Abstract:
Objective
Polycystic ovarian syndrome (PCOS) is a common disorder that leads to infertility in reproductive-aged females. HOTAIR is highly expressed in various gynecological diseases and is associated with a poor prognosis. We aimed to explore the role of HOTAIR in PCOS.
Methods
First, PCOS rats were induced using dehydroepiandrosterone and then treated with si-HOTAIR. Next, HOTAIR mRNA expression and serum hormone levels were detected. HE staining was applied to observe estrus cycle, ovarian morphology and count the number of follicles. Apoptosis in the ovary was detected by TUNEL. Thereafter, ovarian granulosa cells (GCs) were isolated from PCOS rats, transfected with si-HOTAIR and treated with LY294002 (Akt inhibitor) or IGF-1. CCK-8 and flow cytometry assays were used to evaluate cell viability and apoptosis. IGF-1, apoptosis- and PI3K/Akt pathway-associated protein expressions in ovary and GCs were also detected.
Results
In in vivo experiments, si-HOTAIR decreased serum T, E2 and LH levels but increased FSH level, restored estrus cycle, ovarian morphology and inhibited apoptosis of ovary in PCOS rats. Meanwhile, in vitro assays showed that si-HOTAIR upregulated the viability but inhibited the apoptosis of PCOS GCs. Furthermore, both in vivo and in vitro assays revealed that si-HOTAIR increased Bcl-2 expression but suppressed Bax, Bad, IGF-1 expressions and PI3K, AKT phosphorylation. However, the aforementioned effects of si-HOTAIR in vitro were further enhanced by LY294002 and partially reversed by IGF-1.
Conclusions
HOTAIR knockdown improved PCOS, and the mechanism may relate to IGF-1-mediated PI3K/Akt pathway, indicating HOTAIR may be a novel therapeutic target for PCOS.
Introduction
Polycystic ovarian syndrome (PCOS) is a common metabolic and endocrine disorder in gynecology and is predominantly manifested with hyperandrogenism, follicular dysplasia, aberrant ovarian function as well as insulin resistance clinically [Citation1]. It is estimated that the incidence of PCOS is 5–20% in women of childbearing age, and approximately 74% of patients with PCOS experienced infertility [Citation2]. PCOS-induced infertility can accentuate patients’ psychological disorders, such as depression, anxiety, and other mental health problems, which will ultimately result in metabolic syndrome [Citation3], type 2 diabetes (T2DM) [Citation4], obesity [Citation5], and cerebrovascular diseases [Citation6]. In addition, epidemiologic studies have shown that women with PCOS have a higher risk of developing ovarian cancer (odds ratio: 2.5, 95% confidence interval 1.1–5.9) [Citation7]. At present, the most widely used drugs for PCOS therapy are clomiphene (CC) and gonadotropin-releasing hormone agonist (GnRHa); however, the effects of CC and GnRHa are not ideal (ovulation, pregnancy, and miscarriage rates are 50%, 23.9%, and 25.8%, respectively) [Citation8]. Therefore, understanding the pathogenesis of PCOS and identifying novel therapeutic targets for PCOS are crucial for improving PCOS patients’ outcomes.
Long non-coding RNAs (lncRNAs) are a kind of molecule longer than 200 nucleotides and don’t encode any protein [Citation9]. There is mounting evidence suggesting that dysregulated lncRNA expression is linked to the pathogenesis of ovarian disorders, including PCOS [Citation10]. HOTAIR, a well-studied lncRNA, has been reported to regulate the tumorigenicity and metastasis of epithelial ovarian cancer in vitro and acts as a promising target for treating epithelial ovarian cancer in humans [Citation11]. Moreover, a published study has found that HOTAIR rs920778 polymorphism is associated with the prognosis and susceptibility of ovarian cancer by collecting blood samples from 1009 subjects, and extracting their genomic DNA for genotyping [Citation12]. The mRNA and microRNA profiles of patients with PCOS were extremely similar to those of patients with ovarian cancer [Citation13], which indicates that the same molecular mechanisms may be involved in patients with ovarian cancer and PCOS. Therefore, we speculated that HOTAIR may also play a critical role in PCOS.
