Abstract
Background
Neurokinin B (NKB) belongs to the tachykinin family of proteins who’s regulation is essential for proper function of the reproductive system. It has been shown that patients with functional hypothalamic amenorrhea (FHA) exhibit decreased levels of serum kisspeptin. As kisspeptin secretion is regulated by NKB signaling, it is reasonable to suspect that patients with FHA will also have abnormal NKB secretion.
Aim
To assess NKB levels in patients with FHA and to determine whether NKB signaling is affected in these patients. We hypothesized that decreased NKB signaling is a factor contributing to the development of the FHA.
Materials and methods
A total of 147 patients with FHA and 88 healthy age-matched controls were enrolled. Baseline blood samples were drawn from both groups to measure serum concentrations of NKB, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), prolactin (PRL), thyroid-stimulating hormone (TSH), free thyroxine (fT4), cortisol, dehydroepiandrosterone sulfate (DHEA-S), testosterone (T), glucose, and insulin.
Results
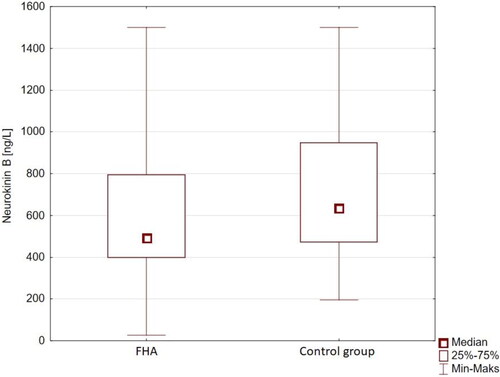
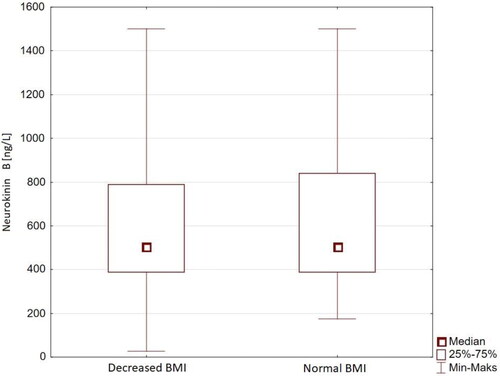
Mean serum NKB levels were found to be decreased significantly in the FHA group when compared with the control group (628.35 ± 324.92 vs. 721.41 ± 337.57 ng/L, respectively p = 0.002). No statistical difference was observed in NKB-1 levels within the FHA group when selecting for normal and decreased body mass index.
Conclusions
Patients with FHA were found to have decreased serum NKB concentrations when compared to healthy controls. Abnormal NKB secretion is likely a key factor contributing to development of FHA.
Introduction
In 2009, Topaloglu et al. [Citation1] observed that loss of function mutations in the genes encoding neurokinin B (NKB) or neurokinin B receptor (NK3R) led to the development of hypogonadotropic hypogonadism. This discovery initiated intensive research into NKB as an essential regulator in the process of sexual maturation and the control of gonadotropin secretion in humans.
NKB belongs to the tachykinin family of proteins. They share a common C-terminal amino acid sequence (Phe-X-Gly-Leu-Met-NH2) and include substance P, neurokinin A, and NKB, as well as neuropeptide K, neuropeptide γ, and hemokinin-1 [Citation2]. NKB mRNA-expressing neurons have been identified in both the basal forebrain and hypothalamus, mainly in the arcuate nucleus (ARCN) and the preoptic area (POA) but are also found in the septal and basal regions of Meynert, terminal striatum, amygdala, and neocortex [Citation3]. Beyond humans, NKB-expressing neurons have also been found in the arcuate nucleus of monkeys, sheep, goats, and mice, indicating a high degree of homology of these nuclei between species [Citation4–7].
NKB-secreting neurons co-express kisspeptin and dynorphin (Dyn) [Citation8], and for this reason are known as kisspeptin/neurokinin B/dynorphin (KNDy) neurons [Citation9]. They co-express NK3R receptors, Dyn receptors (kappa opioid receptor), α-estrogen receptors (ER-α), progesterone receptors (PR), and testosterone (T) receptors [Citation10, Citation11]. The abundance of expressed receptors and regulatory proteins by KNDy neurons suggests at the crucial role these neurons play in regulating not only the hypothalamic-pituitary-gonadal axis but of reproductive function in general [Citation11].
