Abstract
Objective: The aim of this study was to investigate the endometrial proteomic profiles of patients with polycystic ovary syndrome (PCOS) with and without insulin resistance (IR). Method of Study: We collected 40 endometrial samples, including PCOS-IR (n = 21), PCOS-non-IR (n = 12), and control (n = 7). Data-independent acquisition (DIA)-based proteomics method is used to identify the expressed proteins among the three groups. The correlation between pregnancy outcomes and identified proteins was analyzed by Lasso regression. Results: A total of 5331 proteins were identified, while 275 proteins were differentially expressed in the PCOS vs. control group and 215 proteins were differentially expressed in the PCOS-IR vs. PCOS-non-IR group. Platelet degranulation, neutrophil degranulation, and very long-chain fatty acid catabolic processes have been found to play important roles in the endometrium of patients with PCOS-IR. Lasso regression analysis found that ACTR1A, TSC22D2, CKB, ABRAXAS2, and TAGLN2 were associated with miscarriage in patients with PCOS. ACTR1A and CKB were higher in the PCOS-IR group and were positively correlated with HOMA-IR (p < .05). Conclusion: In this study, a panel of proteins was found to be differently expressed in the endometrium. ACTR1A and CKB may be considered as PCOS-IR candidate biomarkers.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders that affects between 8% and 13% among women of reproductive age [Citation1]. The characteristics of PCOS including hyperandrogenism, ovulatory dysfunction, and polycystic ovaries, is often accompanied by insulin resistance (IR) [Citation2,Citation3].
IR is one of the important metabolic signatures of PCOS, appearing in multiple tissues in patients with PCOS, and endometrial IR was previously strongly associated with fertility disorders. Insulin stimulates ovarian theca cells to produce and secrete androgens by reducing the sex hormone binding protein (SHBG) in the liver [Citation4]. IR decreases in nitric oxide (NO) and increases in endothelin-1 (ET-1) in arterial endothelial cells, increases the risk of cardiovascular metabolic diseases. IR increases the likelihood of developing type II diabetes [Citation5]. Insulin also inhibits apoptosis and promotes cell proliferation, inducing endometrial hyperplasia and promoting the growth of endometrial cancer cells, increasing the long-term risk of endometrial cancer in patients with PCOS [Citation6]. However, the mechanism of long-term endometrial cancer in patients with PCOS has not been fully elucidated.
Fertility is a major requirement for people with PCOS [Citation7]. It has been shown that even when age, BMI, and embryo quality are controlled, patients with PCOS-IR have lower implantation and clinical pregnancy rates than non-IR patients, suggesting that local IR in the endometrium of patients with PCOS may be an important factor in the decrease in endometrial receptivity [Citation8]. Currently, it is widely believed that the important cause of endometrial IR in patients with PCOS is the signal transduction disorder after the binding of insulin to its receptor, which is mainly manifested by the inhibition of endometrial insulin receptor substrate, abnormal phosphorylation of PI3K, inactivation of Akt, expression, and translocation of GLUTs. It was found that downregulation of GLUT4 was associated with decreased FoxO1 function, an important biomarker for evaluating endometrial receptivity. Therefore, it has been speculated that one of the causes of reduced endometrial receptivity in patients with PCOS is endometrial local IR. However, more evidence is needed to clarify the mechanism by which local IR affects the expression of tolerance-related molecules [Citation9]. Thus, the present study conducted a proteomics-based approach to identify and select novel proteins associated in the endometrial of patients with PCOS-IR.
