Abstract
Objective: To investigate the expression and localization of Vasorin (Vasn) in human female reproductive system. Methods: The presence of Vasorin was evaluated by RT-PCR and immunoblotting analyses in patient-derived endometrial, myometrial and granulosa cells (GCs) primary cultures. Immunostaining analyses were performed to detect Vasn localization in primary cultures and in ovarian and uterine tissues. Results: Vasn mRNA was detected in patient-derived endometrial, myometrial and GCs primary cultures without significant differences at the transcript level. Otherwise, immunoblotting analysis showed that Vasn protein levels were significantly higher in GCs than proliferative endometrial stromal cells (ESCs) and myometrial cells. Immunohistochemistry performed in ovarian tissues revealed that Vasn was expressed in the GCs of ovarian follicles at different stages of development with a higher immunostaining signal in mature ovarian follicles such as the antral follicle or on the surface of cumulus oophorus cells than in early-stage follicles. The immunostaining of uterine tissues showed that Vasn was expressed in the proliferative stroma endometrium while it was significantly less expressed in the secretory endometrium. Conversely, no protein immunoreactivity was revealed in health myometrial tissue. Conclusions: Our results revealed the presence of Vasn in the ovary and the endometrium. The pattern of Vasn expression and distribution suggests that this protein may have a role in the regulation of processes such as folliculogenesis, oocyte maturation, and endometrial proliferation.
Introduction
Vasorin (Vasn) is a type I transmembrane glycoprotein of 673 amino acids; it is also known as Slit-like 2 (Slitl2) due to its strong structural similarities with the Slit family of proteins [Citation1]. Vasn has been identified in numerous species such as rodents, zebrafish, and human [Citation2]. Although the high degree of structural similarity between murine and human protein suggests a highly conserved function of Vasn throughout evolution, to date no human disease or phenotype has been directly associated with variants in the vasn gene [Citation1].
According to new evidence Vasn seems to be a potential biomarker for nephropathy [Citation3–5] and tumorigenesis. In adult human tissues, Vasn is expressed at highest levels in aorta, at intermediate levels in kidney and placenta, and much less so in other tissues such as liver, brain, heart, lung, and skeletal muscle [Citation6]. Ikeda et al. [Citation6] have first reported that Vasn modulates the vascular response to injury by counteracting Transforming growth factor beta (TGF-β) signaling in vivo. TGF-β plays an important role in vascular pathophysiology regulating cell growth and differentiation, extracellular matrix accumulation and inflammation [Citation7]. Moreover, TGF-β1 signaling contributes to the development of smooth muscle cells from embryonic stem cells [Citation8]. It has been demonstrated that Vasn extracellular domain can be released from cell surface by metalloprotease ADAM17-mediated cleavage [Citation9]. The soluble extracellular domain of Vasn (sVasn) negatively modulates TGF-β signaling by sequestering the ligand in a complex, thereby preventing ligand–receptor interactions and subsequent downstream signalings such as SMAD-2/3 phosphorylation and collagen production [Citation10].
Several reports also showed Vasn overexpression in different cancer cells, such as hepatocellular carcinoma [Citation11, Citation12], breast cancer, human gliomas, especially glioblastoma multiforme [Citation13]. More recently, studies performed in human lung cancer tissues and cell lines revealed that the increase in Vasn expression was inversely associated with lung cancer patient survival [Citation14]. In the light of these data, the role of Vasn in carcinogenesis process has been deeply investigated and it has been shown that its expression promotes tumor progression [Citation15]. Furthermore, the protein seems to be involved in the escaping cell death, which represents a crucial event promoting tumor growth. In particular, Vasn is also referred as ATIA (anti-TNFa-induced apoptosis) since it may act as an antiapoptotic factor by suppressing reactive oxygen species (ROS) production and protecting cells against TNFα- and hypoxia-induced apoptosis [Citation14]. In addition to being expressed on cell membrane and being secreted, Vasn is also able to translocate into the mitochondria where it binds to thioredoxin-2 (TRX2) and suppresses ROS production [Citation16].
