Abstract
Objective
Polycystic ovary syndrome (PCOS) is a common endocrine disease in women of reproductive age, with complex pathological symptoms and mechanisms. This study explored the mechanism of action of Chao Nang Qing prescription (CNQP) in PCOS.
Methods
CNQP-medicated serum was prepared for culturing KGN granulosa cells. GATA3 knockdown, MYCT1 overexpression, and MYCT1 knockdown vectors were constructed to transfect KGN cells. Cell proliferation and apoptosis, as well as the expression of autophagy-related LC3-II/I, Beclin-1, and p62, were analyzed. ChIP was used to detect the binding of GATA3 and the MYCT1 promoter, and dual-luciferase reporter assay was used to analyze the influence of GATA3 on MYCT1 promoter activity.
Results
CNQP treatment reduced proliferation, increased apoptosis, elevated LC3-II/I, Beclin-1, GATA3, and MYCT1 expression, and decreased p62 expression in KGN cells. GATA3 bound to the MYCT1 promoter to promote MYCT1 expression. MYCT1 overexpression impeded proliferation and stimulated apoptosis and autophagy in KGN cells. Compared to CNQP treatment alone, GATA3 or MYCT1 knockdown before CNQP treatment promoted proliferation and reduced apoptosis and autophagy in KGN cells.
Conclusion
CNQP may modulate KGN cell activity by upregulating GATA3 and MYCT1 expression, thereby slowing down the progression of PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a complex metabolic condition with endocrine and fertility-related impacts at all stages of life, which probably results from a mismatch between ancient biology and modern lifestyle [Citation1]. The etiology and pathology of PCOS encompass both external and internal factors, such as environmental toxicants, physical and emotional stress, insulin resistance, an excess amount of androgens, and obesity [Citation2]. Moreover, the multiple pathogenic features of PCOS may increase the risks of cardiovascular disease, diabetes mellitus, nonalcoholic fatty liver disease, and other illnesses [Citation3–6]. Granulosa cells are a crucial somatic composition of the ovary that surround oocytes, promote oocyte development, and generate sex steroids and growth factors, overall making a contribution to normal maturation of ovarian follicles and menstrual cycle [Citation7]. However, granulosa cells may abnormally proliferate in the pathogenesis of PCOS, resulting in excess antral follicles and anovulatory infertility [Citation8]. In this condition, promotion of apoptosis may reduce excessive proliferation in granulosa cells to facilitate the treatment of PCOS. Traditional Chinese doctors commonly use herbal formulas or single herbs to treat women with PCOS [Citation9]. Some of the widely used herbal prescriptions for PCOS show regulatory effects on granulosa cell apoptosis [Citation10,Citation11].
This study used an original prescription of traditional Chinese medicine (TCM), named Chao Nang Qing prescription (CNQP) (‘Chao’ means the ovary, ‘Nang’ means cysts, and ‘Qing’ means cleansing), which consists mainly of Cyperus rotundus L., Curculigo orchioides Gaertn., and “Chongweizi” (fruits of Leonurus japonicus Houtt.). C. rotundus L. is an important component of TCM prescriptions for PCOS [Citation11–13]. Curculigo rhizome and active ingredients from L. japonicus Houtt. also show therapeutic effects on ovary-related disorders [Citation14,Citation15]. We confirmed the therapeutic effect of CNQP on PCOS at the clinical level, but the mechanism of action of CNQP remained unknown. Previous work showed that Cyperus rhizome and stachydrine hydrochloride (an ingredient from L. japonicus Houtt.) reduced GATA binding protein 3 (GATA3) expression in regulating Th1/Th2 lineage differentiation [Citation16,Citation17], but it was an unexplored topic that whether the herbal components affected GATA3 expression in granulosa cells when treating PCOS.
GATA3 is a zinc-finger transcription factor that has established pioneering function in multiple developmental pathways and cellular reprogramming systems [Citation18]. It identifies G-A-T-A-containing sequences in target genes and binds to target DNA via two zinc-finger domains [Citation19]. Mutations of GATA3 are associated with multiple diseases, such as breast cancer, autoimmune arthritis, and T cell acute lymphoblastic leukemia [Citation20–22]. Qi et al. has discovered reductions in the level of GATA3 in the intestines of mice receiving fecal microbiota from women with PCOS [Citation23]. This finding suggests that upregulation of GATA3 expression may relieve PCOS. Using bioinformatics tools, we selected a downregulated gene, MYC target 1 (MYCT1), in patients with PCOS, which happened to be a predicted target gene of GATA3. MYCT1, first cloned in myeloid cells, is a target of c-Myc that recapitulates multiple c-Myc phenotypes, such as promotion of apoptosis [Citation24]. Therefore, we hypothesized a connection between GATA3 and MYCT1 in the regulation of cell apoptosis in PCOS.
