Abstract
Aims: To investigate the underling mechanisms of liver dysfunction in patients with polycystic ovary syndrome (PCOS).Materials and methods: PCOS patients were enrolled according to the Amsterdam criteria while PCOS animal model was established by dihydrotestosterone (DHEA) sustained release tablet implantation on its neck. Further liver damage and iron overload were detected by HE and Prussian blue staining. The liver related enzymes, mRNA and protein levels of hepcidin and GPX4 were tested by ELISA, qRT-PCR and Western blot. RNA interference and miR-761 transfection were routinely performed while the regulation of miR-761 on hepcidin and GPX4 was confirmed by luciferase reporter gene analysis.Results: We found that a part of PCOS patients and animal model had unexplained liver damage, which is independent of nonalcoholic fatty liver disease (NAFLD) and accompanied by increased ferrum (Fe) deposition. Besides, the expression of hepcidin and GPX4 that is important effector proteins for ferroptosis was down regulated in liver, showing the importance of iron metabolism in this unexplained liver damage. Based on the miR-761-hepcidin/GPX4 axis, we systematically studied the effects of miR-761 on ferroptosis and Fe deposition, which further influence the phenotype and liver function of PCOS model. From both in vivo and in vitro levels, changes in PCOS disease phenotype and ferroptosis were observed through hierarchical antagonism or overexpression of miR-761, hepcidin and GPX4.Conclusions: our results provide a novel explanation for unexplained liver damage in PCOS and a potential therapeutic target.
Keywords:
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age. The prevalence rate is as high as 12%–18% [Citation1]. PCOS patients often have abnormal liver function, mostly attributing to nonalcoholic fatty liver disease (NAFLD) [Citation2,Citation3]. However, there is still a non-negligible portion of PCOS patients with unexplained liver damage, reaching approximately 19.2% in our clinical investigation, which increases therapeutic difficulty and becomes a high risk factor for acute liver failure during pregnancy [Citation4]. Therefore, exploring the underling etiology for unexplained liver dysfunction in PCOS is of clinical importance.
Ferrum (Fe) is essential material for human body and involved in various diseases. Fe homeostasis is sophisticatedly regulated, where the hepatic master regulator hepcidin plays an important role [Citation5]. Previous reports showed that hepcidin down-regulation leads to increased Fe uptake in intestinal epithelium and hence causes Fe overload [Citation6] while decreased serum hepcidin was identified in PCOS patients [Citation7]. Although there is an academic consensus that hepcidin is significantly increased in response to Fe overload, inflammation and infection, there are also many studies showing its declination with different causes including testosterone [Citation8–13], where hyper-androgenism is the major clinical feature of PCOS. Additionally, the indirect regulation of miR-130a on hepcidin by inhibiting BMP pathway [Citation14,Citation15] was also reported. Nevertheless, the direct miRNA regulation on hepcidin has not been explored. Our team previously identified the expression profile of serum miRNAs of PCOS patients by microarray analysis [Citation16]. Through further combined application of Targetscan and literature review, we found several miRNAs targeting hepcidin, including upregulated miR-873-5p in NAFLD [Citation17]; upregulated miR-637 and miR-548b-3p, downregulated miR-761 and miR-486-3p in hepatocellular carcinoma [Citation18–21]; as well as upregulated miR-214-3p in hepatic fibrosis [Citation22].
Ferroptosis is a novel identified Fe-dependent form of cell death in mammalian cells. The key processes of ferroptosis are the accumulation of intracellular Fe and the requirement of lipid peroxidation. The ferroptotic cells show condensed mitochondrial membrane densities, vanishing of mitochondria crista and rupture of outer mitochondrial membrane at the ultrastructural level [Citation23]. Ferroptosis has recently been intensively investigated for putative involvement in numerous pathophysiological process [Citation24,Citation25]. However, its role in liver damage of PCOS patients remains unclear. Therefore, we hypothesize that downregulated hepcidin in PCOS patients leads to hepatic Fe deposition, which in turn activates ferroptosis, causing NAFLD independent liver damage. Besides, microRNA may exert regulatory function on hepcidin and further participate in PCOS associated liver damage. By using PCOS mice model and cell line, we tried to reveal the underlying mechanism and provide novel ideas and targets in prevention and treatment of PCOS patients with liver damage.
Methods and materials
Ethics statement
The protocol was approved by the institutional review board at red cross hospital of Hangzhou and conducted in accordance with the Declaration of Helsinki. The study design and manuscript preparation fully followed guideline from the STROBE statement. All written informed consent was collected.