The involvement of TGFβ − superfamily members in the regulation of ovarian folliculogenesis and ovulation has been widely described [Citation17–19]. However, the expression of the TGF-β inhibitory binding protein Vasn and its function in the ovarian physiology and female fertility have been poorly investigated. In particular, the role of this protein in the reproductive tissues was only investigated in mouse model system by Rimon-Dahari et al. [Citation20]. Their studies demonstrated that somatic cells of mouse ovarian follicles express the protein, whose expression is up regulated by Luteinizing Hormone. For the first time, this study presents Vasn as a new potential regulator of murine folliculogenesis, by participating in the regulation of antral follicle survival, and the establishment of the ovarian follicle pool. Interestingly, the presence of the protein was also highlighted in a proteomic analysis of human follicular fluid from fertile women [Citation21]. All these promising data and the lack of information on the topic in human tissues prompted us to investigate the Vasn expression in the human female reproductive system, using ovarian, endometrial, and myometrial tissues and the respective primary cultures.
Materials and methods
Human female reproductive tissues collection
Collected human specimens from healthy patients (mean age of 41 ± 6), who signed informed consent for this study, were obtained from the Department of Obstetrics and Gynecology of Modena University Hospital. Myometrium, endometrium, and ovarian tissues were obtained from eight women who underwent myomectomy (n = 3), hysterectomy (n = 3) or ovariectomy (n = 3) for benign circumstances. This study has been approved by the Local Institutional Review Board.
Primary myometrial and endometrial stromal cell cultures
Myometrium and endometrium fragments were manually cut into small pieces, followed by incubation on a shaker at 37 °C for 3 to 5 h in DMEM containing 1% penicillin/streptomycin, 1 mg/mL collagenase type II. Sigma-Aldrich, St. Louis, MO, USA supplied all reagents for cell cultures’ digestion. The digested tissues were filtered through a sterile 100 μm polyethylene mesh filter to remove undigested tissues and to obtain a single-cell suspension. To assure the purity of the endometrial stromal culture, trypsinization was carried out to eliminate contaminating epithelial cells (TrypsinEDTA10X, GIBCO, Waltham, MA, USA). The immunostaining for the stromal marker Vimentin was performed to confirm the purity of the stromal preparation. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (GIBCO), 1% glutamine (Sigma Aldrich) and 1% Antibiotic-Antimycotic solution (GIBCO).
Tissue slice preparation and VENTANA immunohistochemistry
Tissue samples of ovary, endometrium and myometrium, obtained from the above healthy patients, were fixed in 4% paraformaldehyde/PBS (PFA) (Electron Microscopy Sciences, Hatfield, PA, USA) for 24 h, dehydrated and then paraffin embedded. Sections of 4-5 µm thickness were investigated by immunohistochemical analysis. Immunohistochemistry was carried out on a fully automated VENTANA Benchmark XT (VENTANA Medical Systems; Roche Group, Tucson, AZ, USA) using the pre-diluted VENTANA anti-VASN rabbit polyclonal primary antibody (1:500, Thermo Fisher Scientific, Waltham, MA, USA, PA5-98236), together with the XT ULTRAVIEW DAB detection kit (VENTANA Medical Systems). Negative controls were performed by omitting primary antibody and replacing primary antibody with isotype IgG. Nuclei were counterstained with Carazzi Hematoxylin (Merck KGaA, Milan, Italy). Pictures were acquired at magnification 10-20-40X using Olympus BX53 optical microscope.
GCs isolation and primary cell culture
Patients included in this non-clinical study represent the average population of women undergoing in-vitro fertilization (IVF) at the Department of Obstetrics and Gynecology of Modena University Hospital of advanced maternal age (36 ± 5 years). All patients (n = 20) consented to the donation of GCs, which were otherwise discarded.
hGCs were purified, from follicular aspirates, by centrifugation through a discontinuous Percoll (Amersham, Sweden) gradient [Citation22], and plated in 35-mm dishes at a density of 8x105 cells/dish in DMEM medium supplemented with 5 % fetal bovine serum, 1% glutamine and 1% antibiotic-antimycotic solution. The cells were used for experiments after 6 days of culture to eliminate the hormonal effects suffered by the patient during the pre-IVF in vivo stimulation protocol.
