0.99; 95% CI, −1.66, −0.32; p = 0.004; I2 = 93%) (). Because of the large heterogeneity of the study, a random effects model was used and a subgroup analysis was conducted to analyze the sources of heterogeneity. In subgroup analysis, We found that the combination of antioxidant may be of heterogeneous origin, while we found that coenzyme Q10 significantly reduced T levels ().
Sensitivity analysis
Sensitivity analysis was performed to explain heterogeneity among the studies. We conducted a sensitivity analysis of the outcomes if we detected a high degree of heterogeneity (I2≥ 50%). When we excluded two trials [Citation22,Citation34] from sensitivity analysis, the heterogeneity decreased significantly(I2 < 50%), and the overall estimates remained unchanged, except for the outcome of TC (from p = 0.02 to p = 0.11), indicating that the majority of conclusions were stable and not affected by the quality of the trials included. However, the TC results should be interpreted with caution.
Discussion
Findings for metabolic and sex hormones alteration in PCOS by antioxidants
Metabolic alteration
This systematic review and meta-analysis included 18 randomized controlled trials of 1113 women with PCOS. Overall, antioxidant supplements were significantly related to decreased insulin resistance in patients with PCOS. Several studies [Citation39] have found that antioxidant supplementation can improve insulin sensitivity in patients with insulin resistance, and also improve glucose metabolism in patients with PCOS [Citation16], which are consistent with our findings. Oxidative stress may reduce insulin secretion from pancreatic β-cells by altering glucose uptake by muscle and adipose tissue, thereby inducing IR through intracellular signaling pathway [Citation40], antioxidants may improve glucose metabolism in the above way.
The findings of our meta-analysis showed that antioxidant supplements did not have a significant effect on the lipid profile of patients with PCOS, except for some effect on reducing the lipid index TG. This may differ from some of the results of previous studies [Citation41,Citation42]. Some studies have suggested that oxidative stress is closely related to lipid metabolism and that antioxidants may improve the hyperlipidemic status of patients with PCOS and reduce their cardiovascular disease risk factors [Citation43]. There are several possible explanations for this discrepancy in results. First, the study designs of the meta-analyses were different. The inclusion criteria for other trials were case-control and cohort studies, which may have led to different results [Citation42]. Second, since subgroup analysis is classified according to the type of antioxidant, and most of the previous studies were based on a particular antioxidant, this could cause bias in subgroup analysis results. Therefore, more in-depth and comprehensive studies are required to improve meta-analysis results.
Sex hormones alteration
Hyperandrogenemia is one of the main manifestations of polycystic ovary syndrome. Clinically, total testosterone, free testosterone, and sex hormone binding globulin(SHBG) are often used in the assessment of biochemical androgen excess in women [Citation44].SHBG is a glycoprotein produced by the liver involved in the transport of sex hormones in the blood and has an important role in regulating the circulating free levels of sex hormones [Citation45], it can be used to determine the severity of hyperandrogenism and to assess the efficacy of treatment [Citation46]. In addition, recent studies have concluded that low SHBG levels are strongly correlated with the degree of metabolic syndrome in PCOS patients and its considered to be a strong biomarker associated with the development of insulin resistance and NAFLD in PCOS patients [Citation45,Citation47].
In our study, we found that PCOS patients who received antioxidant interventions had higher SHBG levels with lower T and HOMA-IR levels. Antioxidants may improve insulin resistance in PCOS patients by improving their oxidative stress status, resulting in increased SHBG levels. Thereby increasing SHBG may play a role in insulin resistance by regulating the PI3K/AKT signaling pathway, while possibly reducing testosterone levels by binding to free androgens to alleviate hyperandrogenemia and IR [Citation46]. However, the mechanisms involved are still not fully defined and need to be explored subsequently.
Findings for role of different antioxidants
An association was found in the subgroup meta-analyses by type of supplement in some areas of glucose metabolism ().
Phenolic compounds
Phenolic compounds as natural antioxidants, which are known for their significant antioxidant role, have great structural diversity and variations in chemical composition among plant-derived substances. Polyphenol antioxidants belong to primary antioxidants with high antioxidant properties and usually need a small amount to neutralize a large number of free radicals [Citation48]. The most relevant groups of phenolic compounds are tocopherols, flavonoids, and phenolic acids [Citation14]. In our study, we mainly included vitamin E, curcumin, resveratrol and quercetin. Those phenolic compounds have significant effects on FINS, FPG and HOMA-IR in PCOS patients, which is similar to the results of a previous meta-analysis [Citation41,Citation49–51].
