Abstract
Fertility preservation (FP) for hematological malignancies is difficult because immediate chemotherapy is needed after diagnosis. We report two cases of acute myeloid leukemia (AML) treated with controlled ovarian stimulation (COS) and oocyte cryopreservation using DuoStim after first-line chemotherapy. In Cases 1 and 2, COS and oocyte retrieval (OR) were performed using DuoStim 116 and 51 days after first-line chemotherapy, respectively, and 14 and 6 unfertilized oocytes, respectively, were cryopreserved. Another round of COS and OR was performed using the random-start method 82 days after first-line chemotherapy, and 22 unfertilized oocytes were cryopreserved. DuoStim is useful to maximize OR for patients with a short interval for FP. Many oocytes can be retrieved depending on the timing of recruitment from primary to secondary follicles, although ovarian reserve capacity declines immediately after first-line chemotherapy. Aggressive FP should be performed before allogeneic hematopoietic stem cell transplantation becomes necessary.
Introduction
Oncofertility is attracting attention as cancer prognosis has improved drastically with recent improvements in treatment. Fertility preservation (FP) methods in oncofertility include ovarian tissue cryopreservation, embryo cryopreservation, and oocyte cryopreservation. The first choice for postpubertal women with hematological malignancies is embryo or oocyte cryopreservation with controlled ovarian stimulation (COS) because of the risk of minimal residual disease of malignant cells in ovarian tissuesAs most patients diagnosed with hematological malignancies usually require immediate chemotherapy after diagnosis [Citation1], FP is difficult because of time constraints, resulting in a low pregnancy rate after primary disease treatment [Citation2]. The gonadotoxicity of chemotherapy for acute myeloid leukemia (AML) chemotherapy (idamycin + AraC) is classified by the American Society of Clinical Oncology (ASCO) as low risk (<30%) [Citation3]. However, the remission rate with this therapy is 62% [Citation4]. If remission is not achieved, additional treatments, including hematopoietic stem cell transplantation (HSCT), which is considered to be a high risk (>70%) for gonadotoxicity, are required [Citation3]. Therefore, being proactive regarding FP before HSCT is important.
DuoStim retrieves oocytes twice during the early follicular and luteal phases of the same menstrual cycle and is useful for short-term FP in oncofertility and patients with reduced ovarian reserve capacity [Citation5,Citation6].Therefore, DuoStim can obtain significantly more metaphase (M) II oocytes than the conventional single oocyte retrieval (OR) method [Citation7] and can substantially reduce the interval between first and second ORs compared with conventional ovarian stimulation methods [Citation8]. The random-start protocol for ovarian stimulation during the luteal phase is beneficial in preserving fertility of women with various malignancies [Citation9–11]. Although the gonadotropin-releasing hormone (GnRH) agonist protocol for FP in patients with AML has been reported, no study has reported about the DuoStim method [Citation2,Citation12]. Here, we report our experience with two patients with AML who underwent COS and oocyte cryopreservation with DuoStim after first-line chemotherapy and discuss the optimal timing of OR for FP in patients with AML, along with a literature review.