RNA extraction and RT-PCR analysis
Total RNA was extracted from proliferative ESCs, myometrial cells and GCs using TRI-Reagent (Sigma-Aldrich). RNA concentration and purity were detected using the Nanodrop ND-1000. Total RNA was reverse transcribed in a final volume of 20 μl using the High-Capacity RNA-to-cDNA™ Kit according to the manufacturer’s instructions. Expression of human Vasn was evaluated by semiquantitative RT-PCR using DreamTaq Green PCR Master Mix (2X). Thermo Fisher Scientific supplied all reagents for RNA analysis. All reactions were carried out in triplicate and the following protocol used: hot start for 2 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, annealing for 30 s at 60 °C and extension for 1 min at 72 °C. For each PCR reaction, 15 μl samples were loaded in a 2% agarose gel. Ribosomal protein S7 (RpS7) was used to normalize the intensity of the amplified fragment bands.
Primer’s specificity was confirmed by melting curve analysis and the sequences of the primers used were:
Vasorin: Forward 5′-AACCCCTTCAACTGCGTGTG-3′
Reverse: 5′-AAGTCGGCGTAGTCAAGCTC-3′
RpS7: Forward 5′- AATCTTTGTTCCCGTTCCTCA-3′
Reverse 5′- CGAGTTGGCTTAGGCAGAA-3′
Western blot analysis
Cells were washed with PBS 1X, suspended in 150 μl of lysis buffer 1X (50 mM TrisEDTA, 150 mM NaCl, 0.1% SDS, 0.5% DOC, 0,1% TritonX-100) supplemented with the Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). After incubation for 30 min on ice, protein concentration was measured by BCA Protein Assay Kit (Thermo Fisher Scientific). LDS Sample Buffer (4X) and Sample Reducing Agent (10X) (Thermo Fisher Scientific) were added to the protein sample and boiled for 5 min at 100 °C for denaturing gel electrophoresis. Samples containing equal amounts of proteins (40 μg) were run on 4-12% SDS-polyacrylamide gel (SDS-PAGE) and transferred onto a nitrocellulose membrane with a 0.2 µm pore size (Thermo Fisher Scientific). Membranes were blocked for 2 h in 5% of not-fat milk powder (Sigma-Aldrich) in PBS1X with 0.05% [v/v] Tween® 20 Detergent (Sigma-Aldrich), and then incubated with the antibodies overnight at 4 °C. Primary antibodies used were: rabbit anti-vasorin antibody (1:1000, PA5-98236) and rabbit anti- vinculin (1:1000, # 700062). After the membranes were incubated for 2 h at room temperature with secondary HRP antibodies (goat-antirabbit, 1:10000, G21234). The antibodies used were supplied by Thermo Fisher Scientific. Signals were detected by an ECL immunodetection system (Amersham Corp, Arlington Heights, IL, USA) following the manufacturer’s instruction and visualized by Chemi DocTM XRS 2015 (Bio-Rad Laboratories, CA, USA). Densitometric analysis was performed using Image Lab software and normalized to housekeeping.
Immunocytochemistry analysis
Cells at sub-confluence were fixed with 4% PFA (Electron Microscopy Sciences) for 10 min at 4 °C, washed twice with PBS 1X, and permeabilized with 0.2% TritonX-100 for 1 min at room temperature. The reduction of nonspecific background signal was obtained by incubating cells with block solution consisting of 3% BSA for 1h at room temperature. Cells were incubated with primary antibody (rabbit anti-vasorin 1:200, Thermo Fisher Scientific, PA5-98236; rabbit anti-vimentin 1:200, Novus Biologicals, USA, NBP1-31327) diluted in 3% BSA-0,2% TritonX-100-PBS 1X solution overnight at 4 °C. After washing with PBS 1 × 2-3 times and once with 3% BSA, cells were incubated with species-specific secondary antibody Alexa Fluor 594 Goat anti-Rabbit IgG (H + L) (Thermo Fisher Scientific, A-11012) diluted 1:200 for 2 h at room temperature. Nuclei were counterstained with DAPI solution 1 mg/ml (1:7000, Thermo Fisher Scientific, 62248), and the slides were mounted with buffered glycerol. Pictures were acquired at magnification 10X using a Zeiss AxioObserver D1 and Z1 fluorescence microscope (Carl Zeiss SpA).