This study determined whether CNQP regulates GATA3 expression, apoptosis, and proliferation in granulosa cells and whether its therapeutic effects involve the hypothetical apoptosis-regulatory GATA3/MYCT1 axis.
Materials and methods
Preparation of medicated serum
Intragastrically infused healthy specific pathogen-free six-week-old male Sprague–Dawley rats (Hunan SJA Laboratory Animal Co., Ltd., Hunan, China), after one week of acclimation, with 2 mL of CNQP (100 mg/ml) every day for five consecutive days. Anesthetized the rats by intraperitoneal injection of pentobarbital 1 h after the last drug administration. Collected abdominal aortal blood and spun at 4,000 r/min and 4 °C for 15 min. Sterilized the collected serum with a 0.22-μm filter, heated at 56 °C for 0.5 h, and preserved at –80 °C until use. The Animal Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital approved the animal use.
Cell culture and transfection
Cultured the human ovarian granulosa KGN cells (the Cell Bank of the Chinese Academy of Sciences, Shanghai, China) in Dulbecco’s modified Eagle medium with 10% fetal bovine serum or medicated rat serum, 100 U/mL penicillin, and 100 µg/mL streptomycin (Thermo Fisher Scientific, MA, USA) at 37 °C with 5% CO2.
The GATA3 knockdown vector (sh-GATA3), MYCT1 overexpression vector (OE-MYCT1), MYCT1 knockdown vector (sh-MYCT1), and negative controls (sh-NC and OE-NC) were from RiboBio (Guangzhou, China). Before transfection, discarded the original medium in a 60-mm culture dish and added 2 mL of preheated serum-free medium. Diluted the plasmid DNA (2.5 μg) with 240 μL of HEPES-buffered saline (HBS). Diluted the Lipofectamine 2000 stock solution of 100 μM (11668019; Invirtrogn, New York, USA) ten times with HBS. Mixed the DNA-containing HBS solution with 180 μL of diluted Lipofectamine 2000 solution and incubated the mixture at ambient temperature for 20–30 min. Added the Lipofectamine 2000-DNA mixture (420 μL) to the cell culture medium and incubated the cells for 48 h (37 °C, 5–7% CO2) before subsequent experiments.
5-Ethynyl-2′-deoxyuridine assay
Cultured the KGN cells in 24-well plates (1 × 104 cells/well) for 48 h, incubated with 5-ethynyl-2′-deoxyuridine (EdU) (Beyotime, Shanghai, China), sequentially treated with paraformaldehyde, 0.5% Triton X-100, and 4′,6-diamidino-2-phenylindole (all from Sigma-Aldrich, St. Louis, MO, USA). Took images of the cells with a fluorescence microscope (Olympus, Tokyo, Japan) and counted EdU-positive cells using Image J software.
Cell counting kit-8 assay
Transferred 100 μL of cell suspension containing 104–105 cells after 24, 48, 72, and 96 h of treatment to a 96-well plate (three wells each sample) and cultured at 37 °C with 5% CO2. Next, incubated the cells with 10 μL/well cell counting kit (CCK)-8 solution (C0037; Beyotime) for 120 min. Used a SpectraMax M5 microplate reader (Molecular Devices) to measure absorbance at 450 nm. Took the absorbance of the control group at 24 h as the standard (100%). Tested each group’s absorbance three times to obtain an average value.
Flow cytometry
Briefly, 1 × 106 cells were used at 80% confluence for apoptosis detection with a 88-8005-74 kit from eBioscience (New York, USA). Suspended the cells in 1 × Annexin buffer, incubated with Annexin V-fluorescein isothiocyanate (5 μL) in the dark at ambient temperature for 10 min, washed with precooled phosphate-buffered saline (PBS), and suspended in 300 μL of 1 × Annexin buffer before flow cytometry analysis (Guava® easyCyte 12; Millipore, USA).
Luciferase reporter assay
The MYCT1 promoter sequence and sh-GATA3 were both from RiboBio. Cloned the MYCT1 promoter sequence upstream of the firefly luciferase reporter gene in the pGL3-Basic vector and co-transfected with sh-GATA3 into KGN cells. Forty-eight hours after the transfection, Renilla luciferase activity was detected using a Dual-Glo luciferase assay system (Promega) and normalized to the internal control firefly luciferase activity.