PI3K/Akt pathway is a crucial factor in the onset and development of PCOS. Scholars have found that PI3K/Akt pathway is changed when the body appears resistant to insulin, high level of androgen, and follicular dysplasia [Citation14]. In addition, IGF-1, a factor that can trigger the activity of the PI3K/Akt pathway, has been observed highly expressed in PCOS, and can accelerate granulosa cell (GC) apoptosis, and endocrine disorder [Citation15]. Some studies have reported that HOTAIR can activate IGF-1-mediated PI3K/Akt pathway to regulate the proliferation and apoptosis of endometrial carcinoma cells [Citation16]. In addition, HOTAIR knockdown can reduce drug resistance of breast cancer through the PI3K/Akt/mTOR pathway [Citation17]. However, little is known about the specific relationship between HOTAIR and IGF-1-mediated PI3K/Akt pathway in PCOS.
Hence, in the study, we conducted both in vitro and in vivo experiments to explore whether HOTAIR knockdown can alleviate PCOS via IGF-1-mediated PI3K/Akt pathway, thus providing a solid experimental basis for using HOTAIR as a potential therapeutic target for PCOS.
Materials and methods
Animals
Female Sprague–Dawley rats (3-week-old, 45–85 g) were provided by Shanghai Jihui Laboratory Animal Care Co., Ltd (Certificate No: SCXK(Hu) 2017-0012). The rats were housed in controlled conditions (22 ± 2 °C, 50 − 70% relative humidity, and 12 h light/dark cycle) with free access to food and water. All animal experiments in the study were performed with the approval of the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (Certificate No. SYXK (Zhe) 2021-0033) and in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Model establishment and grouping
After two days of adaptation, the rats were randomly assigned into the Control group (n = 6) and PCOS group. Then, the PCOS rat model was induced by administering dehydroepiandrosterone (DHEA), as previously reported [Citation15]. Briefly, the rats in the PCOS group received subcutaneous injections of DHEA (6 mg/100 g/day) and sesame oil (0.2 mL/day) for 20 days; while the Control rats received only 0.2 mL of sesame oil administered in the same way. After 10 days of injection, vaginal smears of the rats were collected daily and observed under a microscope. The disappeared or disordered estrous cycle indicated the successful establishment of the PCOS model. Then, the rats in the PCOS group were further assigned into the Model, si-NC and si-HOTAIR groups (n = 6) at random. The following day, 15 μg empty plasmid or si-HOTAIR (dissolved in PBS) was injected into the rats of the si-NC or si-HOTAIR group by tail vein, while the rats of the Control and Model groups were treated with equal PBS in the same manner, the injection lasted for consecutive 7 days. Both the empty plasmid and siRNA plasmid against HOTAIR were purchased from Shanghai GenePharma Co., Ltd.
Examination of the estrus cycle
Vaginal smears were performed to determine the estrus phase after the last injection of empty plasmid, si-HOTAIR or PBS. In short, sterile cotton swabs were gently inserted into rats’ vagina to extract vaginal secretions. These secretions were then fixed with ethanol, stained with HE dye at room temperature (RT) and observed under a light microscope. The stages of the estrus cycle were identified by the proportions of cornified squamous epithelial cells, nucleated epithelial cells, and leukocytes.
Sample collection and storage
All rats were euthanized by isoflurane after completing the examination of estrus cycle. The final body weights of the rats were recorded, and blood from the heart as well as ovaries were collected. The harvested blood was centrifuged at 3500 r/min for 15 min, then, the supernatants were stored at –80 °C until use. Furthermore, the dissected ovaries were weighed immediately and then kept at –80 °C for further experiments.
