Abstract
Purpose
We aimed to evaluate the pregnancy outcomes of cleavage-stage embryo transfers (ETs) for the first time and explore optimal number of high-quality cleavage-stage embryos for extended culture to blastocyst-stage in women of advanced maternal age (AMA).
Methods
We retrospectively identified 1646 AMA women ≥ age 38 years for the first fresh ETs between January 2014 and December 2020 at our hospital. Double ETs were divided into three groups as follows: DET-HH (two high-quality embryos), DET-HL (one high-quality and one low-quality embryo), and DET-LL (two low-quality embryos) groups. We mainly analyzed the pregnancy outcomes of double cleavage-stage ETs with different embryo grades and blastocyst-stage ETs with different number of high-quality cleavage-stage embryos on day 3.
Results
Our data indicated that the DET-HH group had significantly higher clinical pregnancy, ongoing pregnancy, and live birth rates than DET-HL and DET-LL groups (p < .05). For extended culture to blastocyst-stage with 2 (D3-2H), 3 (D3-3H), and 4 (D3-≥4H) high-quality cleavage-stage embryos, the D3-≥ 4H group had significantly higher ongoing pregnancy and live birth rates than D3-2H and D3-3H groups (p < .05). We observed that the number of high-quality embryos on day 3 was independently associated with live birth rate for blastocyst transfers (OR: 1.133, 95% CI 1.023–1.256, p = .017). There were no significant differences in the clinical pregnancy, ongoing pregnancy and live birth rates among DET-HH, D3-2H and D3-3H groups (p > .05).
Conclusions
Extended culture to blastocyst-stage for transfer was safe and recommended for AMA women with ≥ 4 high-quality embryos on day 3.
Introduction
Reproductive capacity in women declined with increasing age. The decline in normal fecundity was particularly noticeable over the age of 30, and accelerated between 35 and 40, so that fertility approached almost zero after 45 [Citation1]. Delayed childbearing was becoming increasingly common for a variety of reasons in China. With the deferment of childbearing, an increasing number of couples were turning to assisted reproductive technology (ART) in an attempt to counteract age-related decline in female reproductive capacity. Nevertheless, it was confirmed that a woman’s age was an important determinant of in vitro fertilization (IVF) success and women age 38 years or more had less chance of a successful outcome from IVF treatment [Citation2].
A recent research showed that the live birth rate for the first cycle was significantly low at 3.1% with a plateau in success rates after 4 cycles reaching 21.9% in women older than 40 years [Citation3]. Recurrent embryo implantation failure (RIF) was a disorder with potentially devastating physiological and psychological manifestations for those affected [Citation4]. Psychological stress was increasingly recognized as a risk factor for disease progression and outcome. The discontinuation rate was high for women of advanced maternal age (AMA), which was closed related to the strong decrease in success rate with age induces women of AMA to discontinue [Citation5].
AMA was associated with poor IVF outcome at every step of the procedure, with increasingly poor responsiveness to ovarian stimulation drugs and increased rates of cycle cancelation [Citation6]. Fewer oocytes were retrieved and fewer embryos were available for women of AMA. Furthermore, the cleavage-stage embryo transfer (ET) was always performed with a lower implantation rate and a higher rate of abortion. A recent research showed that the live birth rate was significantly increased after transfer of single high-quality embryo on day 5 and day 6 compared with transfer of single high-quality embryo on day 3 in the vitrified ET cycles [Citation7]. It was reported that the chance of IVF success in women of AMA should not be estimated only on the female age, but also on other predictive factors: number of high-quality embryos on day 3, number of transferred embryos and blastocyst-stage ET [Citation8]. These results suggested that the blastocyst transfers were more beneficial to women of AMA. Nevertheless, it was difficult to predict which cleavage-stage embryos could develop into viable blastocysts. Braga et al. indicated that the probability of blastocyst formation might be impaired when the morphology was compromised at the cleavage-stage [Citation9]. It was demonstrated that the transfer of low-quality embryos on day 3 was a better approach than the transfer at the blastocyst-stage [Citation9].