Patient recruitment
213 patients were diagnosed as PCOS according to the Amsterdam criteria [Citation26] and recruited in red cross hospital of Hangzhou in the past five years, where the inclusion criteria are as followings: menstrual cycle abnormality, amenorrhea, oligomenorrhoea or long cycles, clinical and/or biochemical hyperandrogensim and ultrasound appearance of polycystic ovaries. The diagnosis of NAFLD in PCOS patients is based on the typical liver fat deposition tested by abdominal ultrasound, with exclusion of alcohol hepatitis, chronic hepatitis, autoimmune liver disease, usage of liver toxic drug, hemochromatosis and history of other chronic liver disease. To simplify the study design, we only consider ALT >40 (in our hospital, the normal range is 0–40) as liver damage and not consider the changes of AST, GGT, et al. Besides, since only 129 patients had ferritin level detection, those patients were finally selected for NAFLD and ALT analysis.
Cell culture
Cells were purchased from cell bank of the Chinese academy of sciences (shanghai). AML12 cells were cultured in 10 μg/mL insulin, 10 μg/mL transferrin, 5 ng/mL selenium, 40 ng/mL dexamethasone, 100 U/mL penicillin and 100 mg/mL streptomycin, 10% FBS in DMEM/F-12 medium at 37 °C in a 5% CO2 incubator. L02 cells were routinely cultured in DMEM medium with 100 mg/mL penicillin and 100 mg/mL streptomycin in the same condition. A model of hepatocytes interfering/overexpressing miR-761 was established in AML2 cells by adding miR-761 mimics (50 nM) or inhibitors (100 nM) and were collected 24 h after transfection. AML12 cells were divided into 4 groups: Control group, negative control (NC) group, miR-761-inhibitor group and miR-761-mimic group. L02 cells were incubated with miR-761 mimics for 24 h, followed by ferroptosis inhibitor-Ferrostatin-1 (2 μM) and Fe chelator-DFO (1 mM) addition for 24 h and 48 h. They were divided into 6 groups: Ctrl group (A), NC mic group (B), miR-761-mic group (C), DMSO + miR-761-mic group (D), DFO + miR-761-mic group (E) and Fer-1 + miR-761-mic group (F). The mRNA levels of miR-761, TNF-α; IL-6, IL-1β by qRT-PCR; the protein levels of TNF-α; IL-6; IL-1β by ELISA and GPX4/hepcidin levels by western blot were routinely detected.
Construction and evaluation of PCOS mice model
The SPF grade Balb/c mice aged 4 weeks were raised for 4 weeks, fed adaptively for 3 days and given a normal diet with adequate drinking water. The mice were randomly divided one day before modeling. The PCOS model (n = 15) was established as followings: After hair removing from the neck and back, submerged dihydrotestosterone (DHEA) sustained release tablet of 7.5 mg DHEA (daily dose 83 g/90d) was implanted while the control group (n = 15) was fed routinely, followed by weekly weight measurement and statistical analysis. Besides, serum luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2), progesterone (P), fasting blood glucose (FPG) and testosterone (T) levels in mice were detected by a fully automated biochemical analyzer, with the normal range set in the hospital. Those sex hormones’ changes reflect the typical characteristics of PCOS (Such as increased T level and LH > FSH). Hepcidin and serum Fe levels were detected by conventional ELISA test kits. FPG × FINS/22.5, the insulin resistance index (HOMA-IR), was calculated from test data.
Establishment and experimental grouping of miR-761-interfering mouse models were as followings: the successful constructed PCOS mice model was randomly divided into different groups (n = 5 each). PCOS group was fed regularly, while the other groups were injected with miR-761 and its NC-antagomir through tail vein respectively per time a week, set as Ctrl group (A), PCOS group (B), NC-antagomir + PCOS group (C) and miR-761-antagomir + PCOS group (D). POCS animal model with liver injury was categorized as for Ctrl group (A), PCOS group (B), vehicle + PCOS group (C), NC agomir + vehicle + PCOS group (D), miR-761 agomir + vehicle + PCOS group (E), Fer-1 + PCOS group (F), NC agomir + fer-1 + PCOS group (G) and miR-761 agomir + fer-1 + PCOS group (H).