Materials and methods
Study subjects and sample collection
A total of 33 patients with PCOS aged from 21 to 40 years and meeting Rotterdam diagnostic criteria were recruited at Second Hospital of Lanzhou University from September 2019 to September 2020 and then divided into the PCOS-IR (n = 21) and PCOS-non-IR (n = 12) groups according to their homeostasis model assessment of insulin resistance (HOMA-IR). (HOMA-IR was calculated using the equation: fasting plasma glucose (mmol/L) × fasting insulin (mU/L)/22.5, and HOMA-IR ≥ 2.6 was considered to be IR [Citation10]). The Rotterdam criteria met the following 2–3 items: (1) Oligo- and/or anovulation; (2) Clinical and/or biochemical signs of hyperandrogenism; (3) Polycystic ovaries. At the same time, women were excluded from the study if they had any of the following conditions: hypothyroidism, hyperprolactinemia, adrenal disease, hypertension, diabetes, hormone medication and the drugs affecting glucose metabolism in the last 3 months, pregnancy, and lactation. The control group (n = 7) included women with successful pregnancies due to male factors and a routine ultrasound scan revealed normal ovarian morphology and they had regular menstrual cycles. Demographic characteristics and clinical biochemical parameters of the participants were collected. Demographic characteristics include age and BMI recorded from outpatient medical records. Clinical biochemical indexes included basal testosterone (T), basal luteinizing hormone (LH), and basal follicle-stimulating hormone (FSH), fasting blood glucose (FBG), fasting insulin (FINS), cholesterol (CHO), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and thyroid stimulating hormone (TSH), which were measured at 2 to 5 days of menstruation by the Laboratory of Lanzhou University Second Hospital. We then followed up on the pregnancy outcomes after the patient’s hospital visit, including live births and miscarriages prior to 24 weeks. We recorded the time of endometrial collection and the time of the last menstrual period of pregnancy, in months, and calculated the interval between the time of endometrial collection and the time of final pregnancy. Informal permission was signed by all participants prior to sample collection. The study was approved by the local ethics committee of Lanzhou University Second Hospital. All subjects gave informed consent prior to participation (Ethical Approval Number: 2017 A-057).
The endometrial samples were obtained using a pipelle endometrial aspirator at the time of hysteroscopy when the endometrium was in the proliferative phase (histological examination ensured that samples were taken during the proliferative phase) and during the month in which the blood samples were obtained, then rinsed the surface with phosphate-buffered saline (PBS) to clean the excess blood and stored at –80 °C for proteomic techniques used.
Sample preparation and fractionation, data dependent acquisition (DDA) mass spectrometry assay, mass spectrometry data analysis, and database search were completed by Shanghai Applied Protein Technology (Supplementary document Citation1 provides detailed procedures).
Bioinformatic analysis
The data were normalized using the Limma package of the R language. Differentially expressed proteins (DEPs) between PCOS and control, PCOS-IR and PCOS-non-IR were identified as that with |log2 fold change (log2 FC) |>1 as the cutoff value. Proteins with p < .05 calculated by the Bayes test were included in the DEPs list. The DEPs analysis was performed in R 4.1.0. The ggpubr package was used to map volcanoes to visually show the expression of differential proteins between PCOS and control, PCOS-IR and PCOS-non-IR.
Gene ontology (GO) is an important method and tool to annotate genes and their products, which is beneficial to the integration and utilization of biological data. The annotated, visualized Database DAVID is an online data synthesis tool that lays the foundation for successful high-throughput gene function analysis. The GO and KEGG of the DEPs between PCOS and control, PCOS-IR and PCOS-non-IR, were enriched using DAVID and the results were visualized with the software package ggplot2 in R. A p value < .05 was considered significant, and enriched GO terms or pathways were sorted by p values.
The tool Venn calculator can generate counts and detailed elements for each non-empty intersection for datasets with an arbitrary number of groups. We plot the Venn diagram using PCOS vs. control, PCOS-IR vs. PCOS-non-IR differential proteins, and then perform PCA with the ggprepel package R.
Lasso regression analysis was performed to screen the key proteins affecting the pregnancy outcomes in PCOS patients by R (http://www.R-project.org) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Key proteins were found from raw data and differences in expression levels between groups were analyzed.
Statistical analysis
Normally distributed continuous data are presented as the means ± SE and were compared using the Student’s t test, while non-parametric data were presented as the median (interquartile range) and were compared using the Mann–Whitney U test, categorical variables using the chi-square test or Fisher exact test when appropriate. A two-sided p value < .05 was considered statistically significant for all tests.
