Abstract
Research question
To determine whether blastocyst quality affects the sex ratio at birth through a single blastocyst frozen - thawed embryo transfer (SBFET) cycle.
Design
In this retrospective analysis, we examined 3,041 singleton infants born following SBFET between 2017 and 2020 at a single institution. We compared the sex ratios of these infants with respect to the blastocyst quality, embryo growth rate, and morphology.
Results
The main outcomes of this study were that the sex ratio (M/F) at birth of SBFET was 1.24. Mothers >40 years old had a considerably lower sex ratio than mothers <40 years old (0.39 vs. 1.23–1.28, p < .05). Transplanting high-quality blastocysts significantly increased the proportion of boys born (1.29 vs. 0.88, p < .05). There were no significant differences in the sex ratio with respect to the inner cell mass (ICM) score and expansion degree. Additionally, a high trophoblastic cell (TE) score resulted in a significantly higher sex ratio than the TE score with C (1.62 vs. 1.15 vs. 0.85, p < .001). Multivariable logistic regression analysis was performed to determine which variables were significant factors affecting sex ratio, and the outcomes were consistent with previous findings.
Conclusions
Our study indicated that high-quality, especially good TE score, had a higher chance of resulting in a male infant than a female infant.
Background
Human sex ratio is an important indicator of gender balance between men and women. This is influenced by several factors. Sex ratio at birth, also known as secondary sex ratio (SSR) can be defined as the proportion of males among all live births. The SSR in most gender-neutral countries is approximately 1.05 [Citation15], which is an ideal level for maintaining social stability and promoting economic development.
Over the past decades, with the rapid increase in popularity of assisted reproductive technology (ART) for infertility treatment, more and more children are being born via ART [Citation16]. Gametes and embryos are exposed to external factors during ART, which may have an impact on SSR compared to natural pregnancy.
Most studies have demonstrated that intracytoplasmic sperm injection (ICSI) results in a lower male child birth rate than natural pregnancy and IVF insemination [Citation1,Citation19,Citation23]. It has also been reported that blastocyst stage transplantation might increase SSR when compared with cleavage stage transplantation [Citation8,Citation18,Citation20,Citation23]. Other researchers have also observed a trend of higher SSR in babies born after transfer of thawed embryos than fresh embryos [Citation5].
Many studies have shown that the SSRs are brought about by ART and exploring the influencing factors of SSR. However, there are a few studies on the effect of blastocyst quality on the sex ratio of ART infants. This study aimed to determine whether the differences in blastocyst quality, embryo growth rate, and morphology at our hospital affected the sex ratio of live births via a SBTET cycle.
Methods
Study design and participants
Data were collected from the Center for Reproductive Medicine of the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. This retrospective study reviewed 3,041 singleton infants born following a SBFET cycle between 2017 and 2020. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University. Written informed consent was obtained from all the participants.
ART procedures
Women were monitored and managed according to the hospital’s clinical protocol. Various controlled ovarian stimulation protocols were used with 150–450 IU/day of recombinant follicle stimulating hormone or human menopausal gonadotropin in a gonadotropin-releasing hormone antagonist protocol, a long or short agonist protocol. The protocols were determined according to each patient’s characteristics [age, body mass index (BMI), AFC, and AMH]. Transvaginal oocyte retrieval was scheduled 35–36 h after human chorionic gonadotropin injection. The ART was performed in accordance with the standard operating procedures of the hospital.
Conventional in vitro fertilization (IVF) or ICSI injection was performed, depending on semen characteristics and history of previous fertilization attempts. For conventional IVF, cumulus-oocyte complexes were inseminated with progressively motile spermatozoa in a fertilization culture medium (G-IVF PLUS, Vitrolife, Gothenburg, Sweden). For intracytoplasmic sperm injection, oocytes were denuded 2–3 h after ovum pickup and sperm microinjection was performed 5–6 h after oocyte retrieval. Fertilization was checked approximately 16 h after insemination/injection and was determined by the presence of two pronuclei. Embryos were placed in an incubator (K-MINC-1000; Cook Medical, Bloomington, IN, USA) and cultured in 6% CO2 and 5% O2 at 37 °C. G-1 PLUS and G-2 PLUS (Vitrolife, Gothenburg, Sweden) were used for culturing cleavage- and blastocyst-stage embryos, respectively. The quality of cleavage-stage embryos was assessed approximately 68 h after the insemination/injection, and the quality of blastocyst-stage embryos was evaluated approximately 116 or 140 h after the insemination/injection.