Studies conducted on individuals with mutations in the tachykinin precursor 3 (TAC3) and tachykinin receptor 3 (TACR3) genes provide a unique insight into the role of NKB and NK3R signaling as it relates to regulating the function of the reproductive system. To date, several dozen patients with mutations in the TAC3 and TAC3R gene have been described [Citation1,Citation12–14]. Patients with TAC3 and TAC3R inactivating mutations present normosmic hypogonadotropic hypogonadism which, as in most such cases, is characterized biochemically by low serum estrogen and luteinizing hormone (LH), and clinically by absence of puberty [Citation15]. It is worth noting that this phenotype is generally not observed in mice as reproduction in these cases is only marginally impaired by the loss of TACR3. A much less common TAC3 and TACR3 activating mutation leads to the development of central premature maturation [Citation14].
It has become increasingly apparent that NKB/NK3R signaling is essential for normal activation and regulation of the reproductive system. Pulsatile administration of gonadotropin-releasing hormone (GnRH) in adult males with TAC3 or TACR3 gene mutation restores normal LH levels and normal T secretion. In women with TAC3 or TACR3 mutations pulsatile administration of GnRH stimulates oocyte maturation and ovulation, enabling pregnancy [Citation13].
It is important to note that in patients with TAC3 and TACR3 mutation, follicle stimulating hormone (FSH) secretion is preserved relative to the significant reduction in serum LH [Citation1,Citation13]. This phenomenon is characteristic of a GnRH pulse secretion disorder and can be induced clinically both by slowing GnRH pulses or by the administration of continuous GnRH (which produces an inhibitory effect on the hypothalamus) [Citation16].
Regulation of NKB secretion is closely associated with kisspeptin expression in the ARCN [Citation17]. It has been shown that both patients with functional hypothalamic amenorrhea (FHA) as well as patients suffering from anorexia nervosa have decreased serum kisspeptin levels [Citation18, Citation19].
Considering the synergistic interplay between NKB and Kisspeptin, and that kisspeptin levels are decreased in patients with FHA, we hypothesized that NKB expression in these patients will also be abnormal. Therefore, the aim of this study was to quantify NKB concentration in patients with FHA, and to assess whether decreased signaling of this pathway is contributory to the development of the condition.
Materials and methods
Patients
One hundred and forty-seven (n = 147) patients with FHA, with a mean age of 25 ± 5.0 (mean ± standard deviation [SD]) years were enrolled to the study. Potential study participants were selected from patients referred to the Department of Gynecological Endocrinology, Poznan University Women’s Hospital, Poland due to secondary amenorrhea.
Patients enrolled to the study had been diagnosed with FHA according to the diagnostic criteria established by the Endocrine Society’s task force on FHA guidelines: amenorrhea for at least 90 days, decreased LH (< 5 mIU/mL) and 17-β estradiol concentrations (E2, <50 pg/mL).
In addition to a diagnosis of FHA, patients had to meet the following criteria for enrollment: (1) absence of eating disorders as diagnosable according to Diagnostic and Statistical Manual of Mental Disorders V (DSM-V) classification; (2) absence of underlying endocrine diseases including pituitary, thyroid, or adrenal pathology as determined by a serum hormonal profile; (3) absence of anatomical abnormalities detected on gynecological examination or transvaginal ultrasound; (4) absence of prior hormonal therapy in the 6 months preceding study enrollment; (5) absence of contraindications for hormone replacement therapy (HRT), such as venous thromboembolism, stroke or myocardial infarction in medical history, migraines with aura or acute liver diseases, inherited thrombophilia. In an effort to maintain homogeneity of the study group, only patients with body mass index (BMI) above 18.0 kg/m2 were considered for enrollment.
A control group consisting of 88 healthy age-matched subjects was recruited. Inclusion criteria for the control group were: (1) BMI between 18.5 and 24.9 kg/m2; (2) regular menstrual cycle (28 ± 5 days) for the last 12 consecutive months; (3) stable body weight in the 6 months before enrollment; (4) no history of significant and emotionally stressful life events; (5) negative history of prolonged and intense athletic training for agonistic purposes; (6) negative history of adrenal, thyroid, or pituitary diseases, as determined by serum hormone profiles; (7) negative history of hormonal therapy in the 6 months preceding study enrollment.