Results
Subject clinical characteristics
Clinical and biochemical parameters for all subjects used in the proteomic analysis are presented in . BMI, LH, T, FBG, FINS, HOMA-IR, TG, and the interval between the time of endometrial collection and the time of final pregnancy were statistically different among the three groups, and HDL-C and live birth rates were much lower in the PCOS-IR group than in the PCOS-non-IR group and the control group (p < .05). There were no statistical differences in age, FSH, CHO, LDL-C, and TSH between the three groups (p > .05).
Table 1. Baseline clinical and biochemical parameters in PCOS-IR, PCOS-non-IR, and control groups.
Data processing and screening of differential genes
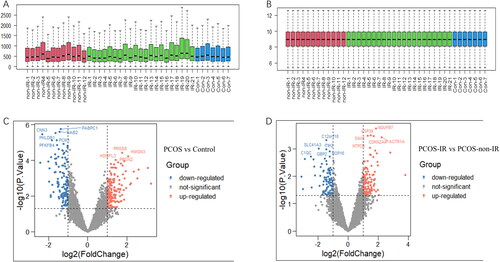
The original dataset is normalized and presented as boxplots before and after normalization (). All samples had 5331 proteins identified, 275 proteins were differentially expressed in the PCOS vs. control group and 215 proteins were differentially expressed in the PCOS-IR group vs. PCOS-non-IR group, differences have been indicated in the volcano diagram ().
Figure 1. Sample expression correction box diagram and volcano diagram. A: Protein expression before normalization. B: Protein expression after sample normalization. The red sample represents the PCOS-non-IR group, the green sample represents the PCOS-IR group and the blue sample represents the control group. C: PCOS vs. Control. D: PCOS-IR vs. PCOS-non-IR. (Red dots represent up-regulated differential proteins, green dots represent down-regulated proteins, and no significantly changed genes are marked as gray dots).
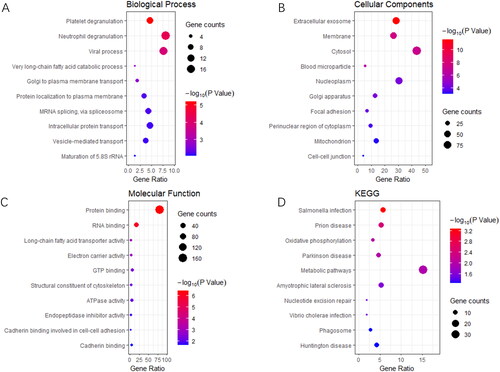
GO and KEGG enrichment of DEPs in endometrial tissues between different groups
All DEPs were uploaded to the DAVID online tool for analysis of GO and KEGG pathway enrichment. Then, the R language ggplot2 package is used for visual processing. The DEPs were classified into three groups including biological process (BP), cellular component (CC), and molecular function (MF).
In the PCOS vs. control group, DEPs of BP were enriched in cellular protein metabolic processes, platelet degranulation, post-translational protein modification, extracellular matrix organization, translational initiation, and cellular response to oxidative stress (). CCs were enriched in extracellular exosomes, blood microparticles, endoplasmic reticulum, extracellular spaces, spectrin-associated cytoskeletons, and focal adhesions (). The MF were enriched in RNA binding, structural constituent of the cytoskeleton, protein binding, spectrin binding, extracellular matrix structural constituent, collagen binding, and actin binding (). KEGG were enriched in coronavirus disease – COVID-19, complement and coagulation cascades, DNA replication, ferroelectricity, ECM-receptor interaction, and carbon metabolism ().
Figure 2. PCOS vs. Control: GO and KEGG enrichment of differentially expressed proteins (DEPs). (A) biological process, (B) cellular component, (C) molecular function, and (D) KEGG pathway analysis.