Fertilized oocytes were cultured and observed for three days after oocyte retrieval. Embryos destined for freezing were selected according to embryonic stage and embryo count. For all embryo freezing procedures, 1–2 cleavage-stage embryo VF was performed if the embryos were available. If not, blastocyst culture was performed, and blastocysts were frozen if available.
The luteal phase was supported by vaginal administration of micronized progesterone (400 mg/day) initiated on the day of ovarian puncture.
Blastocyst assessment
Blastocysts were classified using Gardner’s score according to blastocyst size and morphology of the inner cell mass (ICM) and trophectoderm (TE) [Citation13]. Good-quality blastocysts were defined as those that reached at least grade 3 expansion and grade A or B for the ICM and TE parameters. Poor-quality blastocysts were defined as those having reached at least grade 3 expansion, but with grade C ICM or TE parameters [Citation14].
Outcomes and data variables
Primary outcome was SSR (M/F) after SBFET. A live birth was defined as any birth event in which at least one baby was born alive. When calculating the SSR, only one baby was obtained from each set of monozygotic twins.
Statistical analysis
Statistical analysis was carried out using SPSS software (version 24.0, IBM SPSS, IBM Corp, Armonk, NY, USA). Differences between two categorical variables were analyzed using the chi-squared test, depending on the data distribution. Multivariate logistic regression analysis with adjustment for major covariates (age, BMI, type of infertility, main infertility cause, infertility duration, type of fertilization, and day of embryo transfer) was used to assess whether the sex ratio was affected by various morphological parameters used for grading. The data are reported as adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs). Statistical significance was set at p < .05.
Results
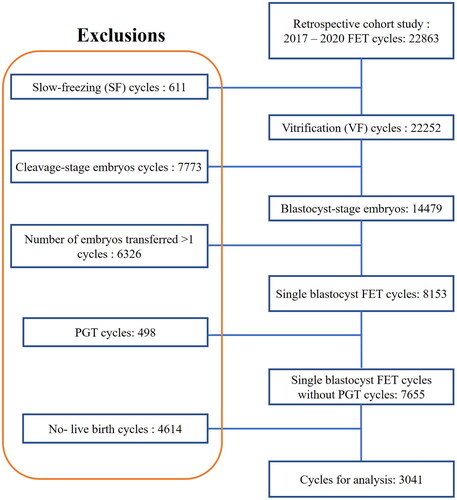
The data selection process for the analysis is shown in . A total of 3,041 infants were included in this study. There were 1,683 male babies and 1358 female babies with an SSR (M/F) of 1.24. Baseline characteristics of the treatment cycles are presented in . The majority of the female population (58.90%) was in the 30 to 39 year old range (mean age, 30.66 ± 3.83 years). Primary infertility is more than secondary infertility, with a ratio of 55.74%, and the major cause of infertility was female sex (65.70%). Most of the women (46.04%) had been infertile for 0–3 years, and 65.77% of the women had a normal BMI.
Table 1. Characteristics of 3041 patients following the SBFET cycles performed from 2017 to 2020.
An analysis of the collected clinical data revealed that there were 2,748 (90.37%) high-quality and 293 (9.63%) low-quality SBFETs, and blastocysts were conceived via 2,244 (73.79%) IVF and 797 (26.21%) ICSI treatments. Among them, 2,628 (86.42%) were D5 embryos and 413 (13.58%) were D6 embryos.
Chi-square test analyses showed that maternal age was associated with SSR (p < .001) (). In terms of maternal age, the babies from the highest paternal age group (>40 years old) had a lower SSR (0.39) than those of younger age groups (<40 years old) (1.23–1.28). No differences in sex distribution among live born infants were noted for type of infertility, infertility cause, infertility duration, maternal BMI, fertilization method, and the day of embryo transfer from all SBFET cycles.
Table 2. SSR stratified by different characteristics.
However, comparing categorical variables of specific ART techniques, we found that high-quality SBFET resulted in a significantly higher sex ratio than poor-quality SBFET (1.29 vs. 0.88, p = .002, ), and more males were born following blastocysts with a high TE score (1.62 and 1.15) than the TE score with C (0.85). The ICM score and degree of expansion had no significant influence on the sex ratio ().
