Abstract
Objective
To establish a cutoff level of AMH which could help for the diagnosis of PCOS, to investigate the predictive value of AMH combined with androgens in Chinese women to diagnose PCOS.
Materials and Methods
This is a prospective case control study, 550 women recruited (aged 20–40 years), in which 450 PCOS women recruited according to the Rotterdam criteria and 100 non-PCOS women in the control group were from the women for the pregnancy preparation examination. AMH were measured by the Elecsys AMH Plus immunoassay. Androgens and other sex hormone were measured. The validity of AMH toward the diagnosis of PCOS, or AMH combined with total testosterone, free testosterone, bioavailable testosterone and androstenedione was estimated by receiver operating characteristic (ROC)curves, and correlations between paired variables was estimated by Spearman’s rank correlation coefficient.
Results
The cutoff value of AMH in Chinese reproductive-age women with PCOS is 4.64 ng/mL, AUC under the curve is 0.938, with 81.6% sensitivity, and 92.0% specificity. Total testosterone, free testosterone, bioactive testosterone, and androstenedione are significantly higher in women with PCOS of reproductive age than in controls. The combination of AMH and free testosterone resulted in a higher AUC of 94.8%, with higher sensitivity (86.1%) and excellent specificity (90.3%) for the prediction of PCOS.
Conclusion
The Elecsys AMH Plus immunoassay, with a cutoff of 4.64 ng/mL, is a robust method for identifying PCOM to aid in PCOS diagnosis. The combination of AMH and free testosterone resulted in a higher AUC of 94.8% for the diagnose of PCOS.
Introduction
Polycystic ovary syndrome is a highly heterogeneous disease often associated with obesity, hyperandrogenemia, insulin resistance and ovulatory dysfunction. The global prevalence of polycystic ovary syndrome is 5% to 20% [Citation1]. The prevalence of abdominal obesity in polycystic ovary syndrome is as high as 38–88% [Citation2,Citation3]. Patients with polycystic ovary syndrome often have a combination of metabolic and endocrine disorders, and polycystic ovary syndrome increases the risk of cardiovascular disease, metabolic disease and reduces the overall quality of life of women [Citation4–6]. Despite the fact that polycystic ovary syndrome is the most common endocrine disorder, it remains undiagnosed in up to 70% of women [Citation7,Citation8]. According to the Rotterdam criteria, polycystic ovarian syndrome is diagnosed by anovulation or amenorrhea, hyperandrogenemia (HA) and/or clinical features of its associated disorders (e.g. hirsutism, acne, and androgenetic alopecia) and polycystic ovarian morphology (PCOM; e.g. follicles 2–9 mm in diameter in one or both ovaries). The diagnosis of polycystic ovarian syndrome is confirmed by at least two of these three criteria [Citation2].
Anti-Müllerian-hormone (AMH) is secreted by granulosa cells of the antral follicles and small sinus follicles and is a member of the transforming-growth factor beta (TGF-β) family, responsible for regulating follicular growth and development [Citation9,Citation10]. Studies have shown a good correlation between anti-müllerian hormone levels and the number of 2–9 mm sinus follicles in the ovary, and that anti-müllerian hormone levels can stably respond to the number of follicles in the primordial follicular pool [Citation11,Citation12]. Anti-Müllerian hormone has recently emerged as the most promising biological marker for quantifying ovarian reserve, and women with polycystic ovary syndrome have higher AMH levels compared to healthy women, while AMH levels decline as women age [Citation13,Citation14], and higher AMH levels are associated with menstrual disorders and ovulatory disturbances [Citation15,Citation16]. A previous study by our team found that AMH values were 2–3 times higher in women with polycystic ovary syndrome compared to those in normal women [Citation17]. We also found that women with polycystic ovary syndrome have an increased probability of developing gestational diabetes, gestational hypertension, and preterm delivery, and preconception hypoandrogenic pretreatment improves adverse maternal outcomes in polycystic ovary syndrome [Citation18]. Therefore, for women of reproductive age with fertility needs, early diagnosis of PCOS is not only relevant to women’s health but also crucial to improve pregnancy outcomes in women with PCOS [Citation19]. However, the current use of anti-mullerian hormone as a biomarker for PCOM still requires the derivation of AMH cutoff/threshold values in large samples and validation in different age groups of female population [Citation20, Citation21].