ELISA
The serum hormone levels, including testosterone (TT), estradiol (E2), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were analyzed using ELISA. Before ELISA, frozen samples were taken out and equilibrated to RT. After that, TT (ml059506), E2 (ml002871), LH (ml064293), and FSH (ml403115) levels in the serum of the rats were evaluated by respective commercial kits. All ELISA kits were bought from Shanghai Enzymelinked Biotechnology Co., Ltd.
HE staining
HE staining was conducted to examine the morphological changes in ovarian follicles among the rats. Specifically, the ovarian tissues were fixed in a 10% formaldehyde solution and then treated with a gradient of ethanol and xylene for dehydration and permeabilization, respectively. After being dipped in wax and embedded in paraffin, the ovarian tissue was sliced into sections (4 μm thickness). Following a series of standard procedures, the tissue sections were stained with HE solution (G1005, Servicebio) and observed under a light microscope.
TUNEL assay
Cell apoptosis of the ovary was detected through TUNEL staining. To begin, the paraffin-embedded sections were deparaffinized with xylene, hydrated in ethanol, followed by incubation with proteinase K. Subsequently, the sections were treated with a TUNEL reaction mixture (G1501, Servicebio) and stained with 4′,6‐diamidino‐2‐phenylindole (G1012, Servicebio). After sealing, the slices were observed and photographed with a fluorescence microscope.
Ovarian granulosa cell isolation and culture
The methods for isolating and culturing ovarian GCs were adapted from previous research with a little modification [Citation18]. After dissecting the ovaries, the bursa, fat, and oviducts of the ovaries were removed using a microscope. Then, the follicles were punctured to release ovarian GCs. The isolated ovarian GCs were collected through filtration with a 200 mesh cell sieve and centrifuged at 1500 rpm for 5 min. After that, the deposited ovarian GCs were cultured in DMEM/F12 medium, which contained 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, at 37 °C with 5% CO2.
Cell grouping and treatment
The collected ovarian GCs in the logarithmic growth stage were divided as follows: Control group (without any treatment), Model group (without any treatment), si-NC group (transfected with empty plasmid), si-HOTAIR group (transfected with siRNA plasmid against HOTAIR), si-HOTAIR + LY294002 group (transfected with siRNA plasmid against HOTAIR, and then treated with 20 μM Akt inhibitor LY294002) and si-HOTAIR + IGF-1 group (transfected with siRNA plasmid against HOTAIR, and then treated with 20 μM IGF-1). All ovarian GCs were extracted from PCOS rats, except for the Control group, which came from the Control rats. Both the empty plasmid and siRNA plasmid against HOTAIR were purchased from Shanghai GenePharma Co., Ltd. Based on the instructions, the cells were transfected with lipofectamine 2000 for 48 h, and then harvested for further experiments. LY294002 (S43088) and IGF-1 (PT0442) were supplied by Shanghai source leaf Biological Technology Co., Ltd and Shanghai Qiming Biological Technology Co., Ltd, respectively.
qRT-PCR
The expression of HOTAIR mRNA in ovarian tissues and GCs were assessed by qRT-PCR. The total RNA of the ovarian tissues and GCs was isolated using Trizol reagent (B511311, Sangon Biotech), following which cDNA was synthesized with reverse transcription kits (CW2569, CWBIO). The synthesized cDNA was quantified by qRT-PCR using SYBR Premix Ex TaqII (RR820A, Takara). GAPDH served as an internal reference, and the relative expression level of HOTAIR mRNA was calculated using the 2–ΔΔCt method. The primer sequences used in this study are presented in .
Table 1. qRT-PCR primers.
CCK-8 assay
Cell viability was evaluated using the CCK-8 assay. Briefly, ovarian GCs were cultured in 96-well plates and then treated with or without 20 μM LY294002 or IGF-1 for 24, 48, and 72, respectively. Subsequently, 10 μL of CCK-8 solution (HY-K0301, MCE) was added to each well followed by 1.5 h incubation. The absorbance of each well was measured with a microplate reader at 450 nm. Afterward, the viability of ovarian GCs under different treatments was calculated based on their corresponding absorbance.