Quantitative PCR assay and western blot analysis
Total RNA was isolated from mice or cells with TRizol reagent (Invitrogen, Carlsbad, CA, USA). The quality of total RNA was checked by Thermo Scientific™ NanoDrop™ 2000 spectrophotometer. The 1 μg total RNA was reversely transcribed using PrimeScript™ RT Master Mix (Perfect Real Time) obtained from TAKARA Co., Ltd. (Dalian, China). Quantitative RT-PCR was routinely performed with the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) on CFX Connect™ Real-Time System (Bio-Rad, USA). All primers used were designed and synthesized by Sangon Biotech, Co., Ltd. China and shown in Supplementary Table 1. Proteins from mice or cells were extracted by RIPA Lysis Buffer (Beyotime, Shanghai, China) on ice, and their concentrations were measured using bicinchoninic acid method. Further WB analysis was routinely performed and the intensities of bands were analyzed by software of Quantityone (Bio-Rad, USA).
Hematoxylin and eosin (HE) and prussian blue staining
The morphological changes of mice liver tissues were observed using HE and prussian blue staining. In brief, the separated tissues were immediately prefixed in the 4% paraformaldehyde and the sections were prepared according to standard pathological procedures. After deparaffinizing in xylene and rehydrating with ethanol, the sections were stained with HE and Prussian blue, respectively. The sections were subsequently dehydrated with graded ethanol, cleared in xylene and mounted with neutral balsam for further microscopic observation.
Elisa and CCK-8 measurement
The serum of mice in each group was collected and the expression levels of Fe, ferritin, hepcidin, TG (Triglyceride), Choline, GSH (L-Glutathione), MDA (Malonydialdehyde), TNF-α, IL-6, IL-1β, MCP-1, AST and ALT were detected strictly according to the detection steps of related kits. To detect cell proliferation level, we used cck-8 measurement, where cells of each group in logarithmic growth period were inoculated in 96-well plate. After treatment of respectively 24, 48, 72 h, the light absorption values of each hole were measured by 450 nm wavelength enzyme labeling instrument (OD). Each group of cells was set up with 5 multiple holes to calculate the cell OD activity.
Statistics
Each experiment was performed in triplicate and data were expressed as means ± SD (standard deviation) when the distribution was normality, otherwise the non-parametric analysis was executed. The student’s t test and Man-Whitney U test for numerical data (comparison among more than two groups) as well as chi-square test for categorical data were executed by SPSS 17.0. The differences were considered statistically significant at p < .05.
Results
Elevated serum ferritin in PCOS patients with liver damage
Intriguingly, though there was no significant difference of ferritin level between PCOS patients with (n = 56) and without (n = 73) NAFLD (191.21 ± 130.98 ng/ml vs 171.25 ± 116.79 ng/ml, p > 0.05), the ferritin level was significantly higher in PCOS patients with liver damage (ALT > 40, n = 53) than those without (n = 76, 308.26 ± 74.77 ng/ml vs 90.41 ± 47.02 ng/ml, p < .01), irrespective of etiologies. Furthermore, ALT and ferritin levels were positively correlated (F = 0.699, p < .01). Therefore, this clinical observation suggested the potential involvement of ferritin metabolism in PCOS patients with unexplained liver dysfunction, though the specific mechanism needs further investigation.
Increased liver Fe deposition in PCOS mice model with unexplained liver damage
To test our clinical hypothesis, PCOS mice model was successfully established by back embedding of sustained-release tablet of DHEA. Compared with control group, the body weight of mice in the model group was increased, where the ovarian atresia follicles increased, the ovarian cyst dilated, the granulosa cell layer became thinner and looser, the number of layers decreased to 2∼ and 4 levels, and the membrane cells proliferated irregularly (). Besides, in PCOS mice, serum LH and T levels were significantly increased while P level was significantly decreased. However, other related hormones were not significantly changed (). Further HE staining of mice liver precluded steatosis in PCOS model while the levels of TG, Choline, ALT and AST in PCOS mice model are significantly higher than control groups (). Meanwhile, compared with control group, liver and serum Fe levels were significantly increased in PCOS mice model. Moreover, Prussian blue staining showed increased Fe deposition in the liver as well ().
Figure 1. Increased Fe deposition in PCOS mice model with unexplained liver damage. (A) The ovarian tissue change demonstrated by HE staining; (B) the hormone change of PCOS mice; (C) HE staining of liver tissue and serum TG, Choline, ALT, AST changes; (D) Prussian blue staining of liver Fe deposition and Serum/liver Fe content analysis. The white arrowhead indicates the accumulated Fe in the liver. The DHEA group in this figure equals PCOS group. *p < .05; **p < .01.
