Abstract
Objective: Gestational diabetes mellitus (GDM) affects 7% of pregnant women worldwide. How to effectively treat GDM has always been a concern of people.Research methods: In this study, a diabetes model was established by drug-induced mice. Subsequently, the blood glucose levels and serum insulin changes of the mice after N-acetyl-l-cysteine (NAC) treatment were observed. At the same time, the effect of NAC on reproduction of GDM mice was recorded.Results of the study: Mice fed NAC showed significantly improved glucose tolerance and insulin sensitivity compared to Diabetic/Control. Total serum cholesterol, serum triglycerides, and serum low-density lipoprotein were significantly reduced, and atherosclerosis index was much lower than in control mice. In addition, Diabetic/Control mice had lower litter sizes and higher birth weights. NAC treatment significantly restored litter size and reduced birth weight in Diabetic/Control mice. It was found in WB assay that the NAC-fed group significantly increased nuclear Nrf2 and HO-1 expression levels.Conclusion: NAC can improve blood glucose tolerance in GDM mice; NAC effectively relieves the symptoms of hyperlipidemia caused by GDM; NAC enhances the expression of Nrf2/HO-1 in the liver, thereby restoring redox homeostasis. NAC can reduce gestational diabetes-related disease indicators by oral administration, and has a beneficial effect on the offspring of pregnant mice (reduces its diabetes disease indicators).
Introduction
Gestational diabetes mellitus (GDM) [Citation1–3] refers to varying degrees of hyperglycemia onset or first detected during pregnancy. GDM is a high-risk pregnancy that affects both the pregnant woman and the fetus. Although most metabolic disturbances in GDM can return to normal postpartum, the odds of developing type 2 diabetes are significantly increased postpartum. In addition, fetuses exposed to high-sugar environments had a significantly increased chance of developing diabetes in the future. Therefore, timely detection, comprehensive intervention, and individualized treatment of GDM have attracted great attention from obstetricians and endocrinologists.
The pathogenesis of GDM is complex, and one of the important factors is the prolonged stimulation of the body by hyperglycemia and hyperlipidemia, which leads to a continuous imbalance between the production of oxygen free radicals and antioxidant defense, and finally leads to severe damage to cells and tissues and the occurrence of disease. The main substance closely related to oxidative stress is reactive oxygen species (ROS). Under normal circumstances, autophagy plays an important role in the homeostasis of the intracellular environment, maintaining the structure and function of islet B cells, and maintaining the normal level of insulin content. In GDM, ROS-induced autophagy has a protective effect on B cells to a certain extent, but the excessive production of ROS leads to the accumulation of autophagosomes and causes autophagic cell death. Clearing the excessively generated ROS, reducing the oxidative stress of the body, and rationally regulating the moderate autophagy of B cells will become the main idea for the prevention and treatment of GDM.
N-Acetyl-l-cysteine (NAC) is a reduced glutathione precursor with strong anti-inflammatory and antioxidant effects. It is widely used in the respiratory system, digestive treatment of diseases of the system, and cardiovascular system. Several studies have shown that NAC can inhibit endothelial cell damage and improve vascular dysfunction by reducing oxidative stress levels and maintaining endothelial nitric oxide synthase (eNOS) activity. Michlin et al. [Citation4] reported the effect of NAC cysteine administered during pregnancy and lactation on the susceptibility of offspring to develop glucose intolerance in adulthood. The experimental results showed that isolated islets of NAC-treated progeny had higher efficacy of glucose-stimulated insulin secretion and higher ability to resist oxidative damage. Glucose tolerance and insulin sensitivity were improved in NAC-treated offspring after high-fat diet feeding. NAC depletion early in life improves glucose tolerance in mice in adulthood. Morgan [Citation5] explored the effects of NAC at different periods on heat stress-induced oxidative stress and inflammation in the hypothalamus of hens. NAC effectively alleviated the morphological changes of the hypothalamus induced by heat stress. Furthermore, NAC attenuated the activity of the Nf-B pathway activated by heat stress and decreased the expression of proinflammatory cytokines Interleukin 6 (IL-6), IL-18, Tumour Necrosis Factor alpha (TNF-α), IkappaB kinase (IKK), and Interferon-gamma (IFN-γ). However, the effect of NAC on GDM-related oxidative stress and vascular function remains unclear. It has been reported that the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling pathway, an important antioxidant stress pathway, plays a crucial role in the prevention of GDM. Studies have found that the Nrf2/HO-1 pathway can be activated by repeated hypoxia reoxygenation treatment and then improve GDM [Citation6]. In addition, NAC activated Nrf2/HO-1 signaling pathway has been considered as a therapeutic approach for diseases such as neuropsychiatric disorders, renal ischemia/reperfusion injury, and liver injury [Citation7–9].