Table 3. SSR stratified by blastocyst quality and morphology.
To further assess the effect of TE grades on the sex ratio, we stratified the data according to the inner-cell mass grade are shown in . In all the ICM groups, Grade A TE showed the highest sex ratio with “statistically significant” results. In the ICM Grade C group, when the TE was grade A, blastocysts showed four times higher probability of being male than when the TE was of Grade B. However, perhaps due to the tiny sample size, this difference was not statistically significant.
Table 4. SSR following SBFET cycles by different grades of trophectoderm (TE)
and inner cell mass (ICM).
After adjusting for potential confounding factors, we identified significant associations between the sex ratio and morphological characteristics of blastocysts. Results of a multivariate analysis revealed that a high-quality blastocyst had higher probability of being male than a poor-quality blastocyst (aOR = 1.466; 95% CI:1.151–1.868, p = .002). Particularly, when the TE was of grade A (aOR = 1.914; 95% CI:1.445–2.537, p < .001), the blastocysts showed higher probability of being male than when the TE was of grade B (aOR = 1.360; 95% CI:1.046–1.769, p < .05) and grade C. Neither the degree of expansion nor the degree of ICM was significantly associated with the SSR ().
Table 5. Multivariate analysis of potential factors associated with SSR.
Discussion
Secondary sex ratio is often used as important indicators of population health and fertility. As far we know, the normal value of SSR is 1.05 [Citation15]. Many population-based retrospective studies confirmed that a specific ART regimen can alter SSR of babies born, and the SSR of babies born through ART was slightly higher [Citation2,Citation8,Citation22]. Previously, research mainly focused on the influence of maternal age, fertilization methods, embryo transfer stage, and types of embryos transferred. However, studies on the effect of blastocyst quality on sex ratio are limited. Here, we conducted a retrospective study to explore blastocyst quality associated with SSR after SBFET.
As the largest retrospective cohort study, our data suggest that there were 1,683 male babies and 1,358 female babies. The SSR of ART babies in the SBFET cycle was 1.24 (), and the SSR was associated with blastocyst quality. Our study results indicate that implantation of high-quality blastocysts, rather than poor-quality blastocysts during SBFET cycles, is associated with increased SSR among live births. In particular, we determined that only TE grade was associated with a significantly higher sex ratio. Similar observations were not observed for blastocoel expansion and ICM grades ().
Possible factors affecting the infant sex ratio in ART include female age, insemination method, embryo transfer stage, and transfer type. Recent evidence from meta-analyses and large population-based studies has suggested that blastocyst embryo transfer is associated with sex ratio imbalance in males [Citation4,Citation12]. For the types of embryos transferred, SSR was significantly lower in the fresh embryo transfer group than in the thaw transfer group. In our study, the SSR of SBFET was 1.24, which was higher than the normal level (1.05). Some studies [Citation6,Citation11] have reported that density gradient centrifugation, one of the preferred sperm methods, is conducive for the enrichment of Y sperm; therefore, the IVF method tends to give birth to males. Similarly, a higher SSR was observed after SBFET with IVF procedures (1.25) but not with ICSI (1.20) in our study; however, the difference was not statistically significant. With an increase in female age, the newborn male-to-female sex ratio will decrease [Citation24]. Consistent with the results of a previous population-based study, our data showed that maternal age was associated with SSR (p < .001). Babies from the highest maternal age group had a lower SSR than those from the youngest age group. This is probably because in women >40 years of age, the uterus and ovaries release a large number of alkaline substances, which is not conducive to the survival of the Y chromosome. This may also be because the stress level of older pregnant women is high, and the male fetus is less able to resist stress than the female fetus; therefore, it is more likely to abort [Citation21].
We found no significant effect of BMI or fertilization on the SSR, as similar values were observed for the groups that received days five and six embryo transfers. Our results are consistent with those of a study published by Elgindy and Elsedeek who reported similar live birth rates after transfer of vitrified embryos on days five and six [Citation10].