Chromatin immunoprecipitation
Used a chromatin immunoprecipitation (ChIP) kit (26156; Thermo Fisher Scientific) for this assay. Incubated 5 × 108 adherent cells with paraformaldehyde (1%) at ambient temperature for 10 min. Stoped the crosslinking reaction by adding glycine. Scraped off the cells, centrifuged, and mixed with protease inhibitor-containing lysis buffer by vortexing for 15 s. Sonicated the chromatin into fragments of 300–1000 bp. Stored 50 μL of supernatant at −20 °C as input. Mixed 500 μL of supernatant with 1 mL of hybridization buffer and incubated with 50 pmol anti-immunoglobulin G (IgG) or anti-GATA3 antibody (5 μg, ab199428; Abcam, Cambridge, UK) at 37 °C for 4 h. Incubated the samples with washed streptavidin beads under rotation at 4 °C overnight. Washed beads were collected by centrifugation once in low-salt, high-salt, and lithium chloride wash buffers. Added elution buffer (120 μL) to the protein A/G magnetic beads to elute the DNA (slowly vortexing at 30 °C for 15 min). Centrifuged the samples at 2,000 × g for 1 min and incubated the supernatant with 4.8 µL of NaCl (5 M) and 2 µL of RNase A (10 mg/mL) under rotation at 65 °C overnight. Disrupted the crosslinks by treating with proteinase K (2 µL, 20 mg/mL) under rotation at 60 °C for 60 min. Purified the DNA and detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR).
RT-qPCR
Briefly, 1 μg of KGN cell total RNA, extracted using TRIzol (15596018; Invitrogen, New York, USA), was taken for cDNA synthesis (3733; Takara, Japan). Used a CFX384 Touch detection system (Bio-Rad, USA) and SYBR Green qPCR master mix (330500; Qiagen, Germany) to perform real-time PCR (each sample in triplicate). Data presented are the averages of 2–ΔΔCt from three independent PCR experiments, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). See primers used for amplification in .
Table 1. Primer sequences used in this study.
Western blotting
Lysed the cells and determined the total protein concentration with a bicinchoninic acid assay kit (23227; Thermo Fisher Scientific). Diluted the protein samples in 5 × sample buffer, electrophoresed on 12% separating gels for 1.5 h, and incubated with 5% (wt/vol) nonfat milk powder in PBS blocking solution on a membrane for 60 min at ambient temperature. Next, incubated the membrane overnight (4 °C) with anti-GATA3 (ab199428), anti-LC3B (ab192890), anti-p62 (ab109012), anti-Beclin-1 (ab207612), anti-GAPDH (ab8245) (all 1:1000; Abcam), and anti-MYCT1 (1:1000, PA5-109999; Invitrogen). After washing, incubated the membrane with a secondary antibody (1:3000, ab150077; Abcam) for 60 min at ambient temperature and imaged using a BioSpectrum system (UVP, USA). With the control group as the standard, quantified the protein expression of experimental groups according to band grayscale.
Statistical analyses
Performed data analyses using GraphPad Prism 8.0, with all quantitative data presented as mean ± SEM. After Shapiro–Wilk test of normality, compared the data in two and more groups using Student’s t test and one-way analysis of variance, respectively. Performed post hoc comparisons using Bonferroni test. p < .05 indicates statistically significant differences.
Results
CNQP promotes KGN cell apoptosis and autophagy
The research team has verified the therapeutic effect of CNQP on PCOS at the clinical level, but its mechanism of action remains unclear. To determine the effects of CNQP on granulosa cells in vitro, we cultured KGN cells with CNQP-medicated rat serum and observed the changes in cell phenotypes. CNQP treatment reduced the proliferation (, p < .05) and stimulated the apoptosis (< .05) of KGN cells. Moreover, it increased LC3-II/I and Beclin-1 expression and decreased p62 expression in KGN cells (< .05), suggesting that CNQP can promote KGN cell autophagy. To find target genes of CNQP, we used the BATMAN database to predict target genes of the main components of CNQP. There were 44 common targets of C. rotundus L., C. orchioides Gaertn., and “Chongweizi” (fruits of L. japonicus Houtt.) (). Among the predicted common targets, GATA3 shows the potential to slow down the progression of PCOS [Citation23]. Therefore, we further detected GATA3 mRNA and protein expression in KGN cells, with findings indicating that CNQP boosted GATA3 expression (, p < .05). From these findings, we speculated that CNQP promotes KGN cell apoptosis and autophagy by upregulating GATA3 expression.
Figure 1. CNQP promotes KGN cell apoptosis and autophagy. KGN cells were cultured with CNQP-containing serum: CCK-8 (A) and EdU (B) were used to detect cell proliferation; (C) flow cytometry to detect cell apoptosis; (D) western blotting to detect autophagy-related protein expression; (E) BATMAN website to predict target genes of the main components of CNQP; and RT-qPCR (F) and western blotting (G) to detect GATA3 mRNA and protein expression. N = 3; **p < .01, compared to the control group.