Antioxidant minerals
Antioxidant minerals are components of antioxidant enzymes that are important for the maintenance of enzyme activity [Citation52]. They have an antioxidant function by inhibiting the creation of new radicals, and oxidative stress levels in patients with PCOS may be improved in this way. Some similar studies have demonstrated that the antioxidant mineral selenium [Citation53] can improve glucose metabolism in PCOS, and some noted that zinc [Citation54] supplementation was beneficial for patients with PCOS as it resulted in a significant decrease in both insulin concentration and HOMA-IR index. The subgroup analysis of our study also found that mineral supplements with antioxidant properties improved glucose metabolism levels in PCOS patients and that mineral elements were superior to other types of antioxidant supplements for HOMA-IR according to the comparison of effect values.
Omega-3 fatty acid(omega-3)
Omega-3 fatty acids are recognized as one of the important polyunsaturated fatty acids, which cannot be produced inside the body and required to be consume through diets [Citation55]. It acts as an antioxidant by regulating antioxidant signaling pathways, several studies have found that omega-3 fatty acids can improve IR by activating the peroxisome proliferator-activated receptor alpha (PPARa).Activation of receptor alpha (PPARa) reduces inflammation and fat accumulation in insulin-sensitive tissues [Citation56]. The results of our subgroup analysis also found that omega-3 improved insulin resistance in pcos patients, with significant improvements in FINS, FPG, HOMA-IR and SHBG in PCOS patients.
Melatonin
Melatonin is a potent and inducible endogenous antioxidant and the cascade reaction of melatonin distinguishes it from other classic antioxidants [Citation57]. Melatonin may prevent type 2 diabetes and its concomitant oxyradical-mediated damage by combining spatiotemporal effects with its cytoprotective properties [Citation58]. In addition, some previous studies have found that melatonin can improves HOMA-IR in women with PCOS [Citation59], which has some similarity with the results of our study.
Coenzyme Q10 (CoQ10)
Coenzyme Q10 (CoQ10) is a fat-soluble vitamin commonly known as ubiquinone [Citation60]. CoQ10 serves as a powerful endogenous antioxidant and directly protects cellular components from free radicals and indirectly regenerates other antioxidants. It plays a critical role in scavenging free radicals and protein oxidation as well as in the production of cellular energy [Citation61]. Our study found that coenzyme q10 was superior to other antioxidants in improving glucose metabolism of PCOS patients. There are some evidence that CoQ10 is effective against several metabolic disorders associated with IR and diabetes [Citation62]. CoQ10 intake may improve insulin metabolism markers by modulating insulin and lipocalin receptors as well as tyrosine kinase (TK), phosphatidylinositol kinase (PI3K) and glucose transporters [Citation60]. CoQ10 was also found can improve insulin sensitivity by increasing the expression of the insulin-related pathway gene PPAR-γ [Citation63].
Limitations
Our study had several limitations. First, PCOS is a disease with a variety of different phenotypes. Our inclusion criteria were to study only RCTs with three diagnostic features of PCOS and did not classify PCOS by phenotype. Thus, the effects of antioxidant supplements on different phenotypes of PCOS could not be described. Future studies should focus on the relationship between particular phenotypes and different antioxidant interventions, so that these effects can be investigated accordingly. Second, the duration of most included trials lasted ≤ 12 weeks, we attempted to analyze this by using an intervention duration of 8 weeks as the node of analysis, but did not find a difference in effect size between subgroup analyses with intervention duration less than or equal to 8 weeks versus greater than 8 weeks (). None of studies mentioned the side effects of antioxidant supplements, so studies with longer follow-up periods would help to comprehensively elucidate the long-term effects and side effects of antioxidant supplements. Finally, we assessed the methodological quality of individual trials using only the data shown in each article. Therefore, we may not have assessed actual performance or biases in individual trials. In the future, more well-designed studies are required to confirm the effect of antioxidant supplements on PCOS to better inform clinicians’ and patients’ treatment decisions.
Table 3. Subgroup analysis of FINS、FPG、HOMG-IR with different intervention times.