Materials and methods
Patients and blood samples
This is a prospective case control study which recruited 550 Chinese women who visited department of Gynecological Endocrinology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, from October 2020 to October 2022, among which 450 patients had polycystic ovary syndrome (study group) and 100 non-PCOS women (control group). Inclusion criteria for polycystic ovary syndrome were based on the Rotterdam criteria for patients who met at least 2 of the following 3 criteria: ovulatory dysfunction, HA, and polycystic ovarian morphology (PCOM) (AFC ≥12 per ovary), [Citation2]. The exclusion criteria were congenital adrenal hyperplasia, Cushing syndrome, androgen-secreting tumors, and the use of oral contraceptives.
The inclusion criteria for the control group were women aged 20–40 years control group were from the women for the pregnancy preparation examination. with regular menstrual cycles (mean 25–35 days), no uterine or ovarian abnormalities on vaginal ultrasound, normal ovarian function, no clinical manifestations of hyperandrogenism/hyperandrogenemia, and no polycystic ovarian changes. Exclusion criteria: (1) current or within 2 months prior to enrollment treatment with sex steroid hormones affecting the hypothalamic-ovarian axis (3 months of treatment with long-acting agents); (2) disorders related to coagulation dysfunction; (3) Cushing’s syndrome; (4) Abnormal thyroid function(TSH ≤ 0.55mIU/L or TSH ≥ 4.78mIU/L); (5) hypothalamic-pituitary amenorrhea; (6) hyperprolactinemia; (7) premature ovarian failure; (8) functional ovarian tumors; (9) follicular membrane cell proliferation syndrome; (10) functional uterine bleeding; and (11) known or suspected pregnancy. This study has been approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, with ethical approval number: 2019-KY-56-01, and all subjects signed an informed consent form.
Clinical and laboratory assessments
Age, height, body mass, body mass index (BMI), waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure were collected. Blood samples were collected on the second to third day of the menstrual cycle, and in amenorrheic cases, blood was drawn when no follicles ≥10 mm in diameter were seen on ultrasound, and blood was collected from the brachial vein between 8:00 to 10:00 am after 12 h of fasting. The antral follicle count was determined using transvaginal ultrasound. In amenorrheic cases, we measure progesterone and human chorionic gonadotropin to exclude possible ovulation or pregnancy. Serum anti-müllerian hormone was determined by the Elecsys AMH Plus immunoassay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), prolactin (PRL), progesterone (P), thyroid stimulating hormone (TSH), cortisol (F) were determined by ADVIA Centaur XP automatic chemiluminescence immunoassay produced by Siemens Company in Germany. Total testosterone (T), free testosterone (FT) (calculated), sex hormone binding globulin (SHBG) (calculated on website http://www.issam.ch/freetesto.htm), bioavailable testosterone (BIOT) (calculated),17-hydroxyprogesterone (17-OHP), and dehydroepiandrosterone sulfate (DHEAS), androstenedione (A2), were determined by liquid chromatography-mass spectrometry tandem method (LC-MS/MS), which were performed using an AB Sciex 5500 mass spectrometer coupled with a Shimadzu Nexera X2 high-performance liquid chromatography (HPLC) system[Citation22].
Statistical analyses
All the data were analyzed by SPSS version 22.0 (IBM Corp., Armonk, NY). Kolmogorov-Smirnov test for normality was performed on continuous variables. The quantitative variables which were normally distributed were presented as mean ± standard deviation (SD), mean differences between case and controls were compared by independent sample t-tests. Values not normally distributed were presented as the median and interquartile range (IQR), Mann–Whitney U-test was used for continuous variables that did not show normal distribution. The validity of AMH toward the diagnosis of PCOS, or AMH combined with free testosterone, total testosterone, BioT and A2 was estimated by receiver operating characteristic (ROC) curves. The area under the ROC curve (AUC) with specificity, sensitivity, and 95% confidence interval (CI) were computed in order to analyze the diagnostic efficacy of the AMH and other indexes for PCOS. The optimal cutoff value was determined by calculating the Youden index. After adjusting for age and BMI, logistic regression analysis was performed to verify the association between the occurrence of PCOS (dependent) and the serum concentration of AMH and other variable(independent). Correlations between paired variables was estimated by Spearman’s rank correlation coefficient. All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Clinical data
There were no significant differences in age between polycystic ovary syndrome and the controls(p = 0.074). Weight, BMI, waist circumference and hip circumference were significantly higher in patients with polycystic ovary syndrome than in the controls (p < 0.001, p < 0.001, p < 0.001, p < 0.001), and systolic and diastolic blood pressure were not statistically different in the PCOS group compared with the control group (p = 0.054, p = 0.100) ().