Case reports
Case 1
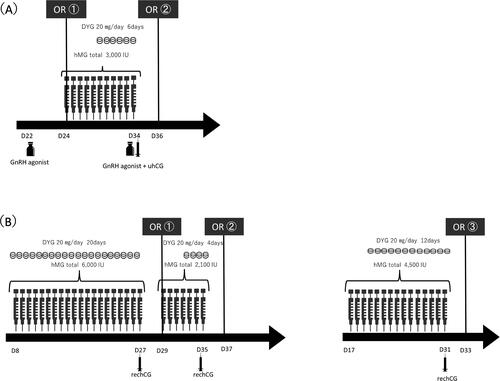
A 20-year-old, unmarried, nulliparous woman was diagnosed with AML and had an intermediate prognosis. Although the patient did not achieve remission after first-line chemotherapy (idarubicin and cytarabine), subsequent chemotherapy (cytarabine, aclarubicin, and granulocyte colony-stimulating factor) led to disease remission. She was referred to our department for FP consultation 116 days after starting first-line chemotherapy because she was scheduled to undergo allogeneic HSCT for disease progression. During her first visit, she was on day 22 of her menstrual cycle, and her serum anti-Müllerian hormone (AMH) was 2.38 ng/mL. We obtained informed consent from the patient to undergo oocyte cryopreservation. Transvaginal ultrasonography showed two follicles >16 mm. Additionally, her serum E2, follicle stimulating hormone(FSH), and Luteinizing Hormone(LH) levels were 618 pg/mL, 1.8 mIU/mL, and 8.1 mIU/mL, respectively. Therefore, GnRH agonist was administered as a trigger on the same day for oocyte maturation, and OR was performed 35 h later (24th day of the menstrual cycle). Immediately after confirmation of only one degenerated oocyte, we switched to DuoStim with COS using progestin-primed ovarian stimulation (PPOS) on the same day. The patient was administered 300 U of human menopausal gonadotropin (hMG) daily by intramuscular injection. Beginning on day 6 of ovarian stimulation, 20 mg/day of oral dydrogesterone was administered concomitantly, and the hMG dose was tapered for a total of 3,000 U of hMG over 11 days. On day 11 of ovarian stimulation, 20 growing follicles (>16 mm) were observed in the left and right ovaries, and her serum E2 level was 4,849 pg/mL. GnRH agonist and urine human chorionic gonadotropin were administered that day as triggers, and OR was performed 35 h later (12 days after the first OR). Since mature oocytes could not be obtained at the first OR, GnRH agonist and uhCG were administered as a double trigger to ensure oocytes were obtained in a limited amount of time. The hCG dose was reduced to 5,000 IU to reduce the risk of ovarian hyperstimulation syndrome. Consequently, 34 oocytes were obtained, including 14 MII oocytes, 1 MI oocyte, and 12 degenerated oocytes (). Fourteen MII and one M. oocytes were cryopreserved. HLA-matched unrelated peripheral blood stem cell transplantation was performed 2 months after OR. Immediately thereafter, the patient became amenorrheic but is currently undergoing periodic hormone replacement therapy.
Case 2
A 25-year-old, unmarried, nulliparous female patient was diagnosed with AML with an intermediate prognosis. The patient was referred to our department for an FP consultation on the day before first-line chemotherapy administration (idarubicin and cytarabine). We decided to proceed with FP after first-line chemotherapy upon obtaining informed consent, considering that the patient could be classified as low risk for gonadotoxicity for first-line chemotherapy for AML. After first-line chemotherapy, the patient achieved disease remission. She was reexamined at our department 30 days after the initiation of first-line chemotherapy. On the day of reexamination, the patient was on the 8th day of her menstrual cycle, and her serum E2, FSH, LH, and AMH levels were 43.7 pg/mL, 5.6 mIU/mL, 2.5 mIU/mL, and 0.17 ng/mL, respectively, and the antral follicle count (AFC) was 2. We decided to perform COS using DuoStim. Twenty milligrams of oral dydrogesterone and 300 U of hMG were administered daily by intramuscular injection using PPOS toward the first OR. Overall, 6,000 U of hMG were administered over 20 days; however, only six growing follicles (>16 mm) were observed, and her serum E2 level value during the OR decision was 54.1 pg/mL. rechCG was administered as a trigger, and the first OR was performed 35 h later (29th day of her menstrual cycle). One MII oocyte and one germinal vesicle (GV) oocyte were retrieved, and the MII oocyte was cryopreserved. COS was resumed on the day of the first OR toward the second OR. Oral administration of dydrogesterone was initiated on day 4 after ovarian stimulation. Overall, 2,100 U of hMG was administered in the first 7 days of ovarian stimulation, during which 11 growing follicles were observed. Her serum E2 level at that time was 247 pg/mL. rechCG was administered as a trigger, and a second OR was performed 35 h later (8 days after the first OR). Five oocytes were retrieved, and four MII oocytes and one MI oocyte were cryopreserved (). High dose-AraC was administered 2 days after the second OR for consolidation therapy. When the patient visited our department again after the completion of treatment (82 days after the start of first-line chemotherapy), we decided to perform a third OR and cryopreservation. On the examination day (17th day of her menstrual cycle), her serum E2, FSH, and LH levels were 41.1 pg/mL, 6.9 mIU/mL, and 8.8 mIU/mL, respectively, and AFC in the right and left ovaries was 7. The random-start method was initiated by administering 300 U of hMG on that day. Beginning on the 4th day of ovarian stimulation, 20 mg/day of oral dydrogesterone was administered, and a total of 4,500 U of hMG was administered over 15 days. Four growing follicles (>16 mm) and nine other follicles (12–16 mm) were observed. Her serum E2 level was 220 pg/mL. rechCG was administered on the same day as the trigger, and the 3rd OR was performed 35 h later (30th day of her menstrual cycle). Overall, 27 oocytes were retrieved, and 22 MII oocytes were cryopreserved (). The patient’s future treatment plan was designed to enable visiting our department when she wishes to become pregnant.