Flow cytometry analysis
Apoptosis of cells was detected by Annexin V-FITC/PI apoptosis detection kit (556547, BD). Generally, ovarian GCs were grown in 6-well plates at a concentration of 1.2 × 106 cells/well and then treated with or without 20 μM LY294002 or IGF-1 for 24 h. Following this, the cells were washed and the density was adjusted again to 1.2 × 106/mL. Subsequently, Annexin V-FITC (5 μL) and PI (10 μL) solution were added to the cells and incubated in the dark room for 15 min. Finally, the apoptotic rate of the cells was assessed using a flow cytometer (C6, BD) after adding 400 μL of binding buffer.
Western blotting
The total protein of ovarian tissues and GCs was extracted by radioimmunoprecipitation buffer (P0013D, Biyuntian), separated with 10% SDS-PAGE and transferred to PVDF membranes. After hindering with 5% nonfat milk, the membranes were incubated overnight at 4 °C with primary antibodies against Bcl-2 (AF6139), Bax (AF0120), Bad (AF6471), IGF-1 (DF6096), PI3K (AF7022), p-PI3K (AF3242), AKT (AF4718), p-AKT (AF0016), and GAPDH (AF7021). Then, the membranes were probed with HRP-conjugated secondary antibody (1:600, DF6096, CST) for 1 h. The bands were visualized using chemiluminescence and quantified by ImageJ software. GAPDH served as an internal control. All primary antibodies were purchased from Affinity and used at a dilution of 1:1000, except for GADPH, which was used at a dilution of 1:10,000.
Statistical analysis
The study’s data were presented as mean ± SD, and analyzed by SPSS 19.0. One-way ANOVA and Tukey tests were conducted to analyze differences among multiple groups. The Kruskal–Wallis H test was applied when variances were not equal. A p < .05 was considered a statistically significant difference.
Results
HOTAIR downregulation recovered the body and ovary weights as well as serum hormone levels in PCOS rats
The body and ovary weights of the rats were detected in the study. It could be seen from treatment with DHEA significantly increased the body and ovary weights of the rats in the Model and si-NC groups (p < .01). However, relative to the Model group, the weights of the body and ovary were obviously decreased in the si-HOTAIR group (p < .05).
Figure 1. HOTAIR downregulation regulated the weights of the body and ovary as well as the levels of serum hormones in PCOS rats. (A) The changes in body weight in rats; (B) Photographs of ovaries and the changes of ovarian weight in rats; (C) The expression of HOTAIR mRNA in the ovarian tissues among the rats. (D) The levels of serum hormones in rats. Unless otherwise specified, all results were presented as mean ± SD. +p < .05, and ++p < .01 vs.Control; *p < .05, and **p < .01 vs. Model; #p < .05, and ##p < .01 vs. si-HOTAIR in all figures. Note: PCOS, polycystic ovarian syndrome; TT, testosterone; E2, estradiol; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
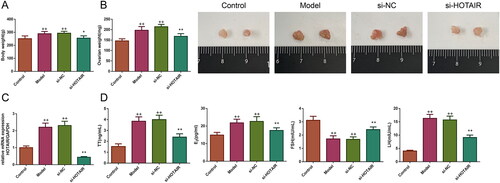
Subsequently, the expression of HOTAIR mRNA in the ovarian tissues was detected. As illustrated in , the result revealed there was a significant upregulation of HOTAIR mRNA expression in the ovarian tissues of the Model and si-NC groups (p < .01). However, following si-HOTAIR treatment, the expression level of HOTAIR mRNA was downregulated significantly (p < .01).
Furthermore, serum TT, E2, LH, and FSH levels were also measured in all rats to test the effect of si-HOTAIR on serum hormones (). The results revealed that the levels of serum TT, E2, and LH were significantly upregulated and the level of serum FSH was greatly downregulated in the rats of the Model and si-NC groups (p < .01), whereas these conditions were reversed after si-HOTAIR injection (p < .01).