Table 1. General parameters of 450 PCOS patients and 100 controls.
Laboratory tests
Serum anti-Müllerian hormone was significantly higher in patients with polycystic ovary syndrome than in controls (8.06 ± 4.02 ng/mL vs 2.39 ± 1.38 ng/mL, p < 0.001) (). The serum LH,T, FT, DHEAS, BioT, and A2 of the PCOS group were 11.46 ± 7.37 IU/L vs 5.00±2.20IU/L, 440 ± 290pg/mL vs 279±123pg/mL, 7.79 ± 6.38 pg/mL vs 4.05±1.80pg/mL, 2516.91(1979.58–3207.37) ng/mL vs 1709.36(1469.21-2234.07)ng/mL, 189 ± 119pg/mL vs 101±45pg/mL, 1.83 ± 0.84 ng/mL vs 1.13±0.46 ng/mL, respectively, which were significantly higher than those in the non-PCOS group (p < 0.001). SHBG was significantly lower in the PCOS group than in the control group (32.9 (19.7–51.5) nmol/L vs 47.7 (30.1–62.6) nmol/L p < 0.001). Prolactin levels were significantly higher in the PCOS group than in the control group (12.35 (7.3–13.4) ng/mL vs 11.9 (8.8–16.7) ng/mL p = 0.011). There was no significant difference in FSH, E2, P, TSH and F between patients with polycystic ovary syndrome and control group ().
Figure 1. Serum AMH concentrations in PCOS versus the controls. Data were presented as mean standard deviation of the mean. AMH: anti-Müllerian hormone; PCOS: polycystic ovary syndrome.
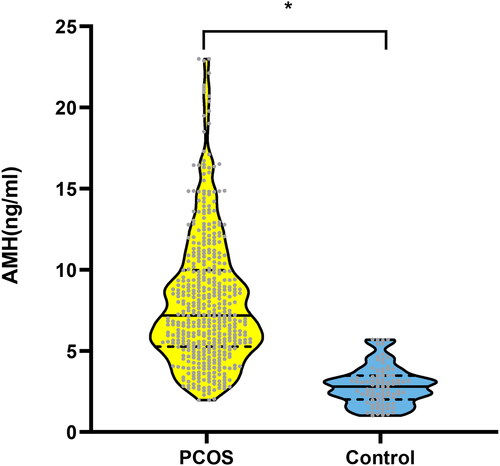
Table 2. The androgens, AMH and LH/FSH ratio of women in the PCOS and controls group.
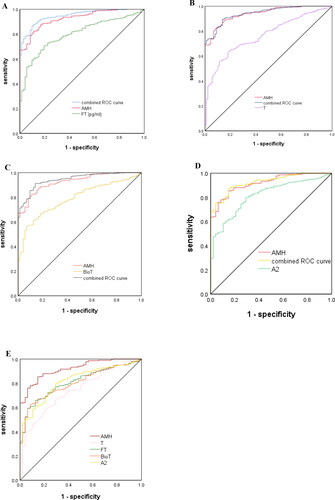
ROC curves were used to estimate the diagnostic performance of AMH, which was then compared and combined with the levels of total testosterone, free testosterone, BioT and A2 (). The AUC of AMH was 93.8% (95%CI 91.7%-95.9%), with a cutoff value of 4.64 ng/mL, and sensitivity and specificity of PCOS prediction of 81.6%(95% CI 78.1%-85.1%) and 92.0%(95%CI 86.3%-97.8%), respectively. However, the combination of AMH and free testosterone resulted in a higher AUC of 94.8%, with higher sensitivity (86.1%) and excellent specificity (90.3%) for the prediction of PCOS (). When AMH and total testosterone were combined, the AUC was 94.3%, with excellent diagnostic sensitivity (89.5%) and specificity (86.4%) for predicting PCOS (). The AUC of PCOS was predicted to be 94.4% for the combination of AMH and BioT, with sensitivity (91.2%) and a moderate diagnostic specificity (86.1.0%) (). The combination of A2 and AMH resulted in the AUC of 92.7%, with sensitivity (87.3%) and moderate specificity (85.4%) for diagnose of PCOS ().
