Abstract
Objective: This systematic review and meta-analysis aimed at summarizing the evidence concerning circulating asprosin, and related endocrine and metabolites in women with and without the polycystic ovary syndrome (PCOS).Method: We performed a comprehensive literature search in Pubmed, Web of Science, Scielo, and Chinese National Knowledge Infrastructure for studies published until May 20, 2022, that evaluated circulating asprosin levels in women with and without PCOS, regardless of language. The quality of studies was assessed with the Newcastle-Ottawa Scale. Random-effects models were used to estimate mean differences (MD) or standardized MD (SMD) and their 95% confidence interval (CI).Results: We evaluated eight studies reporting 1,050 PCOS cases and 796 controls of reproductive age. Participants with PCOS were younger (MD = −2.40 years, 95% CI −2.46 to −2.33), with higher values of asprosin (SMD = 2.57, 95% CI 1.64–3.50), insulin (SMD = 2.73, 95% CI 1.18–4.28), homeostatic model assessment of insulin resistance (SMD = 2.70, 95% CI 0.85–4.55), luteinizing hormone (SMD = 2.33, 95% CI 0.60–4.06), total testosterone (SMD = 4.06, 95% CI 1.89–6.22), dehydroepiandrosterone sulfate (SMD = 2.38, 95% CI 0.37–4.40), and triglycerides (SMD = 1.20, 95% CI 0.13 to 2.27). Moreover, PCOS women had lower circulating levels of sex hormone-binding globulin (SMD = −3.36, 95% CI −4.92 to −1.80), and high-density lipoprotein-cholesterol (SMD = −0.85, 95% CI −1.69 to −0.01); with no significant differences observed for glucose, total cholesterol, and low-density lipoprotein-cholesterol levels.Conclusion: Circulating asprosin levels were significantly higher in women with PCOS as compared to those without the syndrome.
Introduction
Adipose tissue has been considered an inert energy storage organ that provides insulation from the cold. However, it is also an endocrine organ because it synthetizes adipokines or adipose-derived hormones. Asprosin (derived from the Greek word ‘aspros’ which means white) is one of the most recently identified fat hormones that stimulate the liver to release glucose to the circulatory system. It is a 140-amino-acid fragment from the C-terminal of profibrillin (encoded by the FBN1 gene) that stimulates appetite, insulin production, and the liver to release glucose to the circulatory system [Citation1–3]. However, it may also be secreted by the skin, the pancreas, and the submandibular and parotid salivary glands [Citation2, Citation4]. It is a centrally orexigenic hormone that crosses the blood barrier and activates specific orexigenic neurons [Citation5]. Asprosin blood levels are increased in children and adults with insulin resistance, type 2 diabetes mellitus (T2DM), and obesity [Citation3, Citation6].
Maylem et al. [Citation7] have described a possible role of asprosin in ovarian follicular function. They demonstrated that asprosin increases luteinizing hormone (LH) induced theca cell androstenedione production, and reduces insulin growth factor 1 (IGF-1) induced theca cell proliferation, suggesting that the new hormone may regulate ovarian follicle function [Citation8]. Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder during female reproductive years, including a high prevalence of impaired glucose tolerance, hyperandrogenism, and T2DM among those PCOS women with overweight or obesity [Citation9, Citation10]. Both mice and humans with obesity or insulin resistance display increased asprosin levels [Citation2]. However, asprosin results have been inconsistent in studies of PCOS women. Therefore, the purpose of this systematic review and meta-analysis was to study circulating asprosin levels in women with and without PCOS and to evaluate related metabolic and endocrine outcomes.
Methods
This systematic review and meta-analysis of observational studies focused on the evaluation of circulating asprosin levels in women with and without PCOS. We followed the principles of the PRISMA guidelines [Citation11]. The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42021299279). A formal institutional review board approval was not required, since this analysis consisted of the pooling of published studies.