HOTAIR downregulation improved the estrus cycle of PCOS rats
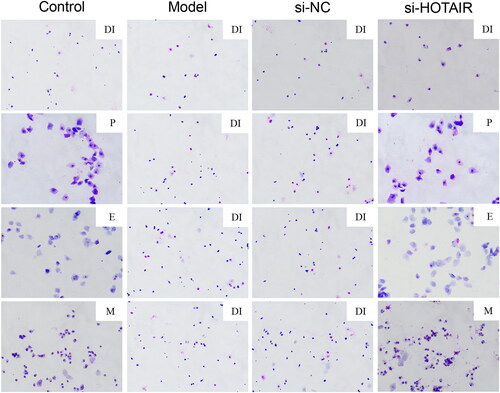
The vaginal smears of the rats were analyzed using HE staining in the study, and the results are presented in . The rats in the Control group displayed a normal estrus cycle, including proestrus (P), estrus (E), metestrus (M), and diestrus (DI) stages. However, the rats in the Model and si-NC groups had irregulated estrous cycle, which were basically in the DI stage. Nevertheless, si-HOTAIR treatment led to a relatively complete estrous cycle in rats with PCOS.
HOTAIR downregulation recovered the ovarian morphology of the PCOS rats
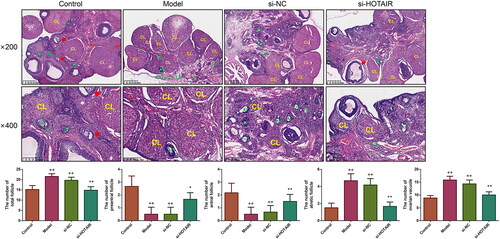
HE staining was applied to observe the impact of si-HOTAIR on ovarian morphological changes as well as follicle number. The results revealed that the ovarian morphology of the Control rats was normal, with visible follicles at different development stages, in addition, there were many lutea and normal oocytes on the surface of ovarian sections (). However, the Model and si-NC groups exhibited significant disordered ovarian structures, including ovarian fibrosis, decreased antral follicle and preantral follicle, as well as increased atretic follicle. After treatment with si-HOTAIR, it could be found that the morphological changes in the ovaries in the Model and si-NC groups were partially restored, the number of GCs was increased and arranged neatly as a radial pattern; furthermore, follicles with different stages also appeared.
Figure 3. HOTAIR downregulation improved the ovarian morphology of the PCOS rats. The ovarian morphology of the rats was observed by HE staining, and the number of total follicle, preantral follicle, antral follicle, atretic follicle as well as ovarian vacuole were also counted under microscope. PF, preantral follicle; AF, atretic follicle; OV, ovarian vacuole; antral follicle was indicated by the red arrow. Magnification, ×200 and ×400.
HOTAIR downregulation suppressed the apoptosis of ovarian tissues in PCOS rats
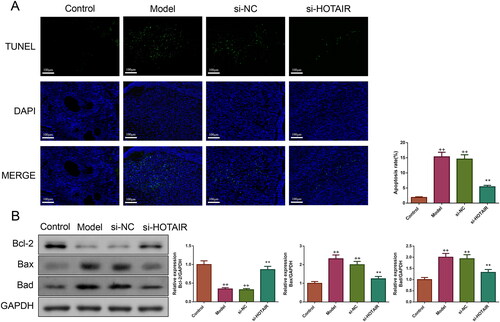
TUNEL staining was conducted to evaluate the impact of si-HOTAIR on cell apoptosis in rat ovarian tissues. The results presented in showed that the rats in the Model and si-NC groups had a higher apoptosis rate in ovarian tissues than those in the Control group. However, si-HOTAIR injection remarkably suppressed the elevated apoptosis rate (p < .01). Then, the expressions of Bcl-2, Bax, and Bad proteins were assessed by western blotting to further verify the effect of si-HOTAIR on the inhibition of apoptosis in PCOS rats. Relative to the Control group, the expressions of Bax and Bad proteins were increased and the expression of Bcl-2 protein was decreased in the Model and si-NC group, but these changes were reversed by si-HOTAIR (p < .01, ).