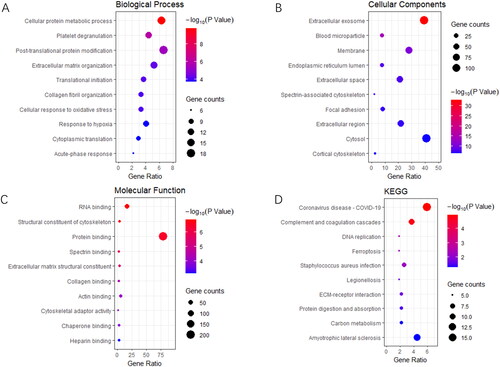
In the PCOS-IR vs. PCOS-non-IR group, the DEPs of BP were enriched in platelet degranulation, neutrophil degranulation, viral processes, very long-chain fatty acid catabolic processes, golgi to plasma membrane transport, protein localization to the plasma membrane, and intracellular protein transport (). CC was enriched in the extracellular exosome, membrane, cytosol, blood microparticles, nucleoplasm, focal adhesions, and perinuclear regions of the cytoplasm (). The MF were enriched in protein binding, RNA binding, long-chain fatty acid transporter activity, electron carrier activity, GTP binding, and ATPase activity (). The KEGG were enriched for salmonella infection, prion disease, oxidative phosphorylation, Parkinson’s disease, and metabolic pathways ().
Identification of key proteins in endometrial tissues between different groups
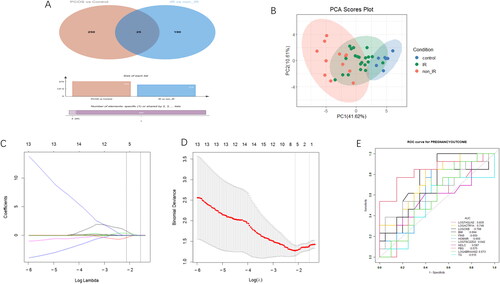
We visually displayed the intersection of differential proteins between PCOS vs. control and PCOs-IR vs. PCOs-non-IR groups using Venn diagrams and found 25 proteins with significant differences between the groups (ACTR1A, ZC3H15, C1QC, NPIPB5, EMC4, TSC22D2, DDX1, BRI3BP, CKB, PRDX2, CNN3, ABRAXAS2, CYBA, MCM7, TMEM167A, GLT8D1, MUC5B, ATRX, TTPAL, HM13, KRT9, TAGLN2, USP5, TUBA4A, and ORM1) (). We performed PCA using 25 PCOS vs. control, PCOS-IR vs. PCOS-non-IR differential proteins and found that the differential proteins were clearly distinguished between PCOS-IR, PCOS-non-IR, and control (). Then using the LASSO regression model, we screened out five DEPs, which ACTR1A, TSC22D2, CKB, ABRAXAS2, and TAGLN2 were associated with live birth of patients with PCOS (). Then using the LASSO regression model, we screened five DEPs, ACTR1A, TSC22D2, CKB, ABRAXAS22, and TAGLN2, for association with live birth in patients with PCOS ().
Figure 4. A: Venn diagrams to visually display the intersection of differential proteins between PCOS vs. control and PCOs-IR vs. PCOs-non-IR groups. B: PCA between the PCOS-IR, PCOS-non-IR and control groups. The red sample represents the PCOS-non-IR group, the green sample represents the PCOS-IR group and the blue sample represents the control group. C and D: The LASSO regression model for live birth of PCOS-IR patients. E: ROC of different factors on pregnancy outcome.
The expression of key proteins between PCOS-IR, PCOS-non-IR, and control groups
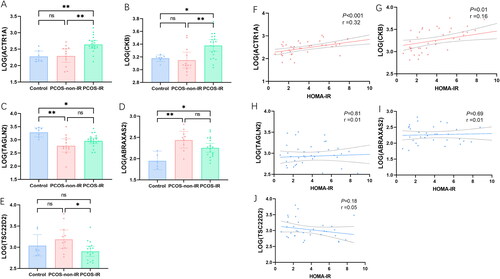
The expression levels of the key proteins were extracted from the raw data, the protein expression was transformed by LOG2 so that the data followed a normal distribution, and the differences between the groups were analyzed. We find that ACTR1A and CKB are higher in the PCOS-IR group, while TSC22D2 is lower in the PCOS-IR group (p < .05) (). ABRAXAS2 was higher in all PCOS groups and TAGLN2 was lower in all PCOS groups (p < .05), but there was no difference between the PCOS-IR and PCOS-non-IR groups (p > .05) (). Pearson correlation analysis shows that ACTR1A and CKB are significantly positively correlated with HOMA-IR and negatively correlated with HOMA-IR, while TAGLN2, TSC22D2, and ABRAXAS2 are not correlated with HOMA-IR ().