Anthropometric assessment of all study participants included measuring height (cm) and body weight (kg), calculation of BMI, and blood pressure (BP). Each subject underwent extensive and detailed physical examination in which the modified Ferriman–Gallwey (FG) score was used to assess hirsutism. Transvaginal ultrasound was used to measure ovarian volume, which was calculated using a simplified ellipsoid formula (0.5 × length × width × thickness).
Prior to enrollment, each participant provided written informed consent for study participation.
Study protocol
Fasting morning blood samples (5 ml) were drawn from all subjects of both the investigative and control groups between 6:00am and 10:00am from either the left or right arm. Baseline serum concentrations of LH, FSH, E2, prolactin (PRL), thyroid stimulating hormone (TSH), free thyroxine (fT4), morning cortisol, dehydroepiandrosterone sulfate (DHEA-S), T, glucose, and insulin were obtained. Patients in the FHA group had blood collected after diagnosis, while blood from patients in the control group was collected between the 10th and 12th day of the menstrual cycle, and after having confirmed the presence of a dominant ovarian follicle.
After samples were collected and clot formation completed, samples were centrifuged at acceleration of 1,500 g for 10 min in order to obtain serum for baseline endocrine evaluation. Blood samples used for NKB assay were collected in EDTA tubes and centrifuged at 1,600 g for 15 min at 4 °C. The serum was stored at −70 °C until the assays were performed
The study protocol was reviewed and approved by Poznan University of Medical Sciences’ Ethics Review Committee in Poznan, Poland.
Assays
NKB was measured with the use of enzyme-linked immunosorbent assay (ELISA) and a Human NKB (Neurokinin B) ELISA Kit (FineTest). The range and sensitivity of each test was 15.625–1,000ng/L and 9.37 ng/L. NKB samples from each subject were analyzed using the same assay unit. Coefficients of variation (CV) for the NKB assay was: intra-assay CV <8% and inter-assay CV <10%.
Serum FSH, LH, PRL, E2, T, cortisol, DHEAS, TSH, fT4, and insulin concentrations were determined by means of electrochemiluminescence immunoassay (Roche Diagnostics). Analysis of these serum hormones was performed using a Cobas E601 analyzer (Roche Diagnostics). Insulin resistance was assessed using the homeostasis model assessment-estimated insulin resistance (HOMA-IR): (fasting glucose (mmol/L) × fasting insulin (mU/L))/22.5. A HOMA-IR threshold ≥ 2.5 was set and interpreted as a measure of insulin resistance. Total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, and glucose levels were measured using standard assay systems at the Central Clinical Laboratory operated by the Poznan University Hospital, Poland.
Statistical analysis
All calculations were performed using Statistica v12.0 (StatSoft, Inc.). Data are expressed as mean ± SD. The normality of data distribution was assessed using the Shapiro-Wilk test. When assessing compliance with normal distribution, the Pearson’s linear correlation coefficient was calculated, otherwise a Spearman’s rank correlation coefficient was performed. For comparisons, Student’s T-test or Mann–Whitney’s U test was used. A p-value of <0.05 was considered a threshold for statistical significance.
Results
NKB was found to be significantly lower at baseline in the FHA group when compared to the control group (mean serum concentration 628.35 ± 324.92 ng/L vs. 721.41 ± 337.57 ng/L, respectively; and ). NKB did not differ significantly between patients with normal and decreased BMI in the FHA group (). Baseline biochemistry was characteristic of hypogonadotropic hypogonadism for all participants in the FHA group, notably decreased PRL and increased morning cortisol (). Patients with FHA were found to have lower insulin and HOMA-IR values, as well as increased total cholesterol levels when compared to healthy controls (). NKB concentration did not correlate significantly with any other measured anthropometric, hormonal, or metabolic parameter.
Table 1. Results of hormonal and metabolic profiles measured in the FHA and control groups.
Discussion
The abnormal pulsatile secretion of GnRH that is observed in FHA leads to lack of stimulation of the hypothalamic-pituitary-ovarian axis. On two occasions, Podfigurna et al. [Citation18] have demonstrated that patients with FHA often have significantly lower serum kisspeptin levels when compared to healthy controls (0.17 ± 0.11 ng/mL vs. 0.3 ± 0.36 ng/mL, p = 0.027) [Citation18]. They observed a similar pattern in patients with restrictive-type anorexia nervosa in which they observed KISS1 to be decreased compared to healthy controls (0.2 ± 0.07 ng/mL vs. 0.3 ± 0.36 ng/mL, p = 0.71) [Citation19].