Search strategy and eligibility criteria
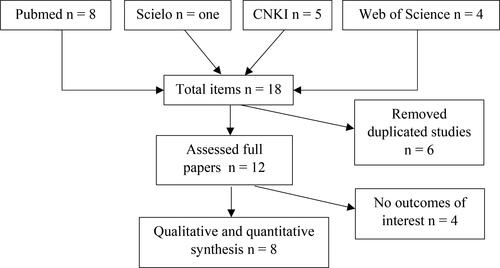
A literature search of electronic databases was performed on PubMed, Web of Science, Scielo, and Chinese National Knowledge Infrastructure to study women with and without PCOS (). We searched for free terms ‘polycystic ovary syndrome’ OR ‘PCOS’ AND ‘asprosin.’ The search strategy using Boolean operators AND or OR is shown in Table S1. The search included articles in any language published from 2016 until May 20, 2022, based on internationally established criteria, either the Androgen Excess and PCOS Society Recommendation [Citation12], the Rotterdam Criteria of the Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus [Citation13], or other validated protocols.
Table 1. Studies comparing circulating asprosin in women with and without polycystic ovary syndrome (PCOS): Authors, study location and period of study, aims of studies, number of participants, age, study design and exclusion criteria, and main findings.
Titles and abstracts of retrieved publications were screened for potentially eligible studies. Eligible for inclusion were relevant studies that: (i) assessed adolescents or women with and without PCOS; (ii) studies reporting information on circulating asprosin levels; and (iii) were in any language irrespective of age, race, and date of publication. References from selected articles were also screened, seeking additional potential publications not captured by the electronic database searches. Articles were excluded if they were narrative reviews, abstracts, conference proceedings, lacked results with validated methods or were non-human studies. All disagreements regarding inclusion/exclusion were discussed and solved by consensus with all authors. Meta-analyses were planned for circulating asprosin levels comparing women with and without PCOS. In addition, other clinical, endocrine, or metabolic parameters traditionally related to PCOS were studied as secondary outcomes if reported in at least three different publications.
Data extraction and risk of bias assessment
The general characteristics of selected articles were screened by study design and asprosin measurement methods. Discrepancies and controversies of extracted data were discussed to reach a consensus. When required the authors of the articles were contacted to obtain any additional clarification. A plot digitizer program was used to obtain numeric results from figures [Citation14] in articles not reporting exact numeric data. Appropriate calculations were also performed to obtain means and standard deviations that served the meta-analysis [Citation15]. Separate meta-analyses were planned for studies assessing endocrine and metabolic outcomes related to PCOS.
Two authors assessed the risk of bias in included studies following the Newcastle-Ottawa Scale (NOS) [Citation16]. Disagreements were solved by discussion between both sides and a third author. This tool evaluates 8 items of selection of exposure and non-exposure comparison, and an additional star can be assigned for comparability based on the analysis. Therefore, the evaluation may reach a maximum number of 9 stars. A total of 7 or more stars suggests that the study has a low risk of bias.
Statistical analysis
Forest plots were planned for outcomes reported in at least three studies using any validated test to assess clinical or laboratory results. Associations among dichotomous outcomes were planned to be expressed as odds ratios and for continuous outcomes mean differences (MDs) or standardized mean differences (SMDs), with their corresponding 95% confidence interval (CI). Because studies might have potential differences in phenotypes, baseline characteristics, lifestyle, and recruitment procedures, we followed the DerSimonian and Laird random-effects model [Citation17] and the inverse variance method. The Hedges’ g method was used to measure effect sizes, interpreting the magnitude of SMDs as small (0.20), moderate (0.50), or large (0.80) [Citation18, Citation19].
We evaluated statistical heterogeneity using the Chi2, the I2 statistic, and the between-study variance using the Tau2 [Citation14, Citation15]. I2 values of 30–65% indicate a moderate level of heterogeneity. A p < 0.1 for the Chi2 defined the presence of heterogeneity; and a Tau2 > 1 defined the presence of substantial statistical heterogeneity. One-study leave-out sensitivity analysis was performed to test the robustness of the overall asprosin result [Citation19]. We planned to estimate the publication bias if at least ten studies reported the same outcome [Citation20]. The Review Manager program (RevMan, version 5.3, Oxford, UK; The Cochrane Collaboration) was used for statistical analyses.