Blastocyst morphology, which is based on blastocoel expansion, TE, and ICM must also be considered when evaluating the outcomes of SBFET. In our study, blastocyst quality was also implicated in SSR. We observed a significantly increasing trend in the sex ratio in the context of high-quality versus poor-quality blastocyst transfers, suggesting that the transfer of more advanced blastocysts may increase the proportion of male infants, which is consistent with previous reports [Citation17]. In addition, Lou et al. [Citation17] proposed that when the TE score was B and C, there was a significant difference between SSR. As the largest retrospective cohort study, we included 3,041 singleton infants in this study and almost three times as many as Lou et al.’s. In addition, Lou et al. had very few good embryos (AA) and a large percentage of fair BB and poor BC embryos, which could have affected the outcomes. In our study, there were 610 AA grade blastocysts, accounting for about 20%. Therefore, they believe that grade B trophectoderm confers benefits in improving the implantation potential of male blastocysts. However, our results show that Grade A trophectoderm was significantly associated with a highest sex ratio. Our data suggest that TE score was an independent influencing factor of SSR, which is consistent with the discovery of Cai et al. [Citation3]. We suspect it may be due to male blastocysts may grow more rapidly and receive better morphological scores than female blastocysts. And because the TE lineage gives rise to the fetal portion of the placenta, female blastocysts may exhibit higher incidence of abnormal trophoblast function and decreased potential for implantation and further development compared with male blastocysts. Unlike the TE score, no effect on SSR was observed compared to the ICM score. Interestingly, our results showed an increasing trend in the sex ratio with blastocyst expansion, but this was not statistically significant, possibly due to the small number of grade 6 blastocyst samples. We noted that male blastocysts may grow more rapidly and receive better morphological scores than female blastocysts. According to Thomas et al. the observed correlations between TE performance and infant sex and the rates of implantation, pregnancy, and pregnancy loss indicate that this measure will eventually be prioritized over the ICM score [Citation9]. Interestingly, we also demonstrated that after controlling for ICM, trophoblast grade A was positively correlated with the highest sex ratio among births. However, these variations clearly emphasize the tendency of male embryos to reach the final stages of blastocyst development more rapidly than that of female embryos [Citation10]. However, two recent studies by Weston et al. [Citation25] and Csokmay et al. [Citation7] retrospectively assessed the mean number of cells and embryo grades of male and female babies, and found no difference in growth and delivery rates between male and female embryos. The authors concluded that BT and selection of higher-grade embryos for transfer did not contribute to the increase in SSR.
This study has some limitations. First, because this is retrospective research, it cannot adequately evaluate a causal relationship between blastocyst quality and SSR. The results from the present study warrant confirmation by prospective studies with a larger cohort. Second, we only obtained samples from one reproductive center. In the future, it is necessary to conduct further analysis with large samples of multi-centers and multi-regions. Further expansion of samples in future analyses is needed to validate our current findings. We also included only single-blastocyst FET cycles to enable us control within-transfer characteristics that are known to affect outcomes.
Conclusions
Our study indicated that high quality, especially a good TE score, had a higher chance of resulting in a male infant than a female infant. In the future, we aim to maintain an equilibrium of sex ratio among offspring without affecting pregnancy rate.
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
Conceptualization: Yuling Mao and Ya-Ming Meng; methodology: Chunyan Wang; formal analysis and investigation: Ming Zeng and Yanfen Luo; writing - original draft preparation: Yuling Mao and Yang Luo; writing – review and editing: Yang Luo and Lei Li; funding acquisition: Lei Li, Ming Zeng, Ya-Ming Meng and Yang Luo. The author(s) read and approved the final manuscript.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Third Affiliated Hospital of Guangzhou Medical University [Ethic no. (2022) 012].
Consent to participate
The data sets in this study cannot be publicly available because of involving the patient privacy but are available from the corresponding author on reasonable request. Each patient has signed informed consent on obtaining and analyzing their clinical data prior to the initiation of IVF/ICSI-ET treatment.
Consent to publish
Not applicable.
Data, materials and/or code availability
The data sets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
| Abbreviations | ||
| Secondary sex ratio | = | SSR |
| Assisted reproductive technology | = | ART |
| Controlled ovarian stimulation | = | COS |
| Body mass index | = | BMI |
| Inner cell mass | = | ICM |
| Trophectoderm | = | TE |
| Single blastocyst frozen thawed embryo transfer | = | SBFET |
Supplemental Material
Download MS Word (13.8 KB)Acknowledgement
The study was performed under the auspices of the in vitro fertilization (IVF) unit of the Third Affiliated Hospital of Guangzhou Medical University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arikawa M, Jwa SC, Kuwahara A, et al. Effect of semen quality on human sex ratio in in vitro fertilization and intracytoplasmic sperm injection: an analysis of 27,158 singleton infants born after fresh single-embryo transfer. Fertil Steril. 2016;105(4):1–6.