In a 2017 study by Bacopoulou et al. [Citation20], the authors observed that in a group of patients with typical anorexia nervosa, the concentration of kisspeptin strongly correlated with BMI (r = −0.60, p = 0.012). Moreover, while a statistically significant difference in kisspeptin concentration was observed between patients with typical and atypical anorexia nervosa (208.2 ng/L [110.0–586.0 ng/L] vs. 436.9 ng/L [167.3–641.4 ng/L], p = 0.055); no significant difference was observed between patients in the group with typical anorexia nervosa and patients in the control group (208.2 ng/L [110.0–586.0 ng/L] vs. 232.8 ng/L [101.9–709.1 ng/L]; p = 0.546). In 2016 Hofmann et al. [Citation21] studied a group of patients with anorexia nervosa and concluded that kisspeptin concentration correlated negatively with physical exercise intensity (r = −0.41, p = 0.01), while correlating positively with BMI in the same group (r = 0.514, p < 0.001). This study also noted that administration of kisspeptin by intravenous infusion restored normal pulsatile LH and FSH secretion in patients with FHA.
To the best of our knowledge, the present study is the first to examine serum NKB concentrations in patients with FHA. As there exists no precedent comparing BMI and NKB levels, there exists no benchmark against which to compare this study and its data can only be interpreted indirectly using the established relationship between BMI and kisspeptin as a guide. The negative correlation established by Bacopoulou et al. [Citation20] between BMI and kisspeptin in patients with typical anorexia nervosa provides a foundation on which to hypothesize a similar relationship between BMI and NKB [Citation20]. It is important to note, however, that despite enrolling a similar group of patients, the negative correlation observed in the Bacopoulou trial was not reproduced in this study group. A positive correlation between kisspeptin and BMI has been reported in certain instances when studying patients with anorexia nervosa. However, these sources have emphasized that this correlation does not hold in other patient groups such as those with polycystic ovary syndrome (PCOS) or obesity [Citation22, Citation23].
With regard to other parameters, Bacopoulou et al. [Citation20] did not observe any additional correlation between kisspeptin concentration and serum FSH, LH, or E2 levels in patients with anorexia nervosa. Furthermore, in the group of healthy patients, no correlation was detected between kisspeptin and E2 in any phase of the menstrual cycle: early follicular, preovulatory, or luteal phases [Citation24]. This group was also found to have no significant correlation between kisspeptin and serum FSH or LH levels [Citation25].
No statistically significant correlation was found between NKB and T, DHEA-S, or morning cortisol in either the FHA group or the control group. It is important to note, however, that due to the scarcity of comparable data in the literature these results remain difficult to interpret. So far, only a positive correlation between kisspeptin and T (r = 0.268, p = 0.001) in patients with PCOS has been demonstrated. Although this finding may itself indirectly suggest a relationship between T and NKB aswell [Citation23], the hormonal dysregulation underlying PCOS is functionally different from that observed in FHA. Observations made in patients with PCOS should be caveated, and care should be taken when extrapolating findings to other conditions such as FHA. Unfortunately, data in the literature on the correlation between NKB and general metabolic parameters is also sparse.
In 2017, Andreozzi et al. [Citation26] found no statistically significant correlation between KISS1 and total cholesterol, HDL-C, triglycerides, fasting glucose, or insulin [Citation26]. However, the authors did note that fasting insulin levels were negatively correlated with serum KISS1 (r = −0.308, p < 0.05) [Citation27] in the study group of PCOS patients. In another study also involving patients with PCOS, it was found that kisspeptin did not significantly correlate with total cholesterol, HDL-C, or fasting glucose, while a positive correlation was noted with triglycerides (r = 0.297, p = 0.001), LDL-C (r = 0.368, p = 0.001), fasting insulin (r = 0.199, p < 0.001), and HOMA-IR values (r = 0.206, p < 0.001) [Citation23]. These findings, however, only indirectly suggest a possible interaction between NKB and general metabolic parameters.
Conclusions
Patients suffering from FHA were found to have decreased serum NKB levels when compared to healthy controls. Within the FHA group, NKB did not correlate to BMI, nor was NKB found to correlate with any biochemical parameter beyond the deviations expected in FHA. In the context of a rapidly expanding market and the development of NKB and kisspeptin analogs, this is a strong potential target for the development of novel treatments. The paucity of robust studies relating to NKB functionality calls for further research in which to strive for a better understanding of the underlying mechanisms involved.