Results
Study characteristics and risk of bias assessment
Our searches yielded 18 potentially eligible articles (). After screening abstracts and deleting duplicates, twelve were assessed as full-text, and eight studies [Citation21–28] were included in the final qualitative and quantitative synthesis. Seven studies [Citation21–24, Citation26–28] followed the ESHRE/ASRM 2003 Rotterdam criteria [Citation13] and one study [Citation25] followed the Chinese Medical Association of Obstetrics and Gynecology Recommendations for PCOS diagnosis [Citation29].
Meta-analyzed studies were performed: four in China [Citation25–28], two in Turkey [Citation21, Citation24], one in Iraq [Citation22], and another in Taiwan [Citation23]. Sample size ranged from 30 [Citation24] to 444 PCOS patients [Citation23], and from 30 [Citation24] to 233 women [Citation28] without the syndrome. Six articles were published in English [Citation21–24, Citation26, Citation27] and two in the Chinese language [Citation25, Citation28]. Seven studies diagnosed PCOS using the Rotterdam Criteria [Citation21–24, Citation26–28] and one study [Citation25] followed the Guideline of PCOS from the Expert Group of Obstetrics and Gynecology of the Chinese Medical Association [Citation29]. Age ranges and other clinical characteristics are displayed in . All studies measured circulating asprosin levels by chemiluminescence assays.
Using the NOS scale, six studies were identified as high-quality [Citation21, Citation24–28], and the other two of moderate quality [Citation22, Citation23] (Table S2). All publications identified the study population, patients were representative of average PCOS cases, and controls were derived from the same population as cases. In all studies, secure patient records were used for the ascertainment of PCOS and assessment of outcomes.
Table 2. Pooled effects reported as mean differences (MDs) or standardized MDs (SMDs) and 95% confidence interval (CI), Z score and p-values, and heterogenity (I 2) in women with and without polycystic ovary syndrome.
Meta-analyses, sensitivity analyses, and publication bias
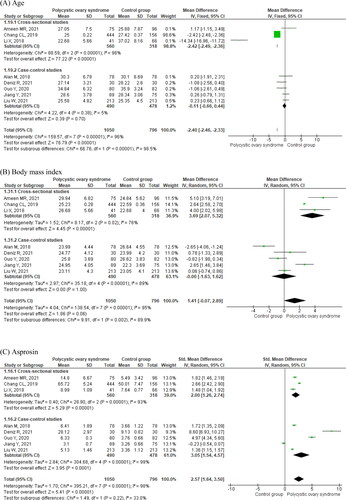
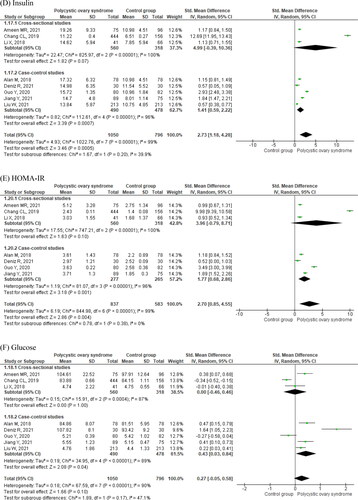
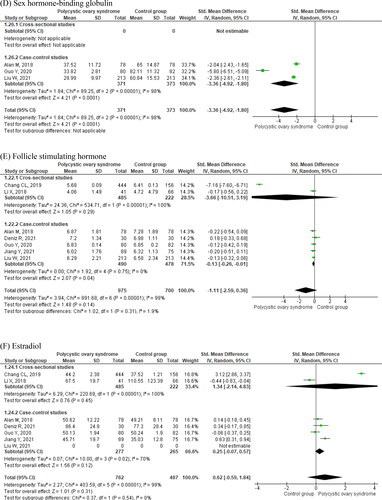
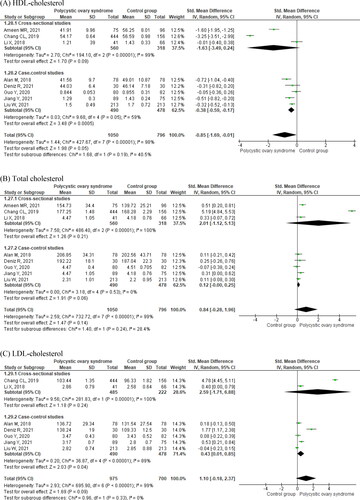
Detailed meta-analysis results of eight studies are displayed in , and . Women with PCOS were younger as compared to controls (MD = −2.40 years, 95% CI −2.46 to −2.33. ; ) and had higher body mass index (BMI; MD = 1.41, 95% CI −0.07 to 2.89. ; ). They had increased circulating levels of asprosin (SMD = 2.57, 95% CI 1.64–3.50. ; ), and insulin (SMD = 2.73, 95% CI 1.18–4.28. ; ). Women with PCOS also displayed higher homeostatic model assessment of insulin resistance (HOMA-IR) values (; ). Circulating glucose levels did not differ between women with and without PCOS (; ). Cross-sectional studies did not display significant differences in insulin (), HOMA-IR (), and glucose () levels in women with and without PCOS as compared to case-control studies. Women with PCOS had significantly higher circulating levels of LH (; ), total testosterone (; ), and DHEA-S (; ), and significantly lower circulating sex hormone-binding globulin (SHBG, ; ) than those without the syndrome. There were no significant differences in circulating follicle-stimulating hormone (FSH, ; ) and estradiol (; ).
Figure 2. Age, body mass index, circulating asprosin and insulin, HOMA-IR and glycemia in women with and without polycystic ovary syndrome.
Figure 3. Luteinizing hormone (A), total testosterone (B), DHEA-S (C), sex hormone-binding globulin (D), follicle-stimulating hormone (E), and estradiol (F) in women with and without polycystic ovary syndrome.
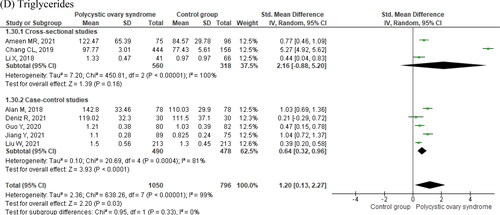
Figura 4. Standardized mean differences of circulating (A) HDL-cholesterol, (B) total cholesterol, (C) LDL-cholesterol, and (D) triglycerides in women with and without polycystic ovary syndrome.
Women with PCOS also had significantly lower circulating levels of HDL-C (; ), whereas there were no significant differences in total cholesterol (; ) and LDL-C between the two groups of women (; ). Finally, women with PCOS had significantly higher triglyceride levels as compared to controls (; ). shows that there was high heterogeneity of effects on all compared outcomes across studies (I2 > 90%).
Subgroup analyses were performed for asprosin and insulin comparing studies from China [Citation25–28] and Taiwan [Citation23] to those from Turkey [Citation21, Citation24] and Iraq [Citation22]. There were similarly increased levels of asprosin (Figure S1) and insulin (Figure S2). No other sub-analyses were possible due to the few available studies.
Sensitivity analyses were performed for both asprosin and insulin, including the removal of studies one by one (Table S3). The asprosin SMD ranged from 1.95 [CI 95%, 1.05 to 2.84] by deleting the publication by Deniz et al. [Citation24], and 2.91 [CI 95%, 2.08–3.74] when deleting the Jiang et al. results [Citation26] (Table S3). The insulin SMD ranged from 1.12 [CI 95%, 0.58–1.66] when omitting the paper by Chang et al. [Citation23], and 3.04 [CI 95%, 1.32 to 4.76] when deleting the Deniz et al. results [Citation24] (Table S3). Therefore, the increased asprosin and insulin levels in women with PCOS are robust findings. The I2 values were very high (> 90%) for both hormones (Table S3).
Since there were only eight studies, there was no option to assess the publication bias risk using funnel plots and Egger tests.