Figure 4. HOTAIR downregulation inhibited the apoptosis of ovarian tissues in PCOS rats. A: The cell apoptosis of the ovarian tissues in the rats was detected by TUNEL. Magnification, ×200. B: Bcl-2, Bax, and Bad protein expressions of the ovarian tissues in the rats were measured by western blotting.
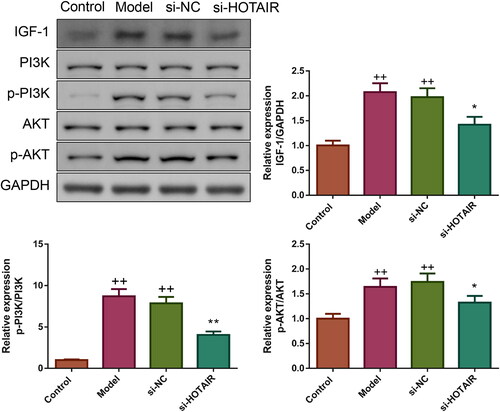
HOTAIR downregulation inhibited IGF-1 expression and PI3K/Akt pathway in PCOS rats
Then, the influence of si-HOTAIR on the expressions of IGF-1 and PI3K/Akt pathway-associated proteins was determined by western blotting. It could be observed that the expression of IGF-1 protein and the ratios of p-PI3K/PI3K and p-Akt/Akt in the Model and si-NC groups were higher than in the Control group (p < .01, ). Yet, compared to the Model group, si-HOTAIR group exhibited reduced IGF-1 protein expression as well as PI3K and Akt phosphorylation (p < .05).
HOTAIR downregulation elevated viability and inhibited apoptosis in ovarian GCs of PCOS
The interference of HOTAIR expression in GCs was verified by qRT-PCR. As indicated in , HOTAIR mRNA expression was increased in both Model and si-NC groups (p < .05). Compared to the Model group, the si-HOTAIR group displayed a clear reduction in HOTAIR mRNA expression, which suggested successful interference of HOTAIR expression in PCOS rats’ ovarian GCs.
Figure 6. HOTAIR downregulation elevated viability and inhibited apoptosis in ovarian GCs of PCOS. A: The expression of HOTAIR mRNA in ovarian GCs was assessed by qRT-PCR. B: The cell viability of ovarian GCs was evaluated by CCK-8. C: The apoptosis of ovarian GCs was measured by flow cytometry. D: The Bcl-2, Bax, and Bad protein expressions of the ovarian GCs were detected by western blotting. Note: GCs, granulosa cells.
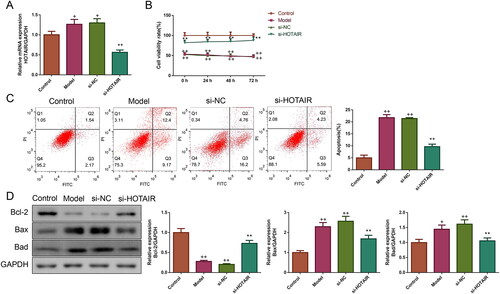
The effect of si-HOTAIR on ovarian GCs’ viability was detected by CCK-8 assay. As illustrated in , the viability of ovarian GCs in the Model and si-NC groups was significantly lower than in the Control group (p < .01). Upon transfection with si-HOTAIR, the viability of ovarian GCs in the si-HOTAIR group was remarkably upregulated (p < .01).
The effect of si-HOTAIR on ovarian GCs’ apoptosis was also valued using a flow cytometry assay. As expected, it could be seen that the apoptosis rate of ovarian GCs in the Model and si-NC groups was obviously increased, but si-HOTAIR transfection significantly downregulated the apoptosis rate (p < .01, ). The expressions of apoptosis-associated proteins (including Bcl-2, Bax and Bad) in the in vitro assay showed similar results to those observed in vivo ().