With this in mind, we aimed to evaluate the pregnancy outcomes of cleavage-stage ETs for the first time and explore optimal number of high-quality cleavage-stage embryos for extended culture to blastocyst-stage in women of AMA.
Methods
Patient population and inclusion criteria
This study included 1646 AMA women ≥ age 38 years for the first fresh ETs between January 2014 and December 2020 at our hospital. The maximal age of study population was 46 years old. 1111 were cleavage-stage ETs and 535 were blastocyst-stage ETs. Single ETs were divided into two groups as follows: SET-H (single cleavage-stage ET with one high-quality embryo; n = 221) and SET-L (single cleavage-stage ET with one low-quality embryos; n = 202) groups. Double ETs were divided into three groups as follows: DET-HH (double cleavage-stage ETs with two high-quality embryos; n = 262), DET-HL (double cleavage-stage ETs with one high-quality and one low-quality embryo; n = 207), and DET-LL (double cleavage-stage ETs with two low-quality embryos; n = 219) groups. The blastocyst-stage ETs were divided into three groups as follows: D3-2H (extended culture to blastocyst-stage with two high-quality cleavage-stage embryos; n = 52), D3-3H (extended culture to blastocyst-stage with three high-quality cleavage-stage embryos; n = 109), and D3-≥4H groups (extended culture to blastocyst-stage with not less than four high-quality cleavage-stage embryos; n = 374) groups.
All female patients were not less than 38 years old, and only the first cycles using fresh embryos were included in the analysis. The patients with endometriosis, in vitro maturation, intrauterine adhesions, polycystic ovary syndrome, rescue-ICSI and no available embryo were excluded in this study. The study protocol was approved by the Ethics Committee of Northwest Women and Children’s Hospital (No. 2022007). Patient consent was not required due to the retrospective nature of the study.
Ovarian stimulation protocol
The ovarian stimulation protocol was described previously [Citation10]. In brief, stimulation protocols were used with a combination of GnRH agonist/GnRH antagonist and recombinant FSH. Ovarian response was monitored by serial ultrasound examination and hormone measurement. Ten thousand units of human chorionic gonadotrophin (hCG) was administered in patients when three follicles were > 18 mm. Oocyte retrieval was performed 36 h later by transvaginal ultrasonography guided aspiration.
Embryo culture and conventional morphology assessment
The OCCs were cultured in the medium (IVF; Vitrolife, Gothenburg, Sweden) after retrieval. The zygotes were shifted to the cleavage medium (G-1; Vitrolife) 5 h after the fertilization of IVF. The embryos to culture blastocyst on day 3 were transferred to the blastocyst medium (G-2; Vitrolife) until day 6. All medium were covered with paraffin oil in a humidified atmosphere at 37 °C for prior 24 h.
The cleavage-stage embryo scoring system used a combination of blastomere number, homogeneous degree of blastomeres, and degree of fragmentation. In this study, the low-quality cleavage-stage embryo should meet the following criteria: (i) 4–5 blastomeres, even homogeneous blastomeres <10% cytoplasmic fragmentation; (ii) 6–7 blastomeres, even homogeneous blastomeres with nearly 15% cytoplasmic fragmentation or one uneven blastomere <5% cytoplasmic fragmentation; (iii) 8–10 blastomeres, even homogeneous blastomeres with nearly 20% cytoplasmic fragmentation or one uneven blastomere <15% cytoplasmic fragmentation or two uneven blastomeres <5% cytoplasmic fragmentation; (iv) >10 blastomeres, even homogeneous blastomeres with nearly 10% cytoplasmic fragmentation or one uneven blastomere <5% cytoplasmic fragmentation [Citation11].
The scoring system for blastocyst evaluation was a combination of the stage of development from 1 to 6 (Early, Blastocyst, Full blastocyst, Expanded, Hatching/Hatched) and of the grade of the inner cell mass (ICM; A, tightly packed, many cells; B, loosely grouped, several cells; or C, very few cells.) and of the trophectoderm (TE; A, many cells forming a cohesive epithelium; B, few cells forming a loose epithelium; or C, very few large cells.) [Citation12]. In this study, the blastocyst scored ≥ 3BB was defined as good blastocyst.