Establishment of MiR-761-hepcidin/GPX4 regulation pathway and the potential involvement of ferroptosis in PCOS mice model with unexplained liver damage
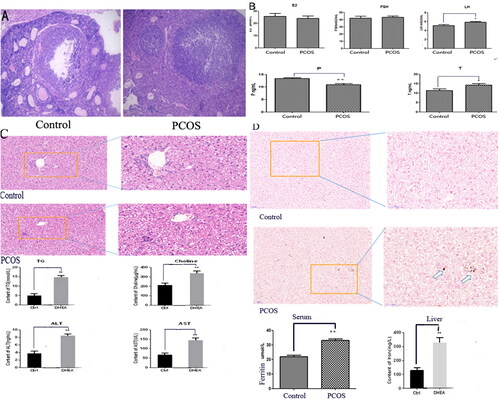
To further explore the underling mechanism for increased Fe deposition in PCOS mice with unexplained liver damage, we tested serum hepcidin and found its significant declination in PCOS mice. Nevertheless, the mRNA and protein levels of liver hepcidin were separately increased and decreased in PCOS model, though not reached statistical significance. Such inconsistent phenomenon suggested the potential existence of post-transcriptional regulation (). Based on bioinformatics prediction and qRT-PCR analysis, we found that miR-873-5p and miR-761 showed an opposite expression level to hepcidin. Furthermore, binding of miR-761 to hepcidin was confirmed by dual luciferase reporter gene analysis, suggesting the regulation of miR-761 on hepcidin ().
Figure 2. MiR-761-hepcidin/GPX4 pathway and the involvement of ferroptosis in PCOS mice model with unexplained liver damage. (A) Change of serum and liver hepcidin in mRNA and protein level; (B) Relative miR-873-5p and miR-761 levels between PCOS and control (left panel), the confirmation of miR-761 regulating on hepcidin by both bioinformatics prediction and luciferase analysis (right panel); (C) mRNA levels of GPX4, ACSL4, PTGS2 and FSP1 in control and model groups (with HFD, DHEA and HFD + DHEA); (D) Relative protein levels of GPX4, ACSL4 and PTGS2 in control and model groups (with HFD, DHEA and HFD + DHEA); (E) the confirmation of miR-761 on GPX4 by both bioinformatics prediction and luciferase analysis; (F) the change of GPX4 and hepcidin after miR-761 antagonizing and overexpression. *p < .05; **p < .01.
Further Elisa analysis showed that GSH and MDA which represent the oxidation-reduction process of ferroptosis had a trend of over-expression. qRT-PCR also showed that GPX4, ACSL4 and PTGS2 which related to ferroptosis in liver tissue were differentially expressed, where the declination of GPX4 reached statistical significance. In contrast, significantly increased ACSL4 and PTGS2 levels were also identified (). Western blot analysis displayed that GPX4, ACSL4 and PTGS2 were differentially expressed in liver tissues. Additionally, Mice with DHEA-HFD and DHEA had lower expression levels GPX4 (), suggesting ferroptosis as an important mechanism of unexplained liver damage in PCOS model mice. More importantly, miR-761 was also identified as the upstream regulator of GPX4 by dual luciferase reporter gene analysis ().
In further verification analysis, miR-761 level was found with significant decrease and increase after its specific knockdown and over-expression in miR-761-inhibitor and mimic groups, indicating the successful establishment of liver cell model with miR-761 interference. Western blot analysis showed that GPX4 was significantly down-regulated after miR-761-mimic treatment, and there was no significant change in hepcidin protein level. On the other hand, hepcidin level was significantly enhanced after miR-761-inhibitor treatment, but there was no significant change in GPX4 protein expression. Therefore, the regulation among miR-761, hepcidin and GPX4 needs further research ().
The effect of miR-761 on Fe deposition in PCOS mice and cell model
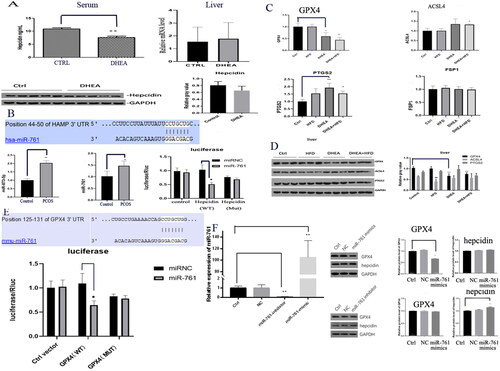
Since the primary regulation of miR-761 on ferroptosis was identified, we hypothesized that it might also participate in Fe deposition. As shown in , Serum AST, ALT, SI, Ferritin, GSH and MDA levels were upregulated in PCOS model group in the order of B = C > D > A, while GSH and Ferritin levels were downregulated in the order of A > D > B = C, indicating the potential regulatory role of miR-761 on iron homeostasis in PCOS mice. Further HE and Prussian Blue staining of mice liver were performed. We found that hepatocytes in control and miR-761-antiagomir + PCOS groups were normal without injury, while hepatocytes in PCOS and NC antiagomir + PCOS groups were significantly injured. This study supported that miR-761 may slow down liver injury in PCOS mice model, which is consistent with the occurrence of Fe deposition in the model ().