Figure 2. (A) Combined curve of AMH with free testosterone. (B) Combined curve of AMH with total testosterone. (C) Combined curve of AMH with BioT. (D) Combined curve of AMH with A2. (E) Diagnostic potential of AMH, total testosterone, free testosterone, BioT and A2 for PCOS estimated using ROC analysis.
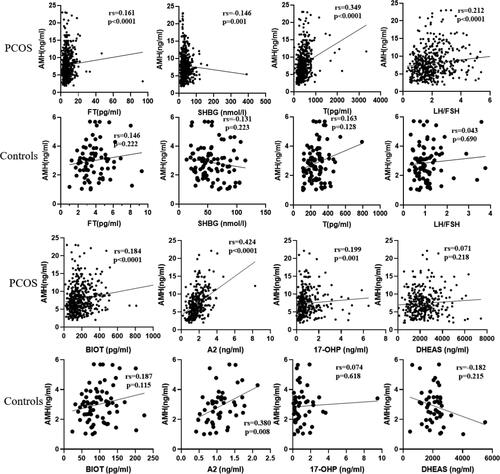
After adjusting age and BMI, binary logistic regression was used to evaluate the effect of AMH, FT, TT, BIOT, and LH/FSH indicators on the diagnosis of PCOS. The logistic regression revealed that an elevation of serum AMH level was correlated with a high risk of PCOS (odds ratio= 2.998; 95% CI= 2.186–4.111 p < 0.001). Relationship of AMH to total and free testosterone, sex hormone binding globulin, bioavailable testosterone, androstenedione, dehydroepiandrosterone sulfate, 17-hydroxypr-ogesterone and LH/FSH ratio in PCOS and controls were showed in . In PCOS patients AMH positively correlated with total and free testosterone, BIOT, A2, LH/FSH, AMH was negatively correlated with SHBG in PCOS and controls. And there were no significant correlations between AMH and androgen and LH/FSH ratio in controls patients.
Figure 3. Relationship of AMH to FT, SHBG, total testosterone, and LH FSH ratio in PCOS (upper panel) and controls (down panel).
Abbreviation: AMH: anti-Müllerian hormone; PCOS: polycystic ovary syndrome; SHBG: sex hormone binding globulin; FT: free testosterone; T:total testosterone; A2:androstenedione; BIOT: bioavailable testosterone; DHEAS: dehydroepiandrosterone sulfate; 17-OHP: 17hydroxypro-gesterone, LH/FSH: LH FSH ratio.
Discussion
In this study, we apply the Elecsys AMH Plus immunoassay ((Roche Diagnostics International Ltd, Rotkreuz, Switzerland) to establish the cutoff value of serum AMH in diagnoses of polycystic ovary syndrome. Our aim was not to predict any metabolic or endocrine abnormalities in this population. Our investigation indicated that serum AMH value are two to three times higher in women with polycystic ovary syndrome compared to controls. Our study result is consistent with previous international studies [Citation23–25]. And the results remained the same when grouped according to BMI, overweight or obese women with PCOS had significantly higher AMH value than controls with comparable BMI, AMH was also significantly higher in lean PCOS than in lean controls. The Elecsys AMH Plus immunoassay, with a cutoff value of 4.64 ng/mL, predictive power of 93.8%, is a robust method for identifying PCOM to aid in PCOS diagnosis. Combining AMH, with free testosterone into a multivariate model showed a predictive power of 94.8% for diagnosing PCOS in reproductive-aged women. The predictive model of AMH combined with free testosterone can improve the diagnostic efficacy of PCOS and provide a robust method for clinician to diagnose PCOS.