ET and pregnancy confirmation
The ET catheter (Cook Ireland Ltd., Limerick, Ireland) was used for transfer. Before transfer, any vaginal and cervical secretions were gently removed from the vagina/cervix with small pledgets of cotton wool, moistened with warm normal saline. The mucus in the cervical canal was wiped away. After transfer, the catheter was checked for retained embryos and the presence of blood. After ET, all patients were given luteal support (Duphaston; progesterone injection).
Serum β-hCG was measured 14 d after cleavage-stage ET and 9 d after blastocyst transfer. Clinical pregnancy was defined by the ultrasound confirmation of an intrauterine gestational sac after 6 weeks of gestation. Ongoing pregnancy was defined as fetal cardiac activity at 12 weeks. Live birth was defined as the delivery of a live-born infant (>24 weeks of gestation).
Statistical analysis
For analysis, parametric continuous variables were expressed as mean values and standard deviation, and then compared with use of Analysis of Variance (ANOVA). Where required, non-parametric variables were analyzed with medians and interquartile range and compared using Fisher’s Exact test or chi-square Goodness of Fit test with post-hoc analysis. Binary logistic regression analysis (stepwise forward entry) was used to determine independent factors that affect the prognostic of future live birth rate of AMA women. The statistical analysis was performed with SPSS version 21 (IBM Corp., Armonk, NY). Differences were considered statistically significant when p< .05.
Results
We observed that the total Gn dosage was significantly lower in the SET-H group than that in the SET-L group (2789 ± 910 versus 3019 ± 1104; p = .010). The SET-H group showed significantly higher clinical pregnancy (25.79 versus 13.37%; p = .001) rate than SET-L group ().
Table 1. Main characteristics of the study population and clinical outcomes.
No significant differences were observed in the female age, BMI, total Gn dosage, Gn stimulation time, basal FSH, and endometrial thickness among DET-HH, DET-HL and DET-LL groups (p > .05). The DET-HH group showed significantly higher number of retrieved oocytes than DET-HL and DET-LL groups (7.14 ± 3.90 versus 6.22 ± 3.56 and 5.92 ± 3.63; p < .001). We observed that the DET-HH group had significantly higher clinical pregnancy (44.66 versus 31.89 and 31.05%; p = .049), ongoing pregnancy (33.59 versus 23.19 and 22.37%; p = .007) and live birth (31.30 versus 21.26 and 20.09; p = .005) rates than DET-HL and DET-LL groups ().
Table 2. Main characteristics of the study population and clinical outcomes.
We further divided patients into the subgroups with different age (38–39, 40–43, and ≥44) and compared the clinical pregnancy, live birth and twin pregnancies rates among three groups (Supplementary Table 1).
We observed there were no significant differences in the female age, BMI, endometrial thickness, and proportion of cycles with no high-quality blastocyst obtained among D3-2H, D3-3H, and D3-≥4H groups (p > .05). The D3-4H group showed significantly lower basal FSH (7.01 ± 2.14 versus 7.62 ± 2.37 and 7.97 ± 2.91; p < .001) and higher number of retrieved oocytes (11.06 ± 4.40 versus 5.83 ± 2.79 and 6.74 ± 3.50; p < .001) than D3-2H and D3-3H groups. The clinical pregnancy rate was significantly lower in the D3-2H group than that in the D3-≥4H group (34.62 versus 52.41%; p = .016). We observed that the D3-4H group showed significantly higher ongoing pregnancy (41.98 versus 25.00 and 28.44%; p = .004) and live birth (39.30 versus 21.15 and 26.61%; p = .011) rates D3-2H and D3-3H groups (). The clinical pregnancy, live birth and twin pregnancies rates were compared among the subgroups with different age (Supplementary Table 2).
Table 3. Main characteristics of the study population and clinical outcomes.