Discussion
This systematic review and meta-analyses included studies reporting women with PCOS, without duplicated populations, assessing circulating asprosin levels as the primary outcome and insulin and HOMA-IR index values as secondary outcomes. Participants with PCOS were younger with higher BMI in comparison to those without the syndrome. They had increased circulating values of asprosin, insulin, HOMA-IR, LH, total testosterone, DHEA-S, and triglycerides; with lower SHBG and HDL-C levels as compared to controls. There were no significant differences in circulating FSH, estradiol, total cholesterol, and LDL-C levels. Many of the hormonal and metabolic findings reported here fit well with what one can expect when comparing young women with and without PCOS.
PCOS is not an exclusive ovarian dysfunction, but a total endocrine and metabolic disorder related to insulin resistance. The meta-analyzed data of PCOS patients displayed increased insulin, glucose, HOMA-IR, triglyceride, and asprosin levels, and reduced HDL-C values. Asprosin regulates lipid and carbohydrate metabolism [Citation2], stimulates the production of reactive oxygen species and inflammation-associated cytokines, and decreases insulin secretion [Citation30, Citation31]. In obese children and adult subjects asprosin secretion is increased, and related to increased waist circumference and triglyceride levels [Citation6, Citation32]. Increased asprosin levels have also been reported in patients with insulin resistance and T2DM [Citation2, Citation32]. Some experimental studies postulate the use of antibodies against asprosin as a way to neutralize metabolic syndrome [Citation33].
Our results show that asprosin secretion is altered in women with PCOS during reproductive years as compared to women without the syndrome. It remains to be determined which are the roles of this new hormone in the development and progress of PCOS. New studies are needed in adolescents and very young women combining the traditional hormonal and metabolic outcomes along with the recently discovered ones which might open a new approach to the PCOS since the early clinical manifestations. Preliminary experimental studies suggest that aerobic exercise may reduce asprosin levels and cardiometabolic risks in rats [Citation34]. In young women with low physical activity and visceral obesity, an 8-week program of exercise improves BMI, insulin resistance, and asprosin levels [Citation35]. The pharmacological inhibition of asprosin to manage excessive body weight and insulin resistance might open a new therapeutic avenue since anti-asprosin antibodies may reduce appetite, body weight, and circulating glucose levels [Citation36]. Previous studies demonstrate the benefits of metformin treatment on PCOS symptoms [Citation37], and future research should identify the effect of metformin on asprosin levels in PCOS patients with different degrees of obesity and insulin resistance.
Strengths and limitations
This meta-analysis has the strength that PCOS patients were diagnosed according to standardized international scientific recommendations, including 1,050 cases without possible duplicated populations. Our meta-analysis provides new information that asprosin is significantly increased in women with PCOS as compared to those without the syndrome in a similar social and healthcare scenario. Our results fill some gaps and controversies from individual studies concerning endocrine and metabolic PCOS knowledge concerning asprosin and its relationships with pituitary and ovarian hormones, androgens, and insulin secretion. Finally, our meta-analysis included a proportion of Chinese women, with their particular cultural peculiarities and lifestyle which are not usually considered in Western scientific literature.
The heterogeneity of studies was very high (I2 > 95%) and represents a limitation, and by the sensitivity analyses, I2 values remained high. This finding may be explained by the polymorphic characteristics of the syndrome which may be related to the wide variability of expression in clinical symptoms, the severity of metabolic and endocrine alterations, BMI, the severity of insulin resistance and hyperandrogenism, inflammatory presence, and lifestyle factors. The inclusion criteria, study designs, and primary publication objectives were quite variable, despite all primary sources being based on validated diagnosis criteria. The heterogeneity of studied PCOS patients, ethnicity, natural history, diet, physical activity, and other lifestyle factors may also contribute to the statistical heterogeneity [Citation35, Citation38], which we tried to sort out using random effects models. However, there remains a heterogeneous source of uncertainty for estimated endpoints that may be related to the difference in PCOS phenotypes, delay of the publication of negative results, and small sample size variability that can significantly contribute to heterogeneity in continuous but not binary outcomes [Citation39–41].