Figure 3. The serum (A) and tissue Prussian Blue staining (B) markers showing the existence of liver Fe deposition and dysfunction. Control group (A), PCOS group (B), NC-antagomir + PCOS group (C) and miR-761-antagomir + PCOS group (D). *p < .05; **p < .01.
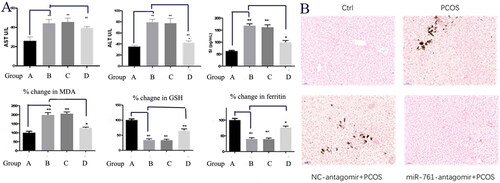
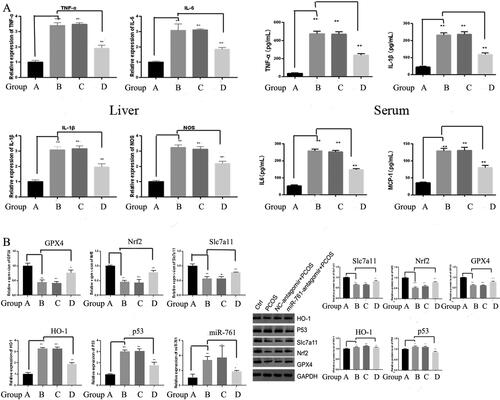
To investigate the existence of ferroptosis, we also detected TNF-α, IL-6, IL-1β, NOS and inflammatory factor levels by qRT-PCR. Their levels were increased in PCOS model, while TNF-α, IL-6, IL-1β and NOS were declined after miR-761-antagomir application with the trend as B = C > D > A. Such phenomenon was also identified in protein level by ELISA (). Moreover, qRT-PCR was used to detect GPX4, Nrf2, Slc7a11, HO-1, P53 and miR-761 levels. GPX4, Nrf2 and Slc7a11 levels were restrained in PCOS model group, further rescued after miR-761-antagomir application, with the trend as B = C < D < A. In contrast, the expression level of HO-1, P53, miR-761 in PCOS model group was higher, and is also rescued after miR-761-antagomir application, with the trend as B = C > D > A. All these results were further confirmed in protein level by western blot ().
Figure 4. Serum and liver markers of inflammation (A) and ferroptosis (B) showing the existence of ferroptosis and inflammation. Ctrl group (A), NC-mimic group (B), NC-antagomir + PCOS group (C) and miR-761-antagomir + PCOS group (D). *p < .05; **p < .01.
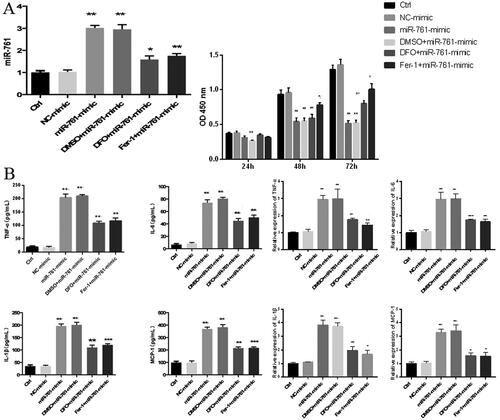
After successful POCS cell model establishment, CCK-8 method was used to compare the proliferation ability of each L02 group. Compared to control group, cells treated with Ferrostatin-1 or DFO showed a significant inhibition of their proliferation capacity after 48 h incubation; however, the OD value of the Fer-1 + miR-761-mimic group was significantly higher than that of the miR-761-mimic group; after 72 h of cell treatment, the OD value of the DFO + miR-761-mimic group was significantly higher than that of the miR-761-mimic group. This result indicates that miR-761 may intervene iron death process and interfere with cell proliferation by affecting iron metabolism (). Further Elisa and qRT-PCR were jointly used to detect the expression level of inflammatory factor TNF-α, IL-6, IL-1β, MCP-1 to reveal the apoptosis situation among groups. Compared with control group, miR-761-mimic group had higher TNF-α, IL-6, IL-1β and MCP-1 level, which was further antagonized after DFO and Fer-1 administration, but still higher than the control group. The order of TNF-α, IL-6, IL-1β, MCP-1 level was C = D > E.F > A = B ().