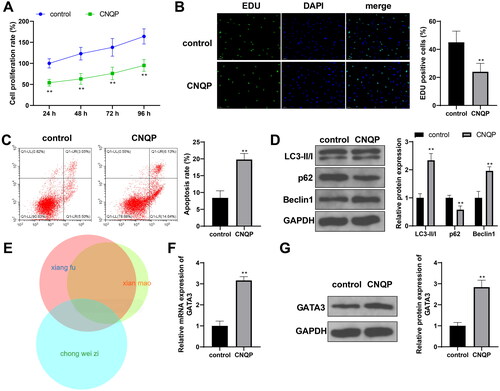
Inhibition of GATA3 eliminates the inhibitory effect of CNQP on KGN cell proliferation
To determine whether CNQP regulates KGN cells by targeting GATA3, we first transfected KGN cells with sh-NC or sh-GATA3. Compared to sh-NC, sh-GATA3 induced a significant reduction in GATA3 expression (, p < .05), which supported its use in subsequent experiments. Next, we cultured sh-NC or sh-GATA3-transfected KGN cells with CNQP-containing serum and divided the cells into CNQP + sh-NC or CNQP + sh-GATA3 group, respectively. The CNQP + sh-GATA3 group showed a higher proliferation rate (, p < .05), a lower apoptosis rate (< .05), decreased LC3-II/I and Beclin-1 levels (< .05), and increased p62 expression (< .05) in comparison with the CNQP + sh-NC group, indicating that knocking down GATA3 expression can partially eliminate the regulatory effects of CNQP on KGN cells.
Figure 2. Inhibition of GATA3 eliminates the inhibitory effects of CNQP on KGN cells. RT-qPCR (A) and western blotting (B) were used to detect GATA3 mRNA and protein expression in KGN cells transfected with sh-NC or sh-GATA3. Next, transfected KGN cells were cultured with CNQP-containing serum: CCK-8 (C) and EdU (D) were used to detect cell proliferation; (E) flow cytometry to detect cell apoptosis; and (F) western blotting to detect autophagy-related protein expression. N = 3; *p < .05 and **p < .01, compared to the control group; #p < .05 and ##p < .01, compared to the CNQP + sh-NC group.
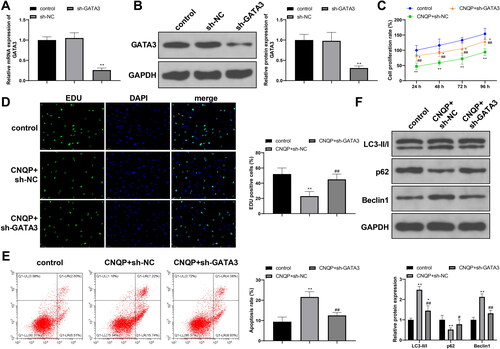
GATA3 promotes transcriptional expression of MYCT1
We screened gene expression datasets of PCOS through the GEO database (https://www.ncbi.nlm.nih.gov/) and selected the GSE98421 dataset for analysis, which contains abdominal adipose tissues from four PCOS patients and four healthy controls. We used the R language limma package to screen differentially expressed genes in the GSE98421 dataset, according to p < .05 and |logFC| > 1. The analysis results showed downregulated expression of MYCT1 in patients with PCOS (< .05). We further detected MYCT1 expression in KGN cells. CNQP treatment increased MYCT1 expression in KGN cells (, p < .05). Compared to the CNQP + sh-NC group, the CNQP + sh-GATA3 group had lower expression of MYCT1 (, p < .05). The detection results suggest that MYCT1 may get involved in CNQP’s regulation of KGN cells and that GATA3 regulates MYCT1 expression.
Figure 3. GATA3 promotes transcriptional expression of MYCT1. (A) Bioinformatics analysis of differentially expressed genes in PCOS. RT-qPCR (B) and western blotting (C) detection of MYCT1 mRNA and protein expression in KGN cells. (D) The binding sites for GATA3 in the MYCT1 promoter. (E) The binding motif of GATA3. (F) ChIP detection of the binding of GATA3 and the MYCT1 promoter. (G) Luciferase reporter vectors containing MYCT1 promoter fragments. (H) Dual-luciferase reporter assay detection of GATA3’s regulation of MYCT1 promoter activity. N = 3; *p < .05 and **p < .01, compared to the control, IgG, or sh-NC group; ##p < .01, compared to the CNQP + sh-NC group.