Therefore, this article constructs a diabetes model by drug-induced mice. The changes of blood glucose level and serum insulin of mice after NAC treatment were observed. The results showed that NAC reduced the glycemic intolerance, hyperlipidemia, and its biochemical indicators caused by diabetes by alleviating the oxidative stress response in gestational diabetic mice. And reduce the prevalence of diabetes indicators in their offspring.
Materials and methods
Animals and experimental setup
Eight-week-old C57BL/KsJ WT and GDM mice were used in this study; mice were ordered from Shanghai Experimental Animal Center. Mice were reared under standard rearing conditions. All mouse studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Hospital Institutional Animal Care and Use Committee.
GDM mouse model construction and NAC administration
Diabetic mice were constructed with reference to the previous literature [Citation10]. Briefly, female rats were housed in a temperature-controlled room (22 ± 1 °C) with alternating 12-h light and dark cycles and fed a standard diet. Diabetes mellitus (Met) was induced by administering a single intravenous dose of streptozotocin (45 mg/kg) in citrate buffer (0.05 M) (pH 4.5). These animals were mated with untreated control animals. On the same day that sperm appeared on the vaginal smear (gestation day 0), they were divided into the following experimental groups: two groups of diabetic animals were studied, one without any supplementation (Diabetic/Contol) and the other by oral administration Supplement with 150 mg/d of NAC (Diabetic/NAC).
Glucose tolerance test and insulin tolerance test
Glucose tolerance test (GTT) and insulin tolerance test (ITT) are performed on day 15 of pregnancy. Treated mice were fasted for 6 h prior to GTT measurements. Administered 2 g of glucose per kg by intraperitoneal (IP) injection. Blood glucose levels were measured at 0, 30, and 60 min after IP. For ITT, treated mice were fasted for 6 h and then injected intraperitoneally with 0.75 U/kg insulin. Blood glucose levels were measured at 0, 30, and 60 min after IP. Blood glucose levels were measured by a glucometer (Roche, Basel, Switzerland) and insulin levels were determined by an insulin mouse ELISA kit (EMINS, Thermo Fisher Scientific, Waltham, USA).
Biochemical index analysis
For blood parameter measurements, GTT and ITT were tested as described above (6-h fast); other measurements were performed under fed conditions. Wild-type and female mice were euthanized on day 19 of gestation. Blood was centrifuged at 1000 g for 10 min at 4 °C to collect serum and then stored at –80 °C for subsequent analysis. Liver tissue was harvested and stored at −80 °C for biochemical analysis. Total serum cholesterol (TChl), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and Malondialdehyde (MDA) levels were measured using a total cholesterol assay kit, serum triglyceride quantification kit, HDL and LDL cholesterol assay kit, and lipid peroxidation MDA assay kit. Liver lysates were prepared as previously described. Liver MDA, Superoxide dismutase (SOD), GPx, Glutathione (GSH), and CAT using Lipid Peroxidation MDA Assay Kit, Total SOD Assay Kit and NBT, Total Glutathione Peroxidase Assay Kit, Glutathione reductase detection kit measurement, and catalase detection kit were purchased from Beyotime Biotechnology (Shanghai, China).
Western blot analysis
Liver tissue (500 mg) was triturated on ice with 2 mL cold buffer A (10 mM Tris-HCl (pH 7.4), 5 mM MgCl2 and 250 mM sucrose) through a cell strainer (40 μm). Centrifuged the tissue lysate at 500 g for 15 min at 4 °C. The pellet was washed one more time with buffer A, then gently resuspended in 8 volumes of buffer B (10 mM Tris-HCl (pH 7.4), 1 mM MgCl2, and 2 M sucrose). Centrifuged the mixture at 16,000 g for 30 min at 4 °C. Carefully removed the upper layer (brown). The granules in the bottom layer (white) are the nuclei. Liver nuclear lysates were analyzed by immunoblotting as previously described. Mouse Nrf2 (1:1000) and HO-1 (1:1000) primary antibodies were purchased from Abcam (Cambridge, UK); histone 3 antibody (1:2000) was obtained from CST, Inc. (Danvers, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism. One-way or two-way analysis of variance (ANOVA) and Bonferroni’s post hoc test was used to analyze differences between groups. Data are presented as mean ± standard deviation (SD).