Discussion
These are the first case reports of patients with AML after remission-induction therapy who successfully had multiple oocytes retrieved and cryopreserved in a short time using DuoStim. Retrieving sufficient oocytes is possible depending on retrieval timing and subsequent AML treatment, although ovarian reserve capacity is reduced temporarily after first-line chemotherapy.
The cumulative pregnancy rate using cryopreserved oocytes retrieved from women aged <35 years increases proportionately to the number of oocytes obtained: if 15 oocytes can be retrieved, the pregnancy rate is 85.2% [Citation13]. Therefore, obtaining as many oocytes as possible is necessary during shorter intervals for FP due to treatment to reach this goal for patients with AML. DuoStim doubles the number of oocytes obtained compared with conventional ovarian stimulation [Citation8,Citation14]. Moreover, DuoStim is useful as a remedial action for such patients [Citation15], as in Case 1, where sufficient oocytes could not be retrieved in the planned single OR. Therefore, we believe that DuoStim is superior in obtaining more oocytes in a short period.
Important questions exist regarding the strengths and limitations of FP in patients with AML because of the low gonadotoxicity of standard chemotherapy for AML [Citation3,Citation16]. Although there is little information regarding the gonadotoxicity of idarubicin and aclarubicin, which were administered in our cases, some studies have reported on the ovarian toxicity of doxorubicin used to treat Hodgkin lymphoma, which belongs to the same anthracycline family and has a similar chemical structural formula. It accumulates in the nucleus and mitochondria of cancer cells; however, it also induces apoptosis of oocytes and granulosa cells of the follicle by generating oxidative stress, causing cell death of the postovulatory MII oocytes and preovulatory GV oocytes and primitive ovarian follicles [Citation12,Citation17]. Moreover, after doxorubicin administration, blood flow to the ovaries and vessel wall constriction is decreased. Therefore, the mechanism of doxorubicin-related ovarian toxicity is believed to be ischemia-induced acute ovarian injury [Citation12,Citation18]. The low peak E2 values during all OR cycles in Case 2 and the large number of degenerated oocytes in the luteal phase OR of the DuoStim cycle in Case 1 suggest that idarubicin induces apoptosis of granulosa cells and primordial follicles by a similar mechanism.
Few oocytes were obtained in the follicular and luteal phase ORs of the DuoStim cycle in Case 1, which was performed immediately after first-line chemotherapy. This low oocyte count may be partly due to the lack of recruitment of primary follicles into antral follicles. Recruitment of primary follicles into secondary follicles requires >120 days, and recruitment of preantral follicles into antral follicles requires 71 days [Citation19,Citation20]. The luteal phase OR of the DuoStim cycle in Case 1 and the third OR of the random-start cycle in Case 2, both performed 3–4 months after idarubicin chemotherapy, resulted in a high number of retrieved oocytes, suggesting that primary follicles were recruited. Previous reports also showed that multiple oocytes were obtained 6–7 weeks after anthracycline administration, although a different drug was used () [Citation2,Citation12]. Therefore, we assume that many oocytes can be obtained 2–4 months after first-line chemotherapy because the number of antral follicles increases due to the recruitment of dormant follicles. However, aggressive FP planning should consider the risk of premature ovarian failure due to follicle depletion caused by recruitment activation resulting from low AMH [Citation21,Citation22] and the possibility of HSCT. Therefore, further case studies are needed to clarify the optimal OR timing in AML.