Statistical analyses
Statistical analyses were performed using one-way analysis of Variance (ANOVA) followed by the Student-Newman-Keuls method to compare multiple groups. Values with p< 0.05 were considered statistically significant.
Results
Vasn mRNA and protein expression in human primary cultures
Characterization of Vasn in human primary cultures may be a starting point for more in-depth future studies on the function of this protein in the ovary and uterus. We used as experimental model GCs isolated from human follicular fluid of women undergoing oocyte retrieval for IVF protocol, myometrial cells, and proliferative ESCs isolated from healthy myometrium and endometrium, respectively. To obtain a pure endometrial stromal culture, trypsinization was carried out to eliminate contaminating epithelial cells. Cells from passages 0 and 1 were evaluated by immunofluorescence assay for the stromal marker Vimentin. At passage 0 both cell types were present, epithelial cells that did not show staining for the stromal marker, and stromal cells that were positive for Vimentin. The cells at passage 1 were all positive for vimentin marker demonstrating only the presence of stromal cells ().
Figure 1. (A) Characterization of proliferative endometrial stromal cells by immunofluorescence assay for the stromal marker Vimentin (red). Scale Bar= 20 µm. (B) Representative RT-PCR analysis of vasn transcript expression in proliferative endometrial stromal cells, granulosa cells and myometrial cells of three independent cell cultures. (C) Representative western blot analysis of Vasn protein expression in proliferative endometrial stromal cells, granulosa cells and myometrial cells. Densitometric absorbance values from three independent experiments were averaged (± SEM) and were expressed as arbitrary units (a.u.). *p ≤ 0.001. ESCs = endometrial stromal cells; GCs = granulosa cells.
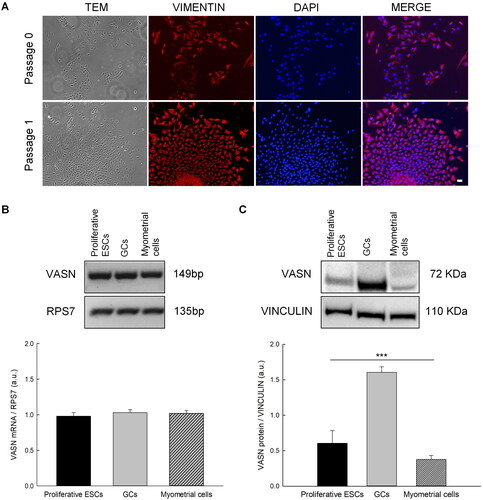
Vasn mRNA expression levels were evaluated by RT-PCR analysis. Vasn mRNA was detected in all cell types without significant differences at the transcript level (). Otherwise, western blotting analysis showed that Vasn protein levels were significantly higher in GCs than ESCs and myometrial cells, thereby indicating that Vasn expression was regulated at post transcriptional level ().
Localization of Vasn in primary cultures of GCs and ESCs
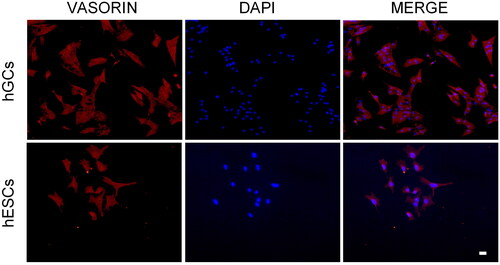
To get more information about Vasn protein we performed a localization analysis on cellular models where Vasn was most expressed as demonstrated by the previous analysis. We stained GCs and proliferative ESCs with an anti-vasorin antibody and, as shown in the , Vasn was located on the cell surface of both cell types.