Figure 5. The effect of miR-761 on Fe deposition in L02 cell model. (A) Change of miR-761 relative mRNA (left panel) and OD (right panel) levels after different reagents; (B) mRNA (left panel) and protein (right panel) levels of TNF-α; IL-6; IL-1β; MCP-1 by qRT-PCR and ELISA methods. Ctrl group (A), NC-mimic group (B), miR-761+ mimic group (C), DMSO + miR-761-mimic group (D), DFO + miR-761- mimic group (E) and Fer-1 + miR-761-mimic group. *p < .05; **p < .01.
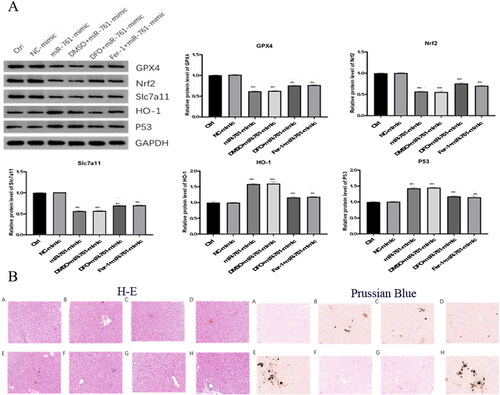
The GPX4, Nrf2 and SLC7A11 levels in miR-761-mimic group were significantly decreased, compared with control. Compared with miR-761-mimic group, GPX4, Nrf2 and SLC7A11 levels in DFO and Fer-1 groups were significantly ameliorated, but still lower than those in control group. The expression of HO-1 and P53-1 was opposite to GPX4, Nrf2 and SLC7A11, which made sense in the process of ferroptosis (). Besides, after successful construction of POCS mice model with liver injury, mice were sacrificed at 2–4 weeks to collect serum and liver tissues. The liver pathology changes were identified by H&E and Prussian Blue staining. H&E staining showed that the degree of tissue damage was E.H > B.C.D > F.G > A, unanimous with the degree of Fe deposition as shown by Prussian Blue staining ().
Figure 6. The effect of miR-761 on ferroptosis and the liver pathology change after different reagents interference. (A) Changes of GPx4, Nrf2, SLC7A11, HO-1 and P53 levels after different mixture of miR-761 interference; (B) the liver pathology change after different reagents interference as shown by both H-E (left panel) and Prussian Blue (right panel) staining. Ctrl group (A), PCOS group (B), vehicle + PCOS group (C), NC agomir + vehicle + PCOS group (D), miR-761 agomir + vehicle + PCOS group (E), Fer-1 + PCOS group (F), NC agomir + fer-1 + PCOS group (G) and miR-761 agomir + fer-1 + PCOS group (H). *p < .05; **p < .01.
The effect of ferroptosis and miR-761 on liver damage of POCS mice model
To reveal the change within the mice experienced ferroptoosis and transfected with miR-761, we detected the expression levels of AST, ALT, SI, ferritin, GSH and MDA by Elisa. Compared with control group, AST, ALT, SI, MDA expression in PCOS model group increased significantly. Compared with PCOS model group, AST, ALT, SI and MDA expression were significantly increased and decreased in miR-761-atomir + vehicle + PCOS group and Fer-1 + PCOS group. Compared with Fer-1 + PCOS group, AST, ALT, SI, MDA expression in miR-761-agomir + Fer-1 + PCOS group was augmented significantly, with the trend as E.H > B.C.D > F.G > A. GSH and ferritin levels showed an opposite trend (). Meanwhile, the expression levels of TNF-α, IL-6, IL-1β and MCP-1 were detected by Elisa. Compared with control group, the level of TNF-α, IL-6, IL-1β and MCP-1 in PCOS model group was increased significantly. Compared with PCOS model group, miR-761-agomir + vehicle + PCOS group had higher TNF-α, IL-6, IL-1β and MCP-1 level, in contrast with the fer-1 + PCOS group. Compared with the Fer-1 + PCOS group, miR-761-agomir + Fer-1 + PCOS group had higher levels of TNF-α, IL-1β, MCP-1 and the expression of IL-6 was significantly declined ().
Figure 7. The Role of ferroptosis and miR-761 in POCS mice model with unexplained liver dysfunction. (A) change of AST, ALT, SI, ferritin, GSH and MDA levels in different groups as shown by ELISA method; (B) change of TNF-α, IL-6, IL-1β and MCP-1 levels in different groups as shown by ELISA method; (C) change of GPx4, Nrf2, SLC7A11, HO-1 and p53 protein levels in different groups as shown by Western blot. *p < .05; **p < .01.