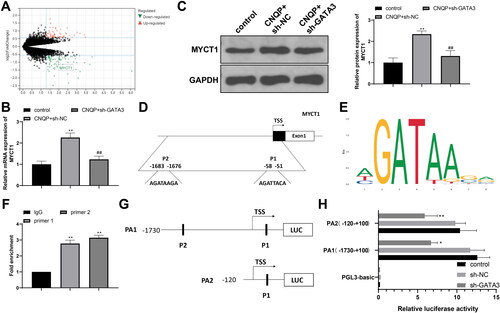
GATA3 is a transcriptional activator that can promote downstream gene expression by recognizing and binding to the promoter of target genes. The JASPAR database (https://jaspar.genereg.net/) showed there are two binding sites for GATA3 in the MYCT1 promoter (), and the binding motif is AGATAAGA (). We performed ChIP experiments to detect the binding of GATA3 and the MYCT1 promoter. As shown in , compared to IgG, GATA3 strongly bound with MYCT1 (p < .01). To observe the effect of GATA3 on MYCT1 promoter activity, we selectively constructed two luciferase reporter vectors containing MYCT1 promoter fragments (). GATA3 knockdown reduced the luminescence of the MYCT1 promoter reporter vectors in KGN cells (, p < .01). Altogether, GATA3 activates MYCT1 transcription by binding to the MYCT1 promoter.
MYCT1 drives KGN cell apoptosis and autophagy
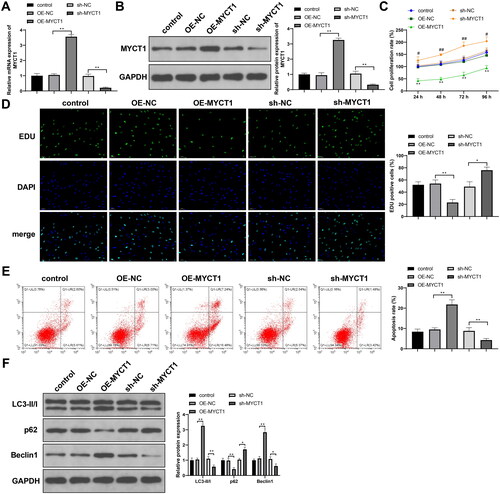
To determine the role of MYCT1 in KGN cells, we transfected KGN cells with sh-NC, sh-MYCT1, OE-NC, or OE-MYCT1. Compared to the corresponding NC, sh-MYCT1 or OE-MYCT1 significantly reduced or increased MYCT1 expression, respectively (, p < .01). MYCT1 overexpression inhibited proliferation, stimulated apoptosis, elevated LC3-II/I and Beclin-1 levels, and decreased p62 expression in KGN cells, whereas MYCT1 knockdown promoted proliferation, inhibited apoptosis, reduced LC3-II/I and Beclin-1 levels, and increased p62 expression in KGN cells (, p < .05). Conclusively, MYCT1 impedes proliferation and drives apoptosis and autophagy in KGN cells.
Figure 4. MYCT1 drives KGN cell apoptosis and autophagy. KGN cells were transfected with sh-NC, sh-MYCT1, OE-NC, or OE-MYCT1: RT-qPCR (A) and western blotting (B) were used to detect MYCT1 mRNA and protein expression; (C) CCK-8 to detect cell proliferation (**p < .01, compared to the OE-NC group; ##p < .01, compared to the sh-NC group); (D) EdU to detect cell proliferation; (E) flow cytometry to detect cell apoptosis; and (F) western blotting to detect autophagy-related protein expression. N = 3; *p < .05, **p < .01.
CNQP stimulates KGN cell apoptosis and autophagy via GATA3/MYCT1
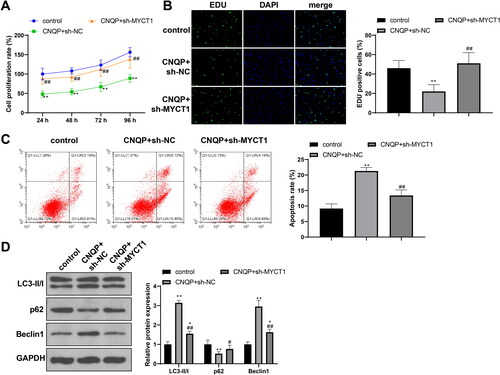
To confirm whether MYCT1 plays a role in CNQP’s effects, we cultured sh-NC or sh-MYCT1-transfected KGN cells with CNQP-containing serum and divided the cells into CNQP + sh-NC and CNQP + sh-MYCT1 groups. The CNQP + sh-MYCT1 group showed a higher proliferation rate, a lower apoptosis rate, decreased levels of LC3-II/I and Beclin-1, and upregulated p62 expression (, p < .05 vs. the CNQP + sh-NC group), indicating that CNQP promotes KGN cell apoptosis and autophagy by raising MYCT1 expression.
Figure 5. CNQP stimulates KGN cell apoptosis and autophagy via GATA3/MYCT1. KGN cells were transfected with sh-NC or sh-MYCT1 and cultured with CNQP-containing serum: CCK-8 (A) and EdU (B) were used to detect cell proliferation; (C) flow cytometry to detect cell apoptosis; and (D) western blotting to detect autophagy-related protein expression. N = 3; **p < .01, compared to the control group; #p < .05 and ##p < .01, compared to the CNQP + sh-NC group.