Results
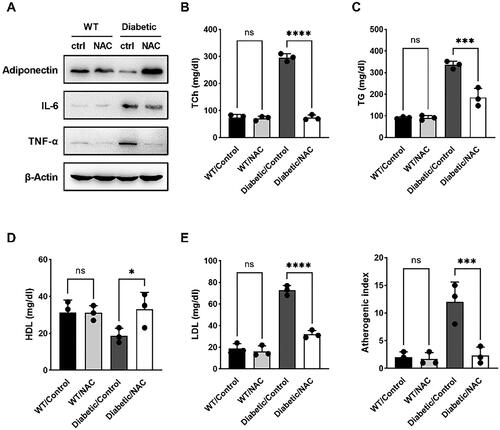
NAC can relieve the symptoms of hyperlipidemia caused by GDM
To further explore the metabolic benefits of NAC administration, a series of biochemical markers were measured in the serum of pregnant mice on day 19 of gestation. The results of Western blot were shown in , the expressions of IL-6 and TNF-α in the Diabetic/NAC group were significantly lower than those in the mice. TCh, serum TG, and serum LDL were significantly reduced in NAC-treated pregnant mice compared with Diabetic/Control (). Furthermore, the atherosclerotic index of NAC-fed pregnant mice was much lower than that of control mice (). This is consistent with the GTT and ITT results, further showing that NAC improved biochemical parameters and improved metabolic homeostasis in GDM mice in the third trimester.
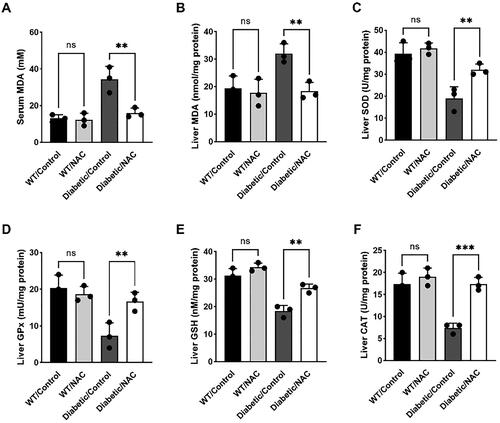
NAC inhibits maternal oxidative stress in pregnant GDM mice
Maternal oxidative stress is the main factor leading to insulin resistance and plays a key role in the occurrence and development of GDM. In this study, we investigated oxidative stress parameters in GDM mice given or not given astaxanthin on day 19 of gestation. Serum and liver MDA levels were elevated in Diabetic/Control mice relative to the WT group. However, they were all significantly inhibited after NAC treatment, and feeding treatment induced a similar trend (). We further examined the activity of first-line defense antioxidants in the liver, including SOD, GPx, and CAT. The results showed that NAC administration significantly increased the activity of these defensive antioxidants in pregnant mice compared to untreated Diabetic/Control mice, which was similar to the positive control group (). Furthermore, the concentration of glutathione, a powerful antioxidant capable of preventing oxidative damage, was significantly elevated in the livers of NAC-treated Diabetic/Control mice (). The above results suggest that NAC administration suppressed maternal oxidative stress in pregnant GDM mice.
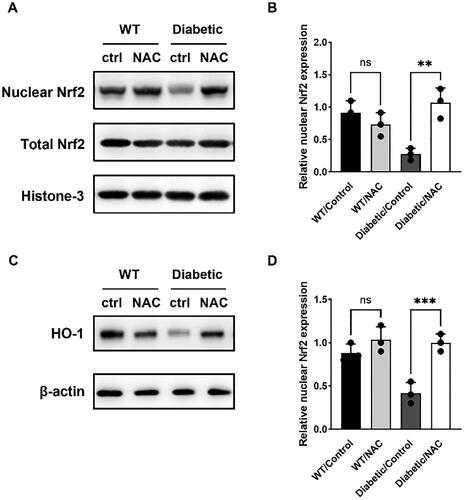
NAC activates Nrf2/HO-1 signaling pathway in pregnant GDM mice
The Nrf2/HO-1 signaling pathway has been reported to be a master transcriptional regulator of antioxidant genes and restoration of redox homeostasis. We investigated the activation of Nrf2/HO-1 signaling pathway in the liver of pregnant mice by astaxanthin using western blotting. Nuclear Nrf2 expression was lower in Diabetic/Control mice compared to wild-type mice (). Thus, Nrf2-dependent gene that removes toxic heme and protects against oxidative damage, heme oxygenase-1 (HO-1), was also downregulated in Diabetic/Control mice, while NAC feeding significantly increased nuclear Nrf2 and HO-1 levels (). These data suggest that astaxanthin activates the Nrf2/HO-1 signaling pathway and upregulates oxidative capacity in GDM mice.