- Bu Z, Chen ZJ, Huang G, et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China – an analysis of 121,247 babies from 18 centers. PloS One. 2014;9(11):e113522.
- Cai H, Ren WJ, Wang H, et al. Sex ratio imbalance following blastocyst transfer is associated with ICSI but not with IVF: an analysis of 14,892 single embryo transfer cycles. J Assist Reprod Genet. 2022;39(1):211–218.
- Chang HJ, Lee JR, Jee BC, et al. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: a systematic review and meta-analysis. Fertil Steril. 2009;91(6):2381–2390.
- Chen M, Du J, Zhao J, et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci Rep. 2017;7(1):7754.
- Claassens OE, Oosthuizen CJ, Brusnicky J, et al. Fluorescent in situ hybridization evaluation of human Y-bearing spermatozoa separated by albumin density gradients. Fertil Steril. 1995;63(2):417–418.
- Csokmay JM, Hill MJ, Cioppettini FV, et al. Live birth sex ratios are not influenced by blastocyst-stage embryo transfer. Fertil Steril. 2009;92(3):913–917.
- Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures – an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002-2006. BJOG. 2010;117(13):1628–1634.
- Ebner T, Tritscher K, Mayer RB, et al. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J Assist Reprod Genet. 2016;33(1):49–57.
- Elgindy E, Elsedeek MSEA. Day 5 expanded blastocysts transferred on same day have comparable outcome to those left for more extended culture and transferred on day 6. J Assist Reprod Genet. 2012;29(10):1111–1115.
- Ericsson RJ, Langevin CN, Nishino M. Isolation of fractions rich in human Y sperm. Nature. 1973;246(5433):421–424.
- Fernando D, Halliday JL, Breheny S, et al. Outcomes of singleton births after blastocyst versus nonblastocyst transfer in assisted reproductive technology. Fertil Steril. 2012;97(3):579–584.
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–311.
- He Y, Chen S, Liu J, et al. Effect of blastocyst morphology and developmental speed on transfer strategy for grade "C" blastocyst in vitrified-warmed cycles. J Ovarian Res. 2021;14(1):51.
- James WH. Hypotheses on the stability and variation of human sex ratios at birth. J Theor Biol. 2012;310:183–186.
- Kissin DM, Jamieson DJ, Barfield WD. Monitoring health outcomes of assisted reproductive technology. N Engl J Med. 2014;371(1):91–93.
- Lou H, Li N, Zhang XK, et al. Does the sex ratio of singleton births after frozen single blastocyst transfer differ in relation to blastocyst development? Reprod Biol Endocrin. 2020;18(1):72.
- Luke B, Brown MB, Grainger DA, Baker VL, Ginsburg E, Stern JE, Society for Assisted Reproductive Technology Writing Group. The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil Steril. 2009;92(5):1579–1585.
- Maalouf WE, Mincheva MN, Campbell BK, et al. Effects of assisted reproductive technologies on human sex ratio at birth. Fertil Steril. 2014;101(5):1321–1325.
- Milki AA, Jun SH, Hinckley MD, et al. Comparison of the sex ratio with blastocyst transfer and cleavage stage transfer. J Assist Reprod Genet. 2003;20(8):323–326.
- Rueness J, Vatten L, Eskild A. The human sex ratio: effects of maternal age. Hum Reprod. 2012;27(1):283–287.
- Supramaniam PR, Mittal M, Ohuma EO, et al. Secondary sex ratio in assisted reproduction: an analysis of 1 376 454 treatment cycles performed in the UK. Hum Reprod Open. 2019;2019(4):hoz020.
- Tarin JJ, Garcia-Perez MA, Hermenegildo C, et al. Changes in sex ratio from fertilization to birth in assisted-reproductive-treatment cycles. Reprod Biol Endocrinol. 2014;12:56.
- Wang M, Liu X, Zhang H, et al. Associated factors of secondary sex ratio of offspring in assisted reproductive technology: a cross-sectional study in Jilin province, China. BMC Pregnancy Childbirth. 2020;20(1):666.
- Weston G, Osianlis T, Catt J, et al. Blastocyst transfer does not cause a sex-ratio imbalance. Fertil Steril. 2009;92(4):1302–1305.