Based on all our experimental findings, CNQP may stimulate ovarian granulosa cell apoptosis and autophagy via the GATA3/MYCT1 axis, thereby delaying the progression of PCOS.
Discussion
PCOS is a heterogeneous metabolic disorder with different phenotypes, influencing about one-sixth of women of childbearing age [Citation25]. Increased apoptosis of granulosa cells often appears in clinical and experimental PCOS, contributing to aberrant follicle maturation [Citation26]. However, abnormal granulosa cell proliferation can disrupt the normal interaction between oocytes and granulosa cells, thereby causing an increase in early growing follicles in PCOS [Citation27]. Therefore, balancing proliferation and apoptosis of granulosa cells during early follicle development may be an important strategy to prevent absence of ovulation in PCOS.
The original Chinese medicine formula, CNQP, used in this study reduced proliferation and provoked apoptosis of KGN granulosa cells. Moreover, CNQP elevated LC3-II/I and Beclin-1 levels and decreased p62 expression in KGN cells, suggesting an autophagy-promoting effect of CNQP. Autophagy is a stress-responsive process involving autophagosome (a double-membrane vesicle) delivery of redundant or potentially dangerous cytosolic entities to lysosomes for degradation [Citation28]. Although its role in PCOS remains controversial, autophagy performs a pivotal role from oocyte origin to fertilization, and defective autophagy in follicular cells appears in PCOS ovaries during different stages of follicles [Citation29]. Many Chinese medicines can regulate granulosa cell autophagy, apoptosis, and viability in PCOS [Citation10–12,Citation30,Citation31]. Cyperus rotundus L., a main component of CNQP, also composes cang-fu-dao-tan decoction, a classic prescription of TCM for the treatment of PCOS. This decoction improves ovarian function and follicular development and suppresses insulin resistance and mitochondria-dependent granulosa cell apoptosis in rats with PCOS [Citation11,Citation12]. Moreover, Qi Gong Wan, another TCM prescription, consisting of C. rotundus L. and other herbs, reduces adipocyte hypertrophy and inflammation in insulin-resistant PCOS [Citation13]. Curculigo rhizome, another major component of CNQP, can ameliorate perimenopausal symptoms in mice [Citation15]. Leonurine, an important active ingredient from L. japonicus Houtt., prevents premature ovarian insufficiency in mice by protecting granulosa cells from pyroptosis [Citation14]. Overall, the three major components of CNQP show therapeutic effects on ovary-related disorders. To determine CNQP’s action mechanism, we used the BATMAN database to predict common target genes of the three major components of CNQP and selected GATA3 for further analysis.
GATA3 is a transcription factor that controls the expression of key lineage-determining genes as well as cell cycle-related genes and participates in various stages of blood formation [Citation32]. In the development of T cells, GATA3 is essential for CD4+ lineage commitment and CD4+ type 2 helper differentiation [Citation33]. GATA3 also plays a crucial role in mammary gland development by maintaining luminal cell differentiation [Citation34]. GATA3 mutations are common in breast cancers, which promote luminal-to-basal-like transformation and acquisition of mesenchymal properties [Citation35,Citation36]. A study by Qi et al. showed that bile acid administration reduces gut microbiota-caused insulin resistance and ovarian dysfunction in PCOS by increasing interleukin-22 production via GATA3 upregulation [Citation23], suggesting a treatment role of GATA3 in PCOS. However, there are no established findings about the function of GATA3 in ovarian granulosa cells. This study found that CNQP treatment increased GATA3 expression in KGN cells. GATA3 knockdown in KGN cells partially eliminated CNQP’s effects. Moreover, GATA3 transcriptionally activated MYCT1, a downregulated gene in patients with PCOS screened by differential expression analysis.
MYCT1 is a target of c-Myc. MYCT1 overexpression recapitulates multiple c-Myc phenotypes, such as promotion of genomic instability, inhibition of differentiation, and stimulation of apoptosis, with or without the cooperation of other c-Myc targets or even c-Myc itself [Citation37]. MYCT1 promotes apoptosis and inhibits proliferation in multiple tumor cells [Citation38–40]. Consistent with the pro-apoptotic role of MYCT1, MYCT1 overexpression impeded proliferation and stimulated apoptosis in KGN cells. Moreover, MYCT1 knockdown in KGN cells nullified CNQP’s effects.