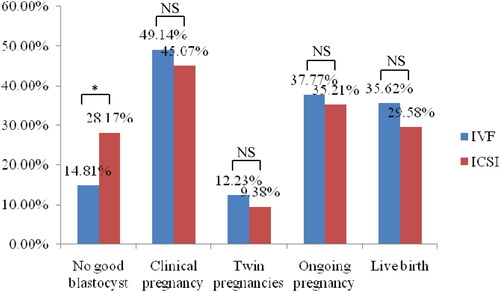
We observed that there were no significant differences in the clinical pregnancy, ongoing pregnancy, and live birth rates among DET-HH, D3-2H, and D3-3H groups (p > .05) (). For patients with day 3 embryos extended culture to blastocyst-stage, no significant differences were observed in the clinical pregnancy, twin pregnancies, ongoing pregnancy, and live birth rates between IVF and ICSI groups (p > .05) (). The IVF group showed significantly lower proportion of cycles with no good blastocyst than the ICSI groups (14.81 versus 28.17%; p < .05).
Figure 1. Comparison of pregnancy outcomes among DET-HH, D3-2H, and D3-3H groups. *Was significantly different; NS: no significant differences.
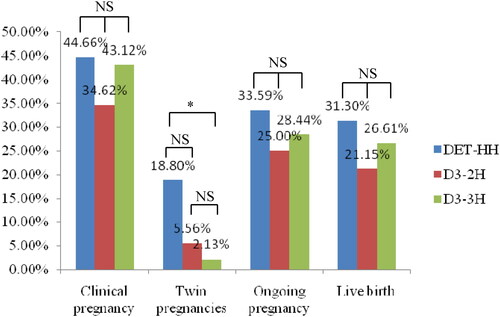
Figure 2. Blastocyst development and pregnancy outcomes of patients with day 3 embryos extended culture to blastocyst-stage between IVF and ICSI groups. *Was significantly different; NS: no significant differences.
We observed that the number of high-quality embryos on day 3 was independently associated with live birth rate for blastocyst transfers (OR: 1.133, 95% CI 1.023–1.256, p = .017) ().
Table 4. Prognostic parameters and odds ratios (OR) obtained from logistic regressions for live birth of patients with day 3 embryos extended culture to blastocyst-stage.
Discussion
It was well known that the female age was an independent factor affecting fertility and pregnancy outcome, and was also a key factor influencing ART success rate.
There was an evident decline in female fecundity with age. The decline was gradual over the reproductive life span of the woman and could be attributed to an increase in the miscarriage rate and a reduction in the conception rate [Citation13]. Thus, the need to optimize treatment for women of AMA has never been more pertinent.
Multiple pregnancies had a high prevalence of adverse pregnancy outcomes such as low birth weight, higher rates of very preterm birth, small for gestational age, perinatal mortality, and congenital malformations [Citation14]. Thus, single ET was increasingly recommended to reduce the twin pregnancies rate. Nevertheless, it was reported that the clinical pregnancy and live birth rates of AMA women showed nearly fourfold decrease when compared to women aged <30 years [Citation8]. Our results indicated that the clinical pregnancy and live birth rates of single cleavage-stage ET were only 19.86% and 12.06% in women of AMA. Due to the low implantation rate in women of AMA, many authors recommended the transfer of higher number of embryos to compensate for this, and hence improved the pregnancy outcomes.
In this study, we observed that the clinical pregnancy and live birth rates reached 36.54% and 24.75% for double cleavage-stage ETs in women of AMA. Especially for AMA women transferred with double high-quality cleavage-stage embryos, the clinical pregnancy, and live birth rates were 44.66% and 31.30%. Nevertheless, the twin pregnancies rate reached nearly 20% even if double low-quality cleavage-stage embryos were transferred which was consistent with previous report [Citation15]. It was concluded that the AMA women with twin pregnancies had higher maternal and perinatal complications with worse outcomes in comparison with younger women [Citation16]. It was recommended that a single ET should be offered in preference to multiple embryos when the AMA women were undergoing ART [Citation17]. Thus, single blastocyst transfer was an effective method to reduce the risk of multiple births without compromising the pregnancy outcomes.