Anti-Müllerian hormone is secreted by granulosa cells of the antral follicles and is a member of the transforming-growth factor beta (TGF-β) family, is recognized as a regulator of initial follicular recruitment from the primordial pool [Citation9,Citation10]. Several previous studies suggested that serum AMH is more sensitive and specific than AFC in diagnosing PCOM and PCOS in adult women [Citation23]. The cutoff value of AMH vary among studies because of the use of different AMH testing assay, different diagnostic criteria for PCOS and genetical and other differences in the patient populations. The Elecsys AMH Plus immunoassay is a fully automated testing method (coefficient of variation within and between batches less than 5%) which is used for serum AMH measurements in this study and this is a standardized, robust assay, which has been approved and is globally accessible [Citation23].
High anti-müllerian hormone levels are associated with menstrual disorders and ovulation disorders. In addition, daughters of women with PCOS have a 60–70% probability of developing PCOS [Citation26,Citation27] and daughters of mothers with PCOS have a 5-fold increased risk of being diagnosed with PCOS [Citation28]. This may be related to hyperandrogenemia and high AMH prenatal exposure in women with PCOS [Citation29]. Therefore, prenatal pretreatment of women with PCOS to reduce androgen levels and AMH levels may improve pregnancy outcomes. AMH test can also be part of method to evaluate the therapeutic effect of PCOS. Serum androgen was detected by LCMS/MS method, which is recommended and considered as the ‘gold standard’ method for female androgen quantification [Citation30,Citation31]. We compared endocrine hormone and androgen in Chinese women of reproductive age with polycystic ovary syndrome and controls, and our study found that AMH levels correlate positively with free testosterone, total testosterone, bioavailable testosterone, androstenedione, 17-hydroxyprogesterone and LH/FSH in PCOS patients.
Notably, The BMI of the PCOS patients were highly significantly than that of the controls (25.03 ± 4.98 kg/m2 vs 22.49 ± 2.89 kg/m2, p < 0.001). 54.27% of the women with PCOS were overweight; among them, 25.4% were obese (definition according to Chinese guidelines: overweight: BMI ≥ 24kg/m2, obesity: BMI ≥ 28 kg/m2). Accordingly, the waist circumference of women with PCOS was higher than that of the control-group (84.91 ± 12.32 cm vs 78.83 ± 9.74 cm, p < 0.001). Both obese and overweight people are increasing worldwide and have detrimental influences on several human body functions including the reproductive health [Citation32]. Particularly, obese women undergo perturbations of the ‘hypothalamic pituitary ovarian axis’, and frequently suffer from menstrual dysfunction leading to anovulation and infertility. Weight loss through lifestyle modification in obese women, have been identified to restore menstrual cyclicity and ovulation and improve the likelihood of conception.
Strengths and limitations
The strength of this study is that all enrolled patients come from the department of Gynecological Endocrinology, Beijing Obstetrics and Gynecology Hospital, and precise data of height, weight, waist circumference and hip circumference are collected. Detailed baseline data for subsequent studies are provided. We use the Elecsys AMH Plus immunoassay, an internationally recognized detection methods to detect AMH. In addition, we use LCMS/MS method to detect androgen, which can accurately detect the androgen level of women, and is useful for the diagnosis of PCOS. The combination of two precise detection methods can help to predict PCOS more accurately.
The limitation of this study is that the included patients are aged between 20 to 40 years old, and did not include adolescents and women over 40 years old. Therefore, it has no predictive ability for PCOS women in adolescents and women over 40 years old. Chinese population included in this study, the AMH cutoff value of PCOS women in different regions are different, and there is no predictive ability for the population in other regions.
Conclusion
The Elecsys AMH Plus immunoassay, with a cutoff of 4.64 ng/mL, is a robust method for identifying PCOM to aid in PCOS diagnosis. The combination of AMH and free testosterone resulted in a higher AUC of 94.8% for the diagnose of PCOS.
Author contributions
All authors have certified the author list and the contribution description. All authors have read and approved the submitted manuscript and any substantially modified version of the manuscript. Contribution to work: Xiangyan Ruan was involved in study conception and design; Lingling Jiang and Yanqiu Li, Muqing Gu, Jiaojiao Cheng, Yuejiao Wang, Yu Yang, Lili Liu, Che Xu were involved in patient recruitment; Lingling Jiang were involved in performing the experiments, and data acquisition, analysis and interpretation; Lingling Jiang drafted the article and Xiangyan Ruan and Alfred O. Mueck critically reviewed and approved the final article; Zhikun Wang contributed to the statistical analysis. The authors especially thank Prof. Xingming Li of Capital Medical University (Beijing, China) for his assistance in the data’s statistical analysis.