Institutional review board statement
The protocol was approved by the local Ethics Committee for Poznan University of Medical Sciences in Poznan, Poland, statement number 244/19. The study was conducted in accordance with the Declaration of Helsinki.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the Central control of reproduction. Nat Genet. 2009;41(3):1–5.
- Almeida TA, Rojo J, Nieto PM, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11(15):2045–2081.
- Chawla MK, Gutierrez GM, Young WS, et al. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384(3):429–442.
- Navarro VM, Gottsch ML, Chavkin C, et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866.
- Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin a in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile Gonadotropin-Releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132.
- Ramaswamy S, Seminara SB, Ali B, et al. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503.
- Foradori CD, Amstalden M, Goodman RL, et al. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–541.
- Szeliga A, Czyzyk A, Podfigurna A, et al. The role of kisspeptin/neurokinin B/dynorphin neurons in pathomechanism of vasomotor symptoms in postmenopausal women: from physiology to potential therapeutic applications. Gynecol Endocrinol. 2018;34(11):913–919.
- Szeliga A, Rudnicka E, Maciejewska-Jeske M, et al. Neuroendocrine determinants of polycystic ovary syndrome. Int J Environ Res Public Health. 2022;19(5):3089.
- Cheng G, Coolen LM, Padmanabhan V, et al. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311.
- Moore AM, Coolen LM, Lehman MN. Kisspeptin/neurokinin B/dynorphin (KNDy) cells as integrators of diverse internal and external cues: evidence from viral-based monosynaptic tract-tracing in mice. Sci Rep. 2019;9(1):14768.
- Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867.
- Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295.
- Tusset C, Noel SD, Trarbach EB, et al. Mutational analysis of TAC3 and TACR3 genes in patients with idiopathic Central pubertal disorders. Arq Bras Endocrinol Metabol. 2012;56(9):646–652. http://www.ncbi.nlm.nih.gov/pubmed/23329188.
- Meczekalski B, Niwczyk O, Bala G, et al. Stress, kisspeptin, and functional hypothalamic amenorrhea. Curr Opin Pharmacol. 2022;67:102288.
- Rance NE, Krajewski SJ, Smith MA, et al. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128.
- Szeliga A, Podfigurna A, Bala G, et al. Kisspeptin and neurokinin B analogs use in gynecological endocrinology: where do we stand? J Endocrinol Invest. 2020;43(5):555–561.
- Podfigurna A, Szeliga A, Meczekalski B. Serum kisspeptin and corticotropin-releasing hormone levels in patients with functional hypothalamic amenorrhea. Gynecol Reprod Endocrinol Metab. 2020;1(1):37–42.
- Podfigurna A, Czyżyk A, Męczekalski B. Serum kisspeptin levels in patients with anorexia nervosa. Pol Merkur Lekarski. 2018;45(265):24–27. http://www.ncbi.nlm.nih.gov/pubmed/30058623.
- Bacopoulou F, Lambrou GI, Rodanaki M-E, et al. Serum kisspeptin concentrations are negatively correlated with body mass index in adolescents with anorexia nervosa and amenorrhea. Hormones (Athens). 2017;16(1):33–41.
- Hofmann T, Elbelt U, Haas V, et al. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite. 2017;108:141–150.
- Rafique N, Latif R. Serum kisspeptin levels in normal and overweight saudi females and its relation with anthropometric indices. Ann Saudi Med. 2015;35(2):157–160.
- Rashad NM, Al-Sayed RM, Yousef MS, et al. Kisspeptin and body weight homeostasis in relation to phenotypic features of polycystic ovary syndrome; metabolic regulation of reproduction. Diabetes Metab Syndr. 2019;13(3):2086–2092.
- Latif R, Rafique N. Serum kisspeptin levels across different phases of the menstrual cycle and their correlation with serum oestradiol. Neth J Med. 2015;73(4):175–178. http://www.ncbi.nlm.nih.gov/pubmed/25968289.
- Katagiri F, Kotani M, Hirai T, et al. The relationship between circulating kisspeptin and sexual hormones levels in healthy females. Biochem Biophys Res Commun. 2015;458(3):663–666.
- Andreozzi F, Chiara Mannino G, Mancuso E, et al. Plasma kisspeptin levels are associated with insulin secretion in nondiabetic individuals. PloS one. 2017;12(16):e0179834.
- Panidis D, Rousso D, Koliakos G, et al. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85(6):1778–1783.