It was reported that older women’s age was associated with delayed embryonic development which was more pronounced at later stages of development [Citation18]. Braga et al. indicated that the probability of blastocyst formation might be impaired when the morphology was compromised at the cleavage-stage [Citation9]. It prompted that the embryologists should be careful to culture the embryos to blastocyst-stage for some patients. In the study, the cycle rate of no high-quality blastocyst obtained was 25.00% and 19.80% for extended culture to blastocyst-stage with 2 and 3 high-quality embryos on day 3. For these patients with 2 and 3 high-quality embryos on day 3, the blastocyst transfers had similar clinical pregnancy, ongoing pregnancy, and live birth rates compared with the cleavage-stage ETs with double high-quality embryos. For patients with 4 or more high-quality embryos on day 3, the blastocyst transfers had significantly higher ongoing pregnancy and live birth rates compared with that for patients with 2 and 3 high-quality embryos on day 3. Thus, we recommended transfer at the blastocyst-stage for AMA women with ≥4 high-quality embryos on day 3.
A multivariate logistic regression analysis performed by Kim et al. on 2362 cycles in women ≥40 years of age showed that the number of high-quality embryos was a significant predictor of live birth which was consistent with our observation [Citation19]. Our data failed to confirm that the number of oocytes was an independent prognostic factor for live birth. Possible explanation was that the number of high-quality embryos prevailed, since it reflected not only quantity, but also quality of oocytes. After all, oocyte quality was the major determinant of embryo developmental competence [Citation20].
It was reported that the general quality of sperm was a good predictor of blastocyst formation, significantly affecting the likelihood of having at least one blastocyst at the end of the cycle [Citation21]. Previous researches indicated that the ICSI technique might result in impaired blastocyst development were not confirmed [Citation22,Citation23]. Subsequent research further indicated that no differences were found on sibling oocytes in the embryo development and blastocyst formation, irrespective of the fertilization procedure [24]. Thus, it was necessary to consider semen quality, fertilization procedure, and the number of high-quality cleavage-stage embryos when forecasting the possibility of blastocyst formation for women of AMA. We observed that the ICSI group showed significantly higher proportion of cycles with no good blastocysts than the IVF group. Nevertheless, the clinical pregnancy, twin pregnancies, ongoing pregnancy, and live birth rates showed no significant differences between the IVF and ICSI groups. Possible explanation was that the number of transferred embryos was increased when no good blastocyst was obtained for some women of AMA.
One of the major strengths of this study was the sample size. In addition, as this was a single-center study, the embryos were cultured in the same media and the strategy of extended-culture to blastocyst-stage was performed by the same standard, allowing elimination of the possible effects of various culture media and techniques on the results. Moreover, embryos were morphologically graded with the same criteria. Nevertheless, this study had some limitations deserve to be underlined. One of the major limitations of this study was the retrospective design. Because we included fresh cycles only, we could not eliminate the negative effects of controlled ovarian hyperstimulation on endometrial receptivity. Finally, because we included some ETs with single or double poor blastocysts, it might make some influence on the accuracy of the results.
In conclusion, extended culture to blastocyst-stage for transfer should be recommended for AMA women with ≥4 high-quality embryos on day 3. We should combine more factors to select the transfer strategy for AMA women with 2 or 3 high-quality embryos on day 3. Further multi-center prospective randomized clinical trials were needed to confirm our suggestion.
Supplemental Material
Download MS Word (29.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data used to support the findings of this study were available from the corresponding author upon request.
Additional information
Funding
References
- Mehmet BC, Linda JS, Claudio B, et al. Reproductive outcome of women 43 years and beyond undergoing ART treatment with their own oocytes in two Connecticut university programs. J Assist Reprod Genet. 2013;30(5):1–5.
- Suchartwatnachai C, Wongkularb A, Srisombut C, et al. Cost-effectiveness of IVF in women 38 years and older. Int J Gynaecol Obstet. 2000;69(2):143–148.