Vasn protein expression in human female reproductive tissues
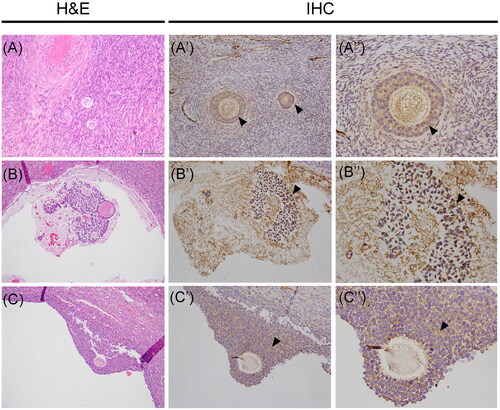
The immunohistochemical staining of the protein was performed in both ovarian and uterine tissues. Regarding human ovarian tissue, the immunostaining revealed the presence of Vasn in follicles at different stages of development, including early-stage follicles. In particular, the immunoreactivity was detected at the level of pre-granulosa cells, as shown in , and it was also maintained during follicle maturation at the surface of GCs, as observed in follicles at advanced stage of development (). Interestingly, the analyses also revealed a high Vasn expression on the surface of cumulus oophorus cells surrounding the oocyte, thus suggesting a role for the protein during the whole process of follicle maturation. Whereas no Vasn expression was observed at level of theca cells in both antral and Graafian follicles.
Figure 3. Vasn immunoreactivity in the human ovarian tissue. (A-B-C) Histological images stained with hematoxylin and eosin (H&E) of human ovarian tissue. Vasn expression was observed on the surface of pregranulosa cells (A’-A’’, arrowhead) and it was also maintained during follicle maturation at the surface of granulosa cells (B’-B’’, arrowhead). High Vasn expression was also present on the surface of cumulus oophorus cells surrounding the oocyte (C’-C’’, arrowhead). Scale bar= 200 µm.
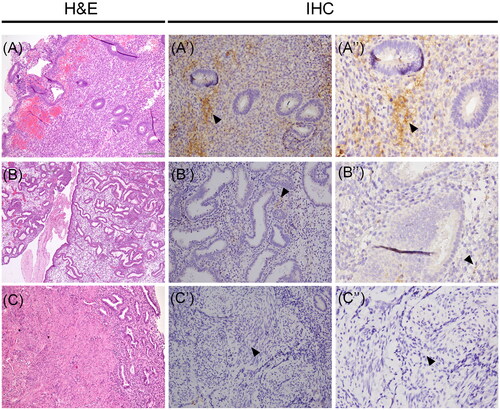
The presence of the protein was also investigated in uterine tissues. In detail, we detected Vasn immunoreactivity only in the stromal compartment of both proliferative and secretory phase endometria with a strong difference in immunoreactivity signal that was much higher in proliferative stroma compared to the secretory one (). Conversely, the expression of the protein was observed neither in proliferative nor in secretory glandular epithelia as well as no immunoreactivity for the protein was revealed in health myometrial tissue ().
Figure 4. Vasn immunoreactivity in the normal proliferative-secretory phase endometrial tissue and normal myometrium. (A-B-C) Histological images stained with hematoxylin and eosin (H&E) of human uterine tissues. Vasn expression was evident in the stromal cells of proliferative endometrium (A’-A’’, arrow). Vasn expression was extremely weak in the stroma of secretory endometrium (B’-B’’, arrow). Vasn expression was absent in the myometrium (C’-C’’). Scale bar = 200 µm.
Discussion
Our work aimed to investigate for the first time the expression of Vasn in the human female reproductive system. Immunohistochemical analyses performed in human ovarian tissue indicated that Vasn was expressed in the GCs of ovarian follicles at different stages of development. Interestingly, the immunostaining signal was much higher in mature ovarian follicles such as the antral follicle or on the surface of cumulus oophorus cells than in early-stage follicles.
In recent years, increasing interest has been observed in the complex intraovarian control mechanisms that, together with other systemic signals, coordinate follicle recruitment, selection, and growth from the primordial stage through ovulation and corpus luteum formation. Several growth factors, many belonging to the TGF-beta superfamily, are expressed by ovarian somatic cells and oocytes and they act as intraovarian regulators of folliculogenesis [Citation17]. Among these factors, activin, Growth differentiation factor-9 (GDF9), and Bone morphogenetic protein 15 (BMP15), are required for the early stages of folliculogenesis. Rimon-Dahari and colleagues, reported that Vasn is expressed in mouse ovaries [Citation20]. Specifically, they found the expression of Vasn mRNA and protein in GCs of all follicular developmental stages: primordial, primary, secondary, antral, and preovulatory follicles.