Ethics statement
This study was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (approval number: 2019-KY-56-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Acknowledgement
The authors express sincere thanks to Prof. Xingming Li, statistical expert from Capital Medical University, Beijing, China, for supporting in statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome, nature reviews. Dis Primers. 2016;2:1.
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility Sterility. 2004;81:19–7.
- Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism. 2019;92:108–120.
- Moran LJ, Brown WJ, McNaughton SA, et al. Weight management practices associated with PCOS and their relationships with diet and physical activity. Hum Reprod. 2017;32(3):669–678.
- Szczuko M, Kikut J, Szczuko U, et al. Nutrition strategy and life style in polycystic ovary syndrome-narrative review. Nutrients. 2021;13(7):2452.
- Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30(7):399–404.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284.
- Detti L, Fletcher NM, Saed GM, et al. Anti-Müllerian hormone (AMH) may stall ovarian cortex function through modulation of hormone receptors other than the AMH receptor. Reprod Sci. 2018;25(8):1218–1223.
- Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–3373.
- Dumont A, Robin G, Dewailly D. Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):377–384.
- Jacobs MH, Reuter LM, Baker VL, et al. A multicentre evaluation of the Elecsys(®) anti-Müllerian hormone immunoassay for prediction of antral follicle count. Reprod Biomed Online. 2019;38(5):845–852.
- Cimino I, Casoni F, Liu X, et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055.
- Evliyaoglu O, Imöhl M, Weiskirchen R, et al. Age-specific reference values improve the diagnostic performance of AMH in polycystic ovary syndrome. Clin Chem Lab Med. 2020;58(8):1291–1301.
- Abbara A, Eng PC, Phylactou M, et al. Anti-Müllerian hormone (AMH) in the diagnosis of menstrual disturbance due to polycystic ovarian syndrome. Front Endocrinol (Lausanne). 2019;10:656.
- Alebic M, Stojanovic N, Dewailly D. Discordance between serum anti-Müllerian hormone concentrations and antral follicle counts: not only technical issues. Hum Reprod. 2018;33(6):1141–1148.
- Tian X, Ruan X, Mueck AO, et al. Anti-Müllerian hormone levels in women with polycystic ovarian syndrome compared with normal women of reproductive age in China. Gynecol Endocrinol. 2014;30(2):126–129.
- Li Y, Ruan X, Wang H, et al. Comparing the risk of adverse pregnancy outcomes of Chinese patients with polycystic ovary syndrome with and without antiandrogenic pretreatment. Fertil Steril. 2018;109(4):720–727.
- Cedars MI. Evaluation of female fertility-AMH and ovarian reserve testing. J Clin Endocrinol Metab. 2022;107(6):1510–1519.
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human Reprod (Oxford, England). 2018;33(9):1602–1618.
- Teede H, Misso M, Tassone EC, et al. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30(7):467–478.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Dietz de Loos A, Hund M, Buck K, et al. Antimüllerian hormone to determine polycystic ovarian morphology. Fertil Steril. 2021;116(4):1149–1157.
- Tal R, Seifer DB, Khanimov M, et al. Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(1):e51-58–59.e8. 59.
- Wongwananuruk T, Panichyawat N, Indhavivadhana S, et al. Accuracy of anti-Müllerian hormone and total follicles count to diagnose polycystic ovary syndrome in reproductive women. Taiwan J Obstet Gynecol. 2018;57(4):499–506.
- Mimouni NEH, Paiva I, Barbotin AL, et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021;33(3):513–530.e8. e518.
- Crisosto N, Ladrón de Guevara A, Echiburú B, et al. Higher luteinizing hormone levels associated with antimüllerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil Steril. 2019;111(2):381–388.
- Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–1904.
- Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846.
- Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract. 2015;21(11):1291–1300.
- Silvestris E, de Pergola G, Rosania R, et al. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22.