Figure 5. Analysis of key proteins expression: (A, B, E) ACTR1A and CKB were higher in PCOS-IR group, while TSC22D2 was lower in PCOS-IR group (p < .05). (C and D) ABRAXAS2 was higher in all PCOS groups and TAGLN2 was low in all PCOS groups (p < .05), but there was no difference between the PCOS-IR and PCOS-non-IR groups (p > .05). Pearson correlation analysis between key proteins and HOMA-IR: (F and G) ACTR1A and CKB was significantly positively correlated with HOMA-IR. (H, I, and J) there was no significant correlation between TAGLN2, ABRAXAS2 and TSC22D2 with HOMA-IR.
Discussion
We found that cellular protein metabolic process, platelet degranulation, post-translational protein modification, extracellular matrix organization, translational initiation, and cellular response to oxidative stress were more active in the endometrium with PCOS compared to controls. Platelet degranulation, neutrophil degranulation, viral process, and very long-chain fatty acid catabolic process was more active in the PCOS-IR group compared to the PCOS-non-IR group.
Platelet degranulation, which is crucial for hemostasis and may be involved in inflammatory responses, was found to be activated in PCOS-IR in this study [Citation11]. New evidence suggests that patients with PCOS have abnormalities in coagulation and fibrinolytic pathways that may explain the increased cardiovascular risk of PCOS, but the effects of PCOS on coagulation and fibrinolysis remain largely unexplored [Citation12]. Limited studies found that platelet count and platelet volume (MPV) of PCOS were higher than that of age- and BMI-matched healthy control, and a positive correlation between MPV and testosterone level was observed [Citation13–15]. Activation of platelets can lead to degranulation and the release of cytokines into plasma that promote the development of a pro-inflammatory state. Platelets also interact with monocytes and neutrophils to further enhance inflammation [Citation16] According to known data, PCOS has chronic low-grade inflammation throughout the body, which is manifested in increased C-reactive protein (CRP), interleukin 18 (IL-18), and tumor necrosis factor (TNF-α). This chronic inflammatory state is aggravated by obesity and hyperinsulinemia [Citation17]. It has shown that excessive uterine inflammation of PCOS may lead to the occurrence of secondary abortion, hyperinsulinemia and insulin resistance will aggravate uterine inflammation, and the molecular regulation of uterine inflammation may provide a new therapeutic target for preventing PCOS abortion [Citation18]. Our study found enhanced platelet degranulation and reduced platelet degranulation in patients with PCOS with IR endometrial tissue, which is expected to improve the inflammatory status of the uterus in patients with PCOS and reduce the abortion rate.
Very long-chain fatty acids as precursors of lipid mediators are intricately regulated to maintain lipid homeostasis [Citation19]. Patients with PCOS exhibit abnormal levels of lipid metabolism, including phosphatidylcholine, FFAs, and PUFA metabolites. Insulin resistance and increased androgen production are important manifestations of PCOS and may adversely affect the lipid profile of patients with PCOS, particularly bioactive lipid metabolites derived from PUFAs [Citation20]. The study also found that increased BMI may be associated with very-long-chain fatty acids [Citation21]. Women with PCOS had lipid-induced increases in plasma inflammatory cytokines, which in turn manifested in paracrine and endocrine effects [Citation22]. Lipid-induced production of reactive oxygen species (ROS) within skeletal muscle promotes mitochondrial dysfunction and the development of IR [Citation23]. In PCOS, mitochondrial dysfunction and abnormal glucose metabolism increase ROS production, induce the release of inflammatory factors, and regulate insulin resistance [Citation24]. In addition, OS can cause an imbalance in the uterine function, which in turn can lead to reduced endometrial receptivity and implant failure in the implantation window [Citation25], is one of the main causes of infertility in patients with PCOS. Very long-chain fatty acid also accumulate during necroptosis [Citation26]. In PCOS, a variety of hormonal and metabolic stimuli can trigger different types of regulated cell death under physiological and pathological conditions, including ferroptosis, apoptosis, and necroptosis. Studies have found that insulin-exposed PCOS pregnant rats exhibited decreased apoptosis in the uterus and increased necroptosis in the placenta [Citation27]. Our study found that for the endometrium of PCOS-IR, the accumulation of very-long-chain fatty acids has occurred before pregnancy, which may indicate that the endometrium of PCOS-IR has already occurred necroptosis before pregnancy, and if this process can be intervened before pregnancy, it may improve the live birth rate of PCOS-IR.