Conclusion
Based on this review, our results suggest that antioxidant supplements improve glucose metabolism in women with PCOS, while having a beneficial effect on improving hyperandrogenemia-related indicators. However, there is no high-quality evidence to support the antioxidant supplement effectiveness in improving lipid profiles in PCOS. The effects of antioxidant treatment were associated with the type of antioxidant according to subgroup analysis, and the antioxidant phenolic compounds and minerals both had a great influence on glucose metabolism in women with PCOS. However, due to the limited number of studies and small sample size, the results should be interpreted with caution. More RCTs with rigorous designs and large samples are needed to confirm the evidence and to further explore the safety and influence of antioxidant supplements in women with PCOS.
Supplemental Material
Download MS Word (298.2 KB)Acknowledgements, avoiding identifying any of the authors prior to peer review 1. This is a note. The style name is Footnotes, but it can also be applied to endnotes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15.
- Park HR, Choi Y, Lee HJ, et al. Phenotypic characteristics according to insulin sensitivity in non-obese korean women with polycystic ovary syndrome. Diabetes Res Clin Pract. 2007;77(3): S233–S237.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284.
- Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62.
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–1650.
- Zhang PY, Xu X, Li XC. Cardiovascular diseases: oxidative damage and antioxidant protection. Eur Rev Med Pharmacol Sci. 2014;18(20):3091–3096.
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761.
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993–999.
- Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10:86.
- Göçer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-related compounds. Arch Pharm (Weinheim). 2013;346(11):783–792.
- Mut-Salud N, Alvarez PJ, Garrido JM, et al. Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid Med Cell Longev. 2016;2016:6719534.
- Neha K, Haider MR, Pathak A, et al. Medicinal prospects of antioxidants: a review. Eur J Med Chem. 2019;178:687–704.
- Gulcin I. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715.
- Nitta H, Kinoyama M, Watanabe A, et al. Effects of nutritional supplementation with antioxidant vitamins and minerals and fish oil on antioxidant status and psychosocial stress in smokers: an open trial. Clin Exp Med. 2007;7(4):179–183.
- Hyderali BN, Mala K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;191:15–22.
- Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(2):319–327.
- Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, et al. The effects of magnesium and zinc Co-Supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Elem Res. 2018;184(2):300–307.
- Mansour A, Samadi M, Sanginabadi M, et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin Nutr. 2021;40(6):4106–4112.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Higgins J. Cochrane handbook for systematic reviews of interventions version 5.1. 0 (updated on 2011). The Cochrane Collaboration; 2015; p. 164–166. Available from: www.cochrane-Handbook.org.
- Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, et al. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322–4328.
- Izadi A, Shirazi S, Taghizadeh S, et al. Independent and additive effects of coenzyme Q10 and vitamin E on cardiometabolic outcomes and visceral adiposity in women with polycystic ovary syndrome. Arch Med Res. 2019;50(2):1–10.
- Rashidi BH, Mohammad Hosseinzadeh F, Alipoor E, et al. Effects of selenium supplementation on asymmetric dimethylarginine and cardiometabolic risk factors in patients with polycystic ovary syndrome. Biol Trace Elem Res. 2020;196(2):430–437.
- Alizadeh M, Karandish M, Asghari Jafarabadi M, et al. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Nutr Metab (Lond). 2021;18(1):57.
- Amini M, Bahmani F, Foroozanfard F, et al. The effects of fish oil omega-3 fatty acid supplementation on mental health parameters and metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Psychosom Obstet Gynaecol. 2018:1–9.
- Ebrahimi FA, Samimi M, Foroozanfard F, et al. The effects of omega-3 fatty acids and vitamin E Co-supplementation on indices of insulin resistance and hormonal parameters in patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2017;125(6):353–359.
- Foroozanfard F, Jamilian M, Jafari Z, et al. Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2015;123(4):215–220.
- Jamilian M, Foroozanfard F, Kavossian E, et al. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. 2020;36:128–133.
- Jamilian M, Razavi M, Fakhrie Kashan Z, et al. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2015;82(6):885–891.
- Khorshidi M, Moini A, Alipoor E, et al. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother Res. 2018;32(11):2282–2289.
- Mirmasoumi G, Fazilati M, Foroozanfard F, et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018;126(4):222–228.