In summary, CNQP promotes apoptosis and autophagy and impedes proliferation in KGN cells by upregulating GATA3 expression. GATA3 promotes the transcription of MYCT1 by binding to the MYCT1 promoter, thereby increasing MYCT1 expression to promote apoptosis in KGN cells. This study supports the application of TCM in the management of PCOS and sheds light on the mechanism of action of CNQP from the aspect of apoptosis promotion. Therefore, CNQP may be more suitable for preventing early growing follicles and absence of ovulation in PCOS. Moreover, this study is the first to elucidate the function of GATA3 and MYCT1 in granulosa cells. GATA3 and MYCT1 may be novel targets for eliminating abnormal granulosa cell proliferation in PCOS. However, we only obtained data from the KGN cell line. Our data needs further validation in primary granulosa cells and animal models.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Parker J, O’Brien C, Hawrelak J, et al. Polycystic ovary syndrome: an evolutionary adaptation to lifestyle and the environment. Int J Environ Res Public Health. 2022;19(3):1336. doi: 10.3390/ijerph19031336.
- Sadeghi HM, Adeli I, Calina D, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. 2022;23(2):583. doi: 10.3390/ijms23020583.
- Choudhury AA, Rajeswari VD. Polycystic ovary syndrome (PCOS) increases the risk of subsequent gestational diabetes mellitus (GDM): a novel therapeutic perspective. Life Sci. 2022;310:1. doi: 10.1016/j.lfs.2022.121069.
- Falzarano C, Lofton T, Osei-Ntansah A, et al. Nonalcoholic fatty liver disease in women and girls with polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107(1):258–8. doi: 10.1210/clinem/dgab658.
- Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30(7):399–404. doi: 10.1016/j.tcm.2019.08.010.
- Zhu T, Cui J, Goodarzi MO. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes. 2021;70(2):627–637. doi: 10.2337/db20-0800.
- Jozkowiak M, Piotrowska-Kempisty H, Kobylarek D, et al. Endocrine disrupting chemicals in polycystic ovary syndrome: the relevant role of the theca and granulosa cells in the pathogenesis of the ovarian dysfunction. Cells. 2022;12(1):174. doi: 10.3390/cells12010174.
- Li Y, Liu YD, Zhou XY, et al. Let-7e modulates the proliferation and the autophagy of human granulosa cells by suppressing p21 signaling pathway in polycystic ovary syndrome without hyperandrogenism. Mol Cell Endocrinol. 2021;535:111392. (doi: 10.1016/j.mce.2021.111392.
- Shen W, Jin B, Pan Y, et al. The effects of traditional Chinese Medicine-Associated complementary and alternative medicine on women with polycystic ovary syndrome. Evid Based Complement Alternat Med. 2021;2021:6619597. (doi: 10.1155/2021/6619597.
- Jiang X, Yuan Y, Shi M, et al. Bu-shen-zhu-yun decoction inhibits granulosa cell apoptosis in rat polycystic ovary syndrome through estrogen receptor alpha-mediated PI3K/AKT/mTOR pathway. J Ethnopharmacol. 2022;288:114862. (doi: 10.1016/j.jep.2021.114862.
- Jiang XL, Tai H, Xiao XS, et al. Cangfudaotan decoction inhibits mitochondria-dependent apoptosis of granulosa cells in rats with polycystic ovarian syndrome. Front Endocrinol (Lausanne). 2022;13:962154. (doi: 10.3389/fendo.2022.962154.
- Wang C, Ding C, Hua Z, et al. Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome via IGF-1-PI3K/Akt-Bax/bcl-2 pathway. Mediators Inflamm. 2020;2020:8865647. 2020(doi: 10.1155/2020/8865647.
- Zheng R, Shen H, Li J, et al. Qi gong wan ameliorates adipocyte hypertrophy and inflammation in adipose tissue in a PCOS mouse model through the Nrf2/HO-1/Cyp1b1 pathway: integrating network pharmacology and experimental validation in vivo. J Ethnopharmacol. 2023;301:115824. doi: 10.1016/j.jep.2022.115824.
- Chi YN, Hai DM, Ma L, et al. Protective effects of leonurine hydrochloride on pyroptosis in premature ovarian insufficiency via regulating NLRP3/GSDMD pathway.Int Immunopharmacol. 2023;114:109520. doi: 10.1016/j.intimp.2022.109520.
- Miao M, Tian S, Bai M, et al. Total glucosides of curculigo rhizome to perimenopausal period mice model. Pak J Pharm Sci. 2017;30(3, Suppl):975–978.
- Bae H, Park N, Kim Y, et al. The modulative effect of cyperi rhizoma on Th1/Th2 lineage development. Cytokine. 2010;51(3):259–265. doi: 10.1016/j.cyto.2010.05.011.
- Li X, Wang B, Li Y, et al. The Th1/Th2/Th17/treg paradigm induced by stachydrine hydrochloride reduces uterine bleeding in RU486-induced abortion mice. J Ethnopharmacol. 2013;145(1):241–253. doi: 10.1016/j.jep.2012.10.059.
- Tanaka H, Takizawa Y, Takaku M, et al. Interaction of the pioneer transcription factor GATA3 with nucleosomes. Nat Commun. 2020;11(1):4136. doi: 10.1038/s41467-020-17959-y.