- Khalife D, Nassar A, Khalil A, et al. Cumulative Live-Birth rates by maternal age after one or multiple in vitro fertilization cycles: an institutional experience. Int J Fertil Steril. 2020;14(1):34–40.
- Wu JX, Lin S, Kong SB. Psychological stress and functional endometrial disorders: update of mechanism insights. Front Endocrinol (Lausanne). 2021;12:690255.
- Soullier N, Bouyer J, Pouly JL, et al. Effect of the woman’s age on discontinuation of IVF treatment. Reprod Biomed Online. 2011;22(5):496–500.
- Marcus SF, Brinsden PR. In-vitro fertilization and embryo transfer in women aged 40 years and over. Hum Reprod Update. 1996;2(6):459–468.
- Wang N, Zhao X, Ma M, et al. Effect of day 3 and day 5/6 embryo quality on the reproductive outcomes in the single vitrified embryo transfer cycles. Front Endocrinol (Lausanne). 2021;12:641623.
- Reljič M, Lovrec VG. Predictive factors for live birth in autologous in vitro fertilization cycles in women aged 40 years and older. Zdr Varst. 2019;58(4):173–178.
- Braga DP, Setti AS, Figueira RC, et al. The importance of the cleavage stage morphology evaluation for blastocyst transfer in patients with good prognosis. J Assist Reprod Genet. 2014;31(8):1105–1110.
- Shi WH, Zhang SL, Zhao WQ, et al. Factors related to clinical pregnancy after vitrified-warmed embryo transfer: a retrospective and multivariate logistic regression analysis of 2313 transfer cycles. Hum Reprod. 2013;28(7):1768–1775.
- Li MZ, Wang H, Ma C, et al. Transferring two grades I cleavage-stageembryo might not be a good protocol. Gynecol Endocrinol. 2017;33(7):557–559.
- Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–382.
- Armstrong S, Akande V. What is the best treatment option for infertile women aged 40 and over? J Assist Reprod Genet. 2013;30(5):667–671.
- Qin JB, Sheng XQ, Wang H, et al. Worldwide prevalence of adverse pregnancy outcomes associated with in vitro fertilization/intracytoplasmicsperm injection among multiple births: a systematic review and meta-analysis based on cohort studies. Arch Gynecol Obstet. 2017;295(3):577–597.
- Scotland GS, McLernon D, Kurinczuk JJ, et al. Minimising twins in in vitro fertilisation: a modelling study assessing the costs, consequences and cost-utility of elective single versus double embryo transfer over a 20-year time horizon. BJOG. 2011;118(9):1073–1083.
- Avnon T, Haham A, Many A. Twin pregnancy in women above the age of 45 years: maternal and neonatal outcomes. J Perinat Med. 2017;45(7):787–791.
- Avnon T, Ovental A, Many A. Twin versus singleton pregnancy in women ≥ 45 years of age: comparison of maternal and neonatal outcomes. J Matern Fetal Neonatal Med. 2021;34(2):201–206.
- Lebovitz O, Michaeli M, Aslih N, et al. Embryonic development in relation to maternal age and conception probability. Reprod Sci. 2021;28(8):2292–2300.
- Kim HO, Sung N, Song IO. Predictors of live birth and pregnancy success after in vitro fertilization in infertile women aged 40 and over. Clin Exp Reprod Med. 2017;44(2):111–117.
- Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103(2):317–322.
- Piccolomini MM, Bonetti TC, Motta E, et al. How general semen quality influences the blastocyst formation rate: analysis of 4205 IVF cycles. JBRA Assist Reprod. 2018;22(2):89–94.
- Shoukir Y, Chardonnens D, Campana A, et al. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: a paternal influence? Hum Reprod. 1998;13(6):1632–1637.
- Schoolcraft WB, Gardner DK, Lane M, et al. Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril. 1999;72(4):604–609.
- Van Landuyt L, De Vos A, Joris H, et al. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83(5):1397–1403.