- Rafraf M, Mohammadi E, Asghari-Jafarabadi M, et al. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr. 2012;31(5):361–368.
- Rahmani E, Samimi M, Ebrahimi FA, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol Cell Endocrinol. 2017;439:247–255.
- Rezvan N, Moini A, Janani L, et al. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: a randomized placebo-controlled double-blind clinical trial. Horm Metab Res. 2017;49(2):115–121.
- Samimi M, Zarezade Mehrizi M, Foroozanfard F, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2017;86(4):560–566.
- Shabani A, Foroozanfard F, Kavossian E, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250:51–56.
- Sohaei S, Amani R, Tarrahi MJ, et al. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201.
- Mancini A, Martorana GE, Magini M, et al. Oxidative stress and metabolic syndrome: effects of a natural antioxidants enriched diet on insulin resistance. Clin Nutr ESPEN. 2015;10(2):e52–e60.
- Mancini A, Bruno C, Vergani E, et al. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: controversies and new insights. Int J Mol Sci. 2021;22(4):1667.
- Chien YJ, Chang CY, Wu MY, et al. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with Meta-Analysis and trial sequential analysis. Nutrients. 2021;13(2):684.
- Yang K, Zeng L, Bao T, et al. Effectiveness of omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16(1):27.
- Gharaei R, Mahdavinezhad F, Samadian E, et al. Antioxidant supplementations ameliorate PCOS complications: a review of RCTs and insights into the underlying mechanisms. J Assist Reprod Genet. 2021;38(11):2817–2831.
- Stanczyk FZ. Diagnosis of hyperandrogenism: biochemical criteria. Best Pract Res Clin Endocrinol Metab. 2006;20(2):177–191.
- Di Stasi V, Maseroli E, Rastrelli G, et al. SHBG as a marker of NAFLD and metabolic impairments in women referred for oligomenorrhea and/or hirsutism and in women with sexual dysfunction. Front Endocrinol (Lausanne). 2021;12:641446.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142–148.
- Karakas SE. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin Chim Acta. 2017;471:248–253.
- Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem. 2020;44(9):e13394.
- Tefagh G, Payab M, Qorbani M, et al. Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in PCOS (polycystic ovary syndrome): a systematic review and meta-analysis. Sci Rep. 2022;12(1):5770.
- Abdelazeem B, Abbas KS, Shehata J, et al. The effects of curcumin as dietary supplement for patients with polycystic ovary syndrome: an updated systematic review and meta-analysis of randomized clinical trials. Phytother Res. 2022;36(1):22–32.
- Pourteymour Fard Tabrizi F, Hajizadeh-Sharafabad F, Vaezi M, et al. Quercetin and polycystic ovary syndrome, current evidence and future directions: a systematic review. J Ovarian Res. 2020;13(1):11.
- He L, He T, Farrar S, et al. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–553.
- Hu KL, Ye X, Wang S, et al. Melatonin application in assisted reproductive technology: a systematic review and Meta-Analysis of randomized trials. Front Endocrinol (Lausanne). 2020;11:160.
- Nasiadek M, Stragierowicz J, Klimczak M, et al. The role of zinc in selected female reproductive system disorders. Nutrients. 2020;12(8):2464.
- Kapoor B, Kapoor D, Gautam S, et al. Dietary polyunsaturated fatty acids (PUFAs): uses and potential health benefits. Curr Nutr Rep. 2021;10(3):232–242.
- Xia Y, Wang Y, Cui M, et al. Efficacy of omega-3 fatty acid supplementation on cardiovascular risk factors in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(6):6425–6437.
- Tan DX, Manchester LC, Esteban-Zubero E, et al. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20(10):18886–18906.
- Cardinali DP, Vigo DE. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol Life Sci. 2017;74(21):3941–3954.
- Li Y, Xu Z. Effects of melatonin supplementation on insulin levels and insulin resistance: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2021;53(9):616–624.
- Miele C, Amin MM, Asaad GF, et al. Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, tyrosine kinase, PI3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats. PloS One. 2014;9(2):e89169.
- Papucci L, Schiavone N, Witort E, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220–28228.
- McCarty MF. Can correction of Sub-optimal coenzyme Q status improve beta-cell function in type II diabetics? Med Hypotheses. 1999;52(5):397–400.
- Rahmani E, Jamilian M, Samimi M, et al. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(3):217–222.