- Khazaeli Najafabadi M, Mirzaeian E, Memar Montazerin S, et al. Role of GATA3 in tumor diagnosis: a review. Pathol Res Pract. 2021;226:153611. doi: 10.1016/j.prp.2021.153611.
- Dai YT, Zhang F, Fang H, et al. Transcriptome-wide subtyping of pediatric and adult T cell acute lymphoblastic leukemia in an international study of 707 cases. Proc Natl Acad Sci USA. 2022;119(15):e2120787119.
- Martin EM, Orlando KA, Yokobori K, et al. The estrogen receptor/GATA3/FOXA1 transcriptional network: lessons learned from breast cancer. Curr Opin Struct Biol. 2021;71:65–70. doi: 10.1016/j.sbi.2021.05.015.
- Patrick AE, Wang W, Brokamp E, et al. Juvenile idiopathic arthritis associated with a mutation in GATA3. Arthritis Res Ther. 2019;21(1):156. doi: 10.1186/s13075-019-1946-3.
- Qi X, Yun C, Sun L, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–1233. doi: 10.1038/s41591-019-0509-0.
- Yin X, Grove L, Rogulski K, et al. Myc target in myeloid cells-1, a novel c-Myc target, recapitulates multiple c-Myc phenotypes. J Biol Chem. 2002;277(22):19998–20010. doi: 10.1074/jbc.M200860200.
- Szczuko M, Kikut J, Szczuko U, et al. Nutrition strategy and life style in polycystic ovary Syndrome-Narrative review. Nutrients. 2021;13(7):2452. doi: 10.3390/nu13072452.
- Liu Y, Li Z, Wang Y, et al. IL-15 participates in the pathogenesis of polycystic ovary syndrome by affecting the activity of granulosa cells. Front Endocrinol (Lausanne). 2022;13:787876. (doi: 10.3389/fendo.2022.787876.
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–378. doi: 10.1093/humupd/dmn015.
- Galluzzi L, Green DR. Autophagy-Independent functions of the autophagy machinery. Cell. 2019;177(7):1682–1699. doi: 10.1016/j.cell.2019.05.026.
- Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–2733. doi: 10.1080/15548627.2021.1938914.
- Liu M, Zhu H, Zhu Y, et al. Guizhi fuling wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol. 2021;270:113821. doi: 10.1016/j.jep.2021.113821.
- Pan X, Liu Y, Liu L, et al. Bushen jieyu tiaochong formula reduces apoptosis of granulosa cells via the PERK-ATF4-CHOP signaling pathway in a rat model of polycystic ovary syndrome with chronic stress. J Ethnopharmacol. 2022;292:114923. doi: 10.1016/j.jep.2021.114923.
- Zaidan N, Ottersbach K. The multi-faceted role of Gata3 in developmental haematopoiesis. Open Biol. 2018;8(11):180152. doi: 10.1098/rsob.180152.
- Callender LA, Schroth J, Carroll EC, et al. GATA3 induces mitochondrial biogenesis in primary human CD4(+) T cells during DNA damage. Nat Commun. 2021;12(1):3379. doi: 10.1038/s41467-021-23715-7.
- Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9(2):201–209. doi: 10.1038/ncb1530.
- Bai F, Zhang LH, Liu X, et al. GATA3 functions downstream of BRCA1 to suppress EMT in breast cancer. Theranostics. 2021;11(17):8218–8233. doi: 10.7150/thno.59280.
- Bai F, Zheng C, Liu X, et al. Loss of function of GATA3 induces basal-like mammary tumors. Theranostics. 2022;12(2):720–733. doi: 10.7150/thno.65796.
- Rogulski KR, Cohen DE, Corcoran DL, et al. Deregulation of common genes by c-Myc and its direct target, MT-MC1. Proc Natl Acad Sci USA. 2005;102(52):18968–18973. doi: 10.1073/pnas.0507902102.
- Fu S, Fu Y, Chen F, et al. Overexpression of MYCT1 inhibits proliferation and induces apoptosis in human acute myeloid leukemia HL-60 and KG-1a cells in vitro and in vivo. Front Pharmacol. 2018;9:1045. doi: 10.3389/fphar.2018.01045.
- Wang HT, Tong X, Zhang ZX, et al. MYCT1 represses apoptosis of laryngeal cancerous cells through the MAX/miR-181a/NPM1 pathway. FEBS J. 2019;286(19):3892–3908. doi: 10.1111/febs.14942.
- Xu XP, Peng XQ, Yin XM, et al. miR-34a-5p suppresses the invasion and metastasis of liver cancer by targeting the transcription factor YY1 to mediate MYCT1 upregulation. Acta Histochem. 2020;122(6):151576. doi: 10.1016/j.acthis.2020.151576.