Studies performed in bovine follicles proved that the proper development and function of ovarian follicles is maintained by continuous angiogenesis and that follicular vascularization appears to be crucial in achieving follicle dominance [Citation23]. Furthermore, it has been demonstrated that GCs play an important role in this process, by producing angiogenic factors [Citation24]. Therefore, investigating the GCs contribute to the development of the blood vessel network during the folliculogenesis, represents an interesting study goal. In this regard, Vasn, which is known as an important angiogenic factor, may be expressed by GCs to promote and support follicular development and oocyte maturation.
The endometrium is mainly composed of two different cell types: epithelial cells and stromal cells. Stromal cells displaying intrinsically migratory and proliferative characters contribute to the regenerative capacity of the endometrium. During the follicular phase of the menstrual cycle, it has been observed that stromal cells proliferate, thus inducing a thickening of the endometrium. On the other hand, during the luteal phase, these cells respond to progesterone exposure and decrease their replication rate. By undergoing a process of differentiation, the stromal cells provide the proper environment for the eventual embryo implantation [Citation25]. We observed that Vasn was highly expressed in the proliferative stroma endometrium, and that it was significantly less expressed in the secretory endometrium. Therefore, we could hypothesize a Vasn contribution to the proliferative process of stromal cells so that the endometrium reaches the right thickness for a possible pregnancy. In the transition from proliferative to secretory endometrium, cell proliferation is reduced in favor of the differentiation process, and this event would explain the observed downregulation of its expression.
Vasn expression, on the other hand, was absent in the myometrium, the middle layer of the uterine wall, consisting mainly of uterine smooth muscle cells. The myometric compartment changes significantly during pregnancy, mainly due to muscle hypertrophy and an increase in lymphatic vessels and blood vessels [Citation26]. Therefore, it could be of interest to investigate a possible Vasn expression in the myometrium selectively during the pregnancy.
In conclusion, our data first showed Vasn expression in the human female reproductive tissues such as ovarian tissue and endometrium. The in vitro analysis located the protein on the cell surface of GCs and ESCs and further investigations are currently ongoing on primary cell cultures to establish the function and molecular mechanisms by which Vasn acts in the human female reproductive system. Identifying the role of Vasn may have important clinical relevance considering the already well-known involvement of many members of the TGF-β superfamily in the pathophysiology of the ovary and endometrium.
Attestation statement
Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bonnet AL, Chaussain C, Broutin I, et al. From vascular smooth muscle cells to folliculogenesis: what About vasorin? Front Med (Lausanne). 2018;5:1. doi: 10.3389/fmed.2018.00335.
- Chen L, Yao JH, Zhang SH, et al. Slit-like 2, a novel zebrafish slit homologue that might involve in zebrafish Central neural and vascular morphogenesis. Biochem Biophys Res Commun. 2005;336(1):364–7. doi: 10.1016/j.bbrc.2005.08.071.
- Ahn JM, Kim BG, Yu MH, et al. Identification of diabetic nephropathy-selective proteins in human plasma by multi-lectin affinity chromatography and LC-MS/MS. Proteomics Clin Appl. 2010;4(6–7):644–653. doi: 10.1002/prca.200900196.
- Taherkhani A, Farrokhi Yekta R, Mohseni M, et al. Chronic kidney disease: a review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and IgA nephropathy biomarkers. Proteome Sci. 2019;17:7. doi: 10.1186/s12953-019-0155-y.
- Samavat S, Kalantari S, Nafar M, et al. Diagnostic urinary proteome profile for immunoglobulin a nephropathy. Iran J Kidney Dis. 2015;9(3):239–248.
- Ikeda Y, Imai Y, Kumagai H, et al. Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc Natl Acad Sci U S A. 2004;101(29):10732–10737. doi: 10.1073/pnas.0404117101.