Table 1. Previously reported cases of controlled ovarian stimulation for fertility preservation in patients with acute myeloid leukemia.
Conclusion
DuoStim is useful to maximize the number of oocytes retrieved from patients with AML requiring a short interval for FP. Since oocytes can be obtained depending on the timing, although the ovarian reserve capacity decreases after first-line chemotherapy, aggressive FP should be performed within the allowed time before allogenic HSCT becomes necessary.
Acknowledgments
The authors thank all the staff of the Reproduction Center of Toho University Omori Medical Center.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ye R, Tomlinson B, de Lima M, et al. Timing embryo preservation for a patient with high-risk newly diagnosed acute myeloid leukemia. Case Rep Hematol. 2018;2018:1.
- Hanne S, Cvancarova M, Møller B, et al. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer. 2011;129(5):1225–4.
- Lee SJ, Schover LR, Partridge AH, et al. American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931.
- The AML Collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br J Haematol. 1998;103(1):100–109.
- Kuang Y, Chen Q, Hong Q, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (shanghai protocol). Reprod Biomed Online. 2014;29(6):684–691.
- Vaiarelli A, Cimadomo D, Petriglia C, et al. DuoStim – a reproducible strategy to obtain more oocytes and competent embryos in a short time-frame aimed at fertility preservation and IVF purposes. A systematic review. Ups J Med Sci. 2020;125(2):121–130.
- Sfakianoudis K, Pantos K, Grigoriadis S, et al. What is the true place of a double stimulation and double oocyte retrieval in the same cycle for patients diagnosed with poor ovarian reserve? A systematic review including a meta-analytical approach. J Assist Reprod Genet. 2020;37(1):181–204.
- Vaiarelli A, Cimadomo D, Conforti A, et al. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the bologna criteria: a case series. Fertil Steril. 2020;113(1):121–130.
- Giulia G, Nicole S, Marta C, et al. Folliculogenesis in random start protocols for oocytes cryopreservation: quantitative and qualitative aspects. Reprod Sci. 2022;29(11):3260–3265.
- Kaitlyn W, Hakan C, Evelyn M, et al. Back-to-back random-start ovarian stimulation prior to chemotherapy to maximize oocyte yield. J Assist Reprod Genet. 2019;36(6):1161–1168.
- Huang H, Itaya Y, Samejima K, et al. Usefulness of random-start progestin-primed ovarian stimulation for fertility preservation. J Ovarian Res. 2022;15(1):2.
- Bar-Joseph H, Ben-Aharon I, Rizel S, et al. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod Toxicol. 2010;30(4):566–572.
- Cobo A, Garcia-Velasco JA, Coello A, et al. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105(3):755–764.e8.
- Demian G, Romina P, Mariana M, et al. How effective are the non-conventional ovarian stimulation protocols in ART? A systematic review and meta-analysis. J Assist Reprod Genet. 2020;37(12):2913–2928.
- Ito A, Katagiri Y, Tamaki Y, et al. DuoStim: a new option for fertility preservation for a woman with turner syndrome. Gynecol Endocrinol. 2020;36(12):1144–1148.
- Chung K, Donnez J, Ginsburg E, et al. Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertil Steril. 2013;99(6):1534–1542.
- Spears N, Lopes F, Stefansdottir A, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. 2019;25(6):673–693.
- Ben-Aharon I, Bar-Joseph H, Tzarfaty G, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20.
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–87.
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21(2):200–214.
- Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385.
- Kalich-Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation and "burnout"; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):185ra62.