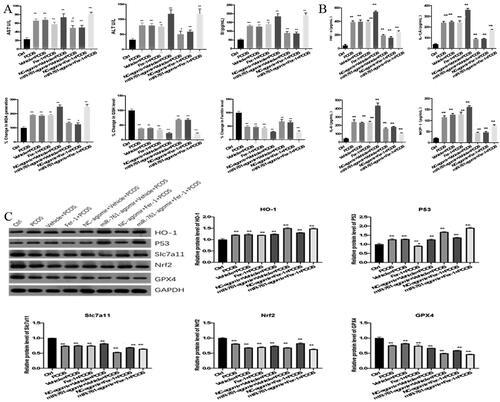
GPX4, Nrf2, SLC7A11, HO-1 and p53 protein levels were detected by Western blot. Compared with control group, the expression of HO-1 and p53 in PCOS model group was significantly increased, while the expression of GPX4, Nrf2 and SLC7A11 was significantly decreased. Compared with PCOS model group, HO-1 and p53 expressions in miR-761-agomir + vehicle + PCOS group were significantly increased, GPX4, Nrf2, SLC7A11 were significantly decreased. Besides, though HO-1, SLC7A11 and GPx4 levels in Fer-1 + PCOS group were not significantly changed, p53 and Nrf2 levels were significantly down-regulated. Compared with Fer-1 + PCOS group, HO-1 and p53 expression in miR-761-agomir + Fer-1 + PCOS group were significantly enhanced, while GPX4, Nrf2 and SLC7A11 levels were significantly decreased as well ().
Discussion
Polycystic ovary syndrome (PCOS) is a common reproductive dysfunction, neuroendocrine and metabolic disorder syndrome characterized by ovulation disorder, hyperandrogenemia (HA) and insulin resistance (IR) in adolescent and reproductive women. Since liver plays an important role in human metabolism, there is a certain probability of liver damage in patients with PCOS, though most of which are related to NAFLD [Citation27,Citation28]. Based on the systematic review of the occurrence and development of PCOS and the observation of clinical cases, we found that some patients with PCOS had unexplained abnormal liver function and serum Fe level.
Clinical and basic research shows that chronic inflammation plays an important role in PCOS related systematic reaction. For instance, abnormal expression of related factors may lead to in situ ovarian inflammation affecting follicular development and ovulation, and cause long-term complications such as IR, obesity, HA, metabolic syndrome, cardiovascular and liver diseases. They also play a role in apoptosis. The core factors like NF-κB, CRP, TNF-α and TGF-β1, the inflammatory factors such as IL-6, IL-10 and IL-18 are closely related to POCS. Each inflammatory factor affects and promotes each other in the process of PCOS, forming a related circulation network and resulting in disease occurrence and persistence [Citation27]. Recently, a study indicated that 1,25(OH)2D3 inhibited ferroptosis in zebrafish liver cells by regulating Keap1-Nrf2-GPX4 and NF-κB-hepcidin Axis [Citation29]. Our results also used GPX4 and hepicidin as the entry point and provided a reasonable interpretation of the phenomenon of unexplained liver injury in PCOS patients.
The phenomenon of chronic inflammation and Fe changes within PCOS patients lead us think whether the liver damage of PCOS may be related to ferroptosis which is a new type of programmed cell death with Fe dependent and different from apoptosis, necrosis and autophagy. The main mechanism of ferroptosis relies on the action of divalent Fe and lipoxygenase. In detail, unsaturated fatty acids that are highly expressed on cell membrane are catalyzed to produce lipid peroxidation, thus inducing cell death. GPX4-mediated neutralization of lipid peroxidation, cellular lipid peroxide levels can be enzymatically enhanced by lipoxygenases. Thus, lipoxygenase and GPX4 have opposite functions in ferroptosis: lipoxygenase causes the formation of lipid peroxides, while GPX4 eliminates them. In addition, GPX4, the core enzyme of antioxidant system (glutathione system) [Citation30] and marker of ferroptosis was decreased in our study.
Noncoding RNA refers to RNA that does not translate proteins, including circ RNA, exRNA, lncRNA, miRNA, piRNA, rRNA, snRNA, snoRNA and tRNA. In addition to rRNA and tRNA, which are well-defined ncRNAs, other ncRNAs have been considered as ‘noise’ in transcription. With accumulating functions of ncRNA being discovered, ncRNA has been proved to have very important regulatory potential in both transcription and post transcription [Citation31]. Recent studies have shown that ncRNA plays an important role in the development of PCOS [Citation14]. Our team also clarified the differentially expressed miRNA profiles in PCOS patients [Citation16]. In this study, we further identified the regulatory role of miR-761 on GPX4 and hepcidin, which further participated in the phenomenon of liver dysfunction in PCOS patients.