Discussion
GDM has become a global public health problem, affecting 7% of pregnant women worldwide. Most women with GDM appear to have chronic insulin resistance and hyperglycemia, suggesting relatively similar causes and symptoms between GDM and diabetes. Given the pathogenic role of oxidative stress in the pathogenesis of insulin resistance and diabetes, it is highly likely that the beneficial antioxidants for diabetes and dyslipidemia such as NAC in this study have similar functions in the prevention and treatment of GDM. Although metformin (Met) and insulin are two commonly used drugs for the treatment of gestational diabetes mellitus, the clinical practice of insulin is inconvenient due to the unpopular route of administration and the potential for weight gain and hypoglycemia. Our findings suggest that NAC is able to significantly alleviate GDM symptoms by activating antioxidant enzymes and restoring the Nrf2/HO-1 signaling pathway. We observed that NAC treatment significantly improved glucose intolerance and β-cell insufficiency in pregnant GDM mice but not in non-pregnant mice.
NAC is an antioxidant that can increase the body’s glutathione (GSH) content, regulate gene expression and signal transduction systems, inhibit and scavenge oxygen free radicals that can penetrate cell membranes, and protect tissues and organs. NAC can react with MGO to generate N-α-acetyl-S-(1-hydroxy-2-oxo-prop-1-yl) cysteine. Therefore, administration of NAC-containing drugs may reduce the levels of highly reactive intermediates, thereby inhibiting the production of Advanced Glycation end products (AGEs) and diabetic damage, thereby benefiting diabetic patients [Citation11]. Of course, NAC can also play an antioxidant role. For example, studies have shown that NAC can improve wound healing in diabetic mice by scavenging free radicals [Citation12]. Kumar et al. [Citation13] found through mouse experiments that pretreatment of cells with NAC could reduce high glucose-induced cytotoxicity by increasing the levels of antioxidant enzymes and reducing the number of apoptotic cells.
Author contributions
Study concept and design: SSX, ZZG, JL; collection of clinical specimen and data: SSX, ZZG, MZ, JFC, LYY, HYH; statistical analysis of data: SSX, ZZG, MZ, YZ; manuscript writing and revision: SSX, ZZG, JFC, LYY, YZ. All authors read and approved the final manuscript
Supplemental Material
Download MS Word (168 KB)Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The original data supporting these findings are available at any time upon request to the corresponding author.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):1.
- Tang XW, Qin QX. miR-335-5p induces insulin resistance and pancreatic islet β-cell secretion in gestational diabetes mellitus mice through VASH1-mediated TGF-β signaling pathway. J Cell Physiol. 2019;234(5):6654–6666.
- Kong M, Liu C, Guo Y, et al. Higher level of GGT during mid-pregnancy is associated with increased risk of gestational diabetes mellitus. Clin Endocrinol. 2018;88(5):700–5.
- Michlin M, Argaev-Frenkel L, Weinstein-Fudim L, et al. Maternal N-Acetyl cysteine intake improved glucose tolerance in obese mice offspring. Int J Mol Sci. 2020;21(6):1981.
- Morgan SC, Relaix F, Sandell LL, et al. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defect Res A. 2008;82(6):453–463.
- Hou X, Zhang J, Ma H, et al. Hypoxia-reoxygenation treatment attenuates gestational diabetes mellitus. Endocr Connect. 2021;10(1):84–91.
- Morris G, Walker A, Walder K, et al. Increasing Nrf2 activity as a treatment approach in neuropsychiatry. Mol Neurobiol. 2021;58(5):2158–2182.
- Zhang L, Zhu Z, Liu J, et al. Protective effect of N-acetylcysteine (NAC) on renal ischemia/reperfusion injury through Nrf2 signaling pathway. J Recept Signal Transduct Res. 2014;34(5):396–400.
- Cai Z, Lou Q, Wang F, et al. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int J Clin Exp Pathol. 2015;8:8655.
- Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31(5):680–701.
- Wild R, Ooi L, Srikanth V, et al. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the-N-acetyl-l-cysteine assay. Anal Bioanal Chem. 2012;403(9):2577–2581.
- Aktunc E, Ozacmak VH, Ozacmak HS, et al. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol. 2010;35(8):902–909.
- Kumar P, Rao GN, Pal BB, et al. Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoG-DNA glycosylase levels in glial cells. Int J Biochem Cell Biol. 2014;53:302–319.