In this study, ACTR1A and CKB were higher in the PCOS-IR group, while TSC22D2 was lower in the PCOS-IR group. An RNA interference study revealed the important role of ACTR1A in the induction of pro-inflammatory cytokines, and identified ACTR1A, part of the dynactin complex, as a novel regulator of TLR2-mediated immune signaling [Citation28]. Studies have shown that TLR2, and TLR4-mediated activation of interferon regulatory factor-7 (IRF-7) and NF-κB signaling, cytokine production, and endometrial inflammation in patients with PCOS compared to patients with non-PCOS [Citation29]. Therefore, ACTR1A may be a discovery in the regulation of PCOS-IR endometrial inflammatory immunity. CKB is overexpressed in hyperplastic endometrial tissue and several types of cancers such as endometrial cancer, ovarian cancer, breast cancer, colon cancer, and colorectal cancer [Citation30–32]. A label-free Proteomics study comparing the endometrial proliferative and receptive phases found that the upregulation of creatine kinase B-type (CKB) in the receptive phase may be related to endometrium receptivity [Citation30]. In our study, endometrium during the PCOS proliferation period was used, and the increased CKB level of PCOS-IR endometrium was found during the proliferation period, suggesting that endometrium development was not synchronous and the receptivity of endometrium had changed, which was not conducive to pregnancy.
Finally, there are some limitations to this study. First, the selected sample is in the proliferative endometrium, and the patient is not pregnant in the current month. The factors that influence a patient’s pregnancy are complex and diverse, and this study can only reflect some of the factors that influence pregnancy outcomes. Second, lifestyle and medication interventions were given according to the patient’s condition after the patient visited the hospital and completed the corresponding checkup, but they were not considered due to heterogeneity in interventions. Third, the sample size is relatively small. In future experiments, we will expand the sample size and conduct an in-depth study.
Conclusion
This study explored the endometrial proteome in PCOS-IR, PCOS-non-IR, and controls. A panel of proteins associated with platelet degranulation, neutrophil degranulation, viral process, and very long-chain fatty acid catabolic process were found in the endometrium of PCOS-IR patients. Moreover, ACTR1A and CKB may be considered as candidate biomarkers of PCOS-IR. The results provide us with a better understanding of the endometrium abnormalities in PCOS-IR.
Ethics approval
The study was approved by the ethics committee of Lanzhou University Second Hospital (Ethical Approval Number: 2017 A-057). All subjects gave informed consent before participating.
Supplemental Material
Download MS Word (20 KB)Acknowledgment
The authors thank Shanghai Applied Protein Technology for supporting this methodology.
Disclosure statement
The authors declare no potential conflicts of interest.
Availability of data and materials
The Trial data used in this manuscript have been deposited to the Proteome Xchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD032383.
Additional information
Funding
References
- Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):1–7.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284.
- Lewandowski KC, Skowrońska-Jóźwiak E, Łukasiak K, et al. How much insulin resistance in polycystic ovary syndrome? Comparison of HOMA-IR and insulin resistance (belfiore) index models. Arch Med Sci. 2019;15(3):613–618.
- Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–332.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940.
- Rosen MW, Tasset J, Kobernik EK, et al. Risk Factors for endometrial cancer or hyperplasia in adolescents and women 25 years old or younger. J Pediatr Adolesc Gynecol. 2019;32(5):546–549.
- Hanson B, Johnstone E, Dorais J, et al. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34(2):167–177.
- Chang EM, Han JE, Seok HH, et al. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf). 2013;79(1):93–99.
- Cho HJ, Baek MO, Khaliq SA, et al. Microgravity inhibits decidualization via decreasing akt activity and FOXO3a expression in human endometrial stromal cells. Sci Rep. 2019;9(1):12094.