Future studies should overcome these limitations to better define the endocrine and metabolic role of asprosin in PCOS patients considering their different phenotypes. To overcome the presence of heterogeneity between studies, we used random effects method that produces wider confidence intervals that reflects the heterogeneity between sample estimates.
Conclusion
We demonstrated that asprosin is significantly increased in women of reproductive age with PCOS compared to those without the syndrome. The studied women with PCOS displayed the usual concomitant endocrine and metabolic alterations for this particular syndrome. Altered asprosin secretion indicates that white fat tissue may have a role in the PCOS origin and maintenance of the typical metabolic alterations and dysfunctional secretion of insulin and steroid hormones. Interventions to reduce asprosin levels, including diet and physical activity or the anti-diabetic drug metformin [Citation42], should be studied along with other metabolites and hormones.
Author contributions
Conceptualization of the study and PROSPERO protocol design, data curation, and risk of bias assessment: MTLB, PC, PGA, and FRPL. Meta-analyses and related methodology were performed by SRV, GPR, and FRPL. The initial manuscript was drafted by FRPL, and all authors contributed with critical intellectual input, reviewing and approving the final manuscript.
| Abbreviations | ||
| BMI | = | Body mass index |
| CI | = | Confidence interval |
| DHEA-S | = | dehydroepiandrosterone sulfate |
| HDL-C | = | High density lipoprotein-cholesterol |
| HOMA-IR | = | Homeostatic model assessment of insulin resistance |
| IGF | = | insulin growth factor |
| LDL-C | = | Low density lipoprotein-cholesterol |
| MD | = | Mean difference |
| NOS | = | Newcastle–Ottawa Scale |
| PCOS | = | polycystic ovary syndrome |
| SD | = | Standard deviation |
| SMD | = | Standardized mean difference |
| T2DM | = | type 2 diabetes mellitus. |
Supplemental Material
Download MS Word (77.6 KB)Acknowledgments
The authors thank Dr. Min Long for providing numeric results from a figure in reference [Citation27].
Disclosure statement
The authors report no conflicts of interest and are alone responsible for the content and the writing of the article.
Data statement
The present meta-analysis was based on published articles. All summary data generated during this study are included in this published article. Raw data used for the analyses are available presented in the original reviewed articles.
References
- Greenhill C. Liver: asprosin – new hormone involved in hepatic glucose release. Nat Rev Endocrinol. 2016;12(6):1.
- Romere C, Duerrschmid C, Bournat J, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165(3):566–11.
- Al-Daghri NM, Alokeel RMI, Alamro A, et al. Serum asprosin levels are associated with obesity and insulin resistance in Arab adults. J King Saud University Science. 2022;34(1):101690.
- Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol. 2019;2019:2521096.
- Duerrschmid C, He Y, Wang C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23(12):1444–1453.
- Wang M, Yin C, Wang L, et al. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab. 2019;75(4):205–212.
- Maylem ERS, Spicer LJ, Batalha I, et al. Discovery of a possible role of asprosin in ovarian follicular function. J Mol Endocrinol. 2021;66(1):35–44.
- Maylem ERS, Spicer LJ, Atabay EP, et al. A potential role of fibrillin-1 (FBN1) mRNA and asprosin in follicular development in water buffalo. Theriogenology. 2022;178:67–72.
- Ollila MM, West S, Keinänen-Kiukaanniemi S, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod. 2017;32(2):423–431.
- Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–467.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. Erratum in: Int J Surg 2010;8:658.12.
- Azziz R, Carmina E, Dewailly D, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS. The Androgen Excess and PCOS Society. Criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2): 456–488.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47.
- Plot Digitizer (version 4.5), https://automeris.io/WebPlotDigitizer/.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Hedges LV. Fitting categorical models to effect sizes from a series of experiments. J Educ Stat. 1982;7(2):119–137.
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group 2010. Chapter 10: Analysing data and undertaking meta-analyses https://training.cochrane.org/handbook/current/chapter-10. Accessibility verified July 2, 2022.