Our experiments started from clinical problem, based on the unique clinical phenomenon that partial of PCOS patients had unexplained liver dysfunction. Nevertheless, the underlining mechanism for this phenomenon is still vague. Our further in vivo and in vitro experiments revealed the involvement of Fe overload and ferroptosis in this phenomenon. We also identified that miR-761 could inhibit hepcidin and GPX4 expression in liver and hence participated in Fe overload and ferroptosis. In detail, based on our analysis, we identified that the down-regulated hepcidin led to increased Fe deposition as well as down-regulated GPX led to antioxidant capacity. To sum up, our study provides a new clue for the pathogenesis and a potential new target for the treatment of PCOS patients with unexplained liver damage.
Supplemental Material
Download MS Word (14.4 KB)Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):1–9.
- Macut D, Tziomalos K, Božić-Antić I, et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum Reprod. 2016;31(6):1347–1353.
- Qu Z, Zhu Y, Jiang J, et al. The clinical characteristics and etiological study of nonalcoholic fatty liver disease in Chinese women with PCOS. Iran J Reprod Med. 2013;11(9):725–732.
- Zhuang X, Cui A-M, Wang Q, et al. Liver dysfunction during pregnancy and its association of with preterm birth in China: a prospective cohort study. EBioMedicine. 2017;26:152–156.
- Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program. 2013;2013:1–8.
- Escobar-Morreale HF. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab. 2012;23(10):509–515.
- Hossein Rashidi B, Shams S, Shariat M, et al. Evaluation of serum hepcidin and iron levels in patients with PCOS: a case-control study. J Endocrinol Invest. 2017;40(7):779–784.
- Latour C, Kautz L, Besson-Fournier C, et al. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology. 2014;59(2):683–694.
- Rishi G, Wallace DF, Subramaniam VN. Hepcidin: regulation of the master iron regulator. Biosci Rep. 2015;35(3):e00192.
- Zmijewski E, Lu S, Harrison-Findik DD. TLR4 signaling and the inhibition of liver hepcidin expression by alcohol. World J Gastroenterol. 2014;20(34):12161–12170.
- Pasricha S-R, Lim PJ, Duarte TL, et al. Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat Commun. 2017;8(1):403.
- Sharp PA, Clarkson R, Hussain A, et al. DNA methylation of hepatic iron sensing genes and the regulation of hepcidin expression. PLoS One. 2018;13(5):e0197863.
- Meng P, Zhang S, Jiang X, et al. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol Environ Saf. 2020;194:110360.
- Zumbrennen-Bullough KB, Wu Q, Core AB, et al. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J Biol Chem. 2014;289(34):23796–23808.
- Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224–4230.
- Ding C-F, Chen W-Q, Zhu Y-T, et al. Circulating microRNAs in patients with polycystic ovary syndrome. Hum Fertil (Camb). 2015;18(1):22–29.
- Fernández-Tussy P, Fernández-Ramos D, Lopitz-Otsoa F, et al. miR-873-5p targets mitochondrial GNMT-Complex II interface contributing to non-alcoholic fatty liver disease. Mol Metab. 2019;29:40–54.
- Zhou X, Zhang L, Zheng B, et al. MicroRNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting mitofusin-2. Cancer Sci. 2016;107(4):424–432.
- Huang Y-H, Lin K-H, Chen H-C, et al. Identification of postoperative prognostic microRNA predictors in hepatocellular carcinoma. PLoS One. 2012;7(5):e37188.
- Du YM, Wang YB. MiR-637 inhibits proliferation and invasion of hepatoma cells by targeted degradation of AKT1. Eur Rev Med Pharmacol Sci. 2019;23(2):567–575.
- Lin L, Wang Y. miR-548b-3p regulates proliferation, apoptosis, and mitochondrial function by targeting CIP2A in hepatocellular carcinoma. Biomed Res Int. 2018;2018:7385426.
- Ma L, Yang X, Wei R, et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing sufu expression. Cell Death Dis. 2018;9(7):718.
- Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285.
- Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043–2053.
- Zhang X, Du L, Qiao Y, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction (Oxford, England). 2004;19:41–47.
- Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol. 2010;26(4):281–296.
- De Leo V, Musacchio MC, Cappelli V, et al. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(1):38.
- Cheng K, Huang Y, Wang C. 1,25(OH)D inhibited ferroptosis in zebrafish liver cells (ZFL) by regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin axis. Int J Mol Sci. 2021;22(21):11334.
- Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30(6):478–490.
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355.