- Hatami H, Montazeri SA, Hashemi N, et al. Optimal cutoff points for anthropometric variables to predict insulin resistance in polycystic ovary syndrome. Int J Endocrinol Metab. 2017;15(4):e12353.
- Cardenas EI, Breaux K, Da Q, et al. Platelet Munc13-4 regulates hemostasis, thrombosis and airway inflammation. Haematologica. 2018;103(7):1235–1244.
- Targher G, Zoppini G, Bonora E, et al. Hemostatic and fibrinolytic abnormalities in polycystic ovary syndrome. Semin Thromb Hemost. 2014;40(5):600–618.
- Dereli D, Ozgen G, Buyukkececi F, et al. Platelet dysfunction in lean women with polycystic ovary syndrome and association with insulin sensitivity. J Clin Endocrinol Metab. 2003;88(5):2263–2268.
- Gursoy A, Ertugrul DT, Pamuk B, et al. Mean platelet volume in patients with polycystic ovary disease. Platelets. 2006;17(7):505–506.
- Kebapcilar L, Taner CE, Kebapcilar AG, et al. High mean platelet volume, low-grade systemic coagulation and fibrinolytic activation are associated with androgen and insulin levels in polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280(2):187–193.
- Singh A, Bisht P, Bhattacharya S, et al. Role of platelet cytokines in dengue virus infection. Front Cell Infect Microbiol. 2020;10:561366.
- Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789.
- Tersigni C, Vatish M, D’ippolito S, et al. Abnormal uterine inflammation in obstetric syndromes: molecular insights into the role of chemokine decoy receptor D6 and inflammasome NLRP3. Mol Hum Reprod. 2020;26(2):111–121.
- Erdbrügger P, Fröhlich F. The role of very long chain fatty acids in yeast physiology and human diseases. Biol Chem. 2020;402(1):25–38.
- Li S, Chu Q, Ma J, et al. Discovery of novel lipid profiles in PCOS: do insulin and androgen oppositely regulate bioactive lipid production? J Clin Endocrinol Metab. 2017;102(3):810–821.
- Thimgan MS, Seugnet L, Turk J, et al. Identification of genes associated with resilience/vulnerability to sleep deprivation and starvation in drosophila. Sleep. 2015;38(5):801–814.
- González F, Considine RV, Abdelhadi OA, et al. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(3):934–946.
- Szczuko M, Kikut J, Szczuko U, et al. Nutrition strategy and life style in polycystic ovary syndrome-narrative review. Nutrients. 2021;13(7):2452.
- Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10:86.
- Ferreira SR, Motta AB. Uterine function: from normal to polycystic ovarian syndrome alterations. Curr Med Chem. 2018;25(15):1792–1804.
- Parisi LR, Li N, Atilla-Gokcumen GE. Very long chain fatty acids are functionally involved in necroptosis. Cell Chem Biol. 2017;24(12):1445–1454.e8.
- Zhang Y, Hu M, Jia W, et al. Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J Endocrinol. 2020;246(3):247–263.
- Kamal AHM, Aloor JJ, Fessler MB, et al. Cross-linking proteomics indicates effects of simvastatin on the TLR2 interactome and reveals ACTR1A as a novel regulator of the TLR2 signal cascade. Mol Cell Proteomics. 2019;18(9):1732–1744.
- Hu M, Zhang Y, Li X, et al. TLR4-Associated IRF-7 and NFκB signaling act as a molecular link between androgen and metformin activities and cytokine synthesis in the PCOS endometrium. J Clin Endocrinol Metab. 2021;106(4):1022–1040.
- Chen Q, Zhang A, Yu F, et al. Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J Proteome Res. 2015;14(4):1831–1842.
- Li XH, Chen XJ, Ou WB, et al. Knockdown of creatine kinase B inhibits ovarian cancer progression by decreasing glycolysis. Int J Biochem Cell Biol. 2013;45(5):979–986.
- Narang P, Chen M, Sharma AA, et al. The neoepitope landscape of breast cancer: implications for immunotherapy. BMC Cancer. 2019;19(1):200.