- Egger M, Davey Smith G, Schneider M, et al. Bias in metaanalysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634.
- Alan M, Gurlek B, Yilmaz A, et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(3):220–223.
- Ameen MR, Sulaiman DM, Muho KH. Serum asprosin levels in women with polycystic ovary syndrome in Duhok city, Kurdistan region of Iraq. J Life Biosci Res . 2021;2(2):36–41.
- Chang CL, Huang SY, Hsu YC, et al. The serum level of irisin, but not asprosin, is abnormal in polycystic ovary syndrome patients. Sci Rep. 2019;9(1):6447.
- Deniz R, Yavuzkir S, Ugur K, et al. Subfatin and asprosin, two new metabolic players of polycystic ovary syndrome. J Obstet Gynaecol. 2021;41(2):279–284.
- Guo Y, Gao Y, Liu J. Serum asprosin level in patients with polycystic ovary syndrome and the influencing factors. Chin J Diabetes. 2020;28(10):720–732.
- Jiang Y, Liu Y, Yu Z, et al. Serum asprosin level in different subtypes of polycystic ovary syndrome: a cross-sectional study. Rev Assoc Med Bras (1992). 2021;67(4):590–596.
- Li X, Liao M, Shen R, et al. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediators of Inflammation. 2018;2018:1–12.
- Liu W, Tang S, Wei S, et al. Relationship between serum asprosin level and insulin resistance in polycystic ovary syndrome. Zhejiang Med J. 2021;43(11):1217–1220.
- Polycystic Ovary, Endocrinology Group and Guidelines Expert Group Chinese Medical Association Obstetrics and Gynecology branch Chinese Journal of Diagnosis and Treatment of Syndrome. 中华医学会妇产科学分会内分泌学组及指南专家组多囊卵巢 综合征中国诊疗指南 中华妇产科杂志. Chin J Obstet Gynecol. 2018;53(1):2–6.
- Lee T, Yun S, Jeong JH, et al. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol. 2019;486:96–104.
- Luís C, Fernandes R, Soares R, et al. A state of the art review on the novel mediator asprosin in the metabolic syndrome. Porto Biomed J. 2020;5(6):e108.
- Wang Y, Qu H, Xiong X, et al. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediators Inflamm. 2018;2018:9471583.
- Mishra I, Duerrschmid C, Ku Z, et al. Asprosin-neutralizing antibodies as a treatment for metabolic syndrome. Elife. 2021;10:e63784.
- Ahmadabadi F, Nakhaei H, Mogharnasi M, et al. Aerobic interval training improves irisin and chemerin levels of both liver and visceral adipose tissues and circulating asprosin in rats with metabolic syndrome. Physiol Int. 2021;108(3):383–397.
- Kantorowicz M, Szymura J, Szygula Z, et al. Nordic walking at maximal fat oxidation intensity decreases circulating asprosin and visceral obesity in women with metabolic disorders. Front Physiol. 2021;12:726783.
- Hoffmann JG, Xie W, Chopra AR. Energy regulation mechanism and therapeutic potential of asprosin. Diabetes. 2020;69(4):559–566.
- Tan S, Hahn S, Benson S, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157(5):669–676.
- Kiconco S, Tay CT, Rassie KL, et al. Natural history of polycystic ovary syndrome: a systematic review of cardiometabolic outcomes from longitudinal cohort studies. Clin Endocrinol (Oxf). 2022;96(4):475–498.
- Alba AC, Alexander PE, Chang J, et al. High statistical heterogeneity is more frequent in meta-analysis of continuous than binary outcomes. J Clin Epidemiol. 2016;70:129–135.
- Usinger L, Reimer C, Ibsen H, Cochrane Hypertension Group Fermented milk for hypertension. Cochrane Database Syst Rev. 2012;18(4):CD008118.
- Calvo F, Karras BT, Phillips R, et al. Diagnoses, syndromes, and diseases: a knowledge representation problem. AMIA Annu Symp Proc. 2003;2003:802.
- Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–574. Erratum in: Hum Reprod Update. 2016 ;22(3):408-9.