- Agrotis A, Kalinina N, Bobik A. Transforming growth factor-beta, cell signaling and cardiovascular disorders. Curr Vasc Pharmacol. 2005;3(1):55–61. doi: 10.2174/1570161052773951.
- Sinha S, Hoofnagle MH, Kingston PA, et al. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol. 2004;287(6):C1560–8. doi: 10.1152/ajpcell.00221.2004.
- Malapeira J, Esselens C, Bech-Serra JJ, et al. ADAM17 (TACE) regulates TGFbeta signaling through the cleavage of vasorin. Oncogene. 2011;30(16):1912–1922. doi: 10.1038/onc.2010.565.
- Pintus G, Giordo R, Wang Y, et al. Reduced vasorin enhances angiotensin II signaling within the aging arterial wall. Oncotarget. 2018;9(43):27117–27132. doi: 10.18632/oncotarget.25499.
- Huang A, Dong J, Li S, et al. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 2015;11(8):961–969. doi: 10.7150/ijbs.11943.
- Li S, Li H, Yang X, et al. Vasorin is a potential serum biomarker and drug target of hepatocarcinoma screened by subtractive-EMSA-SELEX to clinic patient serum. Oncotarget. 2015;6(12):10045–10059. doi: 10.18632/oncotarget.3541.
- Liang W, Guo B, Ye J, et al. Vasorin stimulates malignant progression and angiogenesis in glioma. Cancer Sci. 2019;110(8):2558–2572. doi: 10.1111/cas.14103.
- Chen W, Wang Q, Xu X, et al. Vasorin/ATIA promotes cigarette smoke-induced transformation of human bronchial epithelial cells by suppressing autophagy-mediated apoptosis. Transl Oncol. 2020;13(1):32–41. doi: 10.1016/j.tranon.2019.09.001.
- Yeo HL, Fan TC, Lin RJ, et al. Sialylation of vasorin by ST3Gal1 facilitates TGF-beta1-mediated tumor angiogenesis and progression. Int J Cancer. 2019;144(8):1996–2007. doi: 10.1002/ijc.31891.
- Choksi S, Lin Y, Pobezinskaya Y, et al. A HIF-1 target, ATIA, protects cells from apoptosis by modulating the mitochondrial thioredoxin, TRX2. Mol Cell. 2011;42(5):597–609. doi: 10.1016/j.molcel.2011.03.030.
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. doi: 10.1530/rep.1.01074.
- Kristensen SG, Andersen K, Clement CA, et al. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20(4):293–308. doi: 10.1093/molehr/gat089.
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27(1):14–23. doi: 10.1055/s-0028-1108006.
- Rimon-Dahari N, Heinemann-Yerushalmi L, Hadas R, et al. Vasorin: a newly identified regulator of ovarian folliculogenesis. Faseb J. 2018;32(4):2124–2136. doi: 10.1096/fj.201700057RRR.
- Zamah AM, Hassis ME, Albertolle ME, et al. Proteomic analysis of human follicular fluid from fertile women. Clin Proteomics. 2015;12(1):5. doi: 10.1186/s12014-015-9077-6.
- Nordhoff V, Sonntag B, von Tils D, et al. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod Biomed Online. 2011;23(2):196–203. doi: 10.1016/j.rbmo.2011.04.009.
- Grazul-Bilska AT, Navanukraw C, Johnson ML, et al. Vascularity and expression of angiogenic factors in bovine dominant follicles of the first follicular wave. J Anim Sci. 2007;85(8):1914–1922. doi: 10.2527/jas.2007-0044.
- Chermula B, Brazert M, Izycki D, et al. New gene markers of angiogenesis and blood vessels development in porcine ovarian granulosa cells during short-term primary culture in vitro. Biomed Res Int. 2019;2019:6545210.
- Persoons E, Hennes A, De Clercq K, et al. Functional expression of TRP ion channels in endometrial stromal cells of endometriosis patients. Int J Mol Sci. 2018;19(9):2467.
- Lessey BA, Young SL. Structure, function, and evaluation of the female reproductive tract. In: Yen and Jaffe’s reproductive endocrinology. USA: Elsevier; 2019; p. 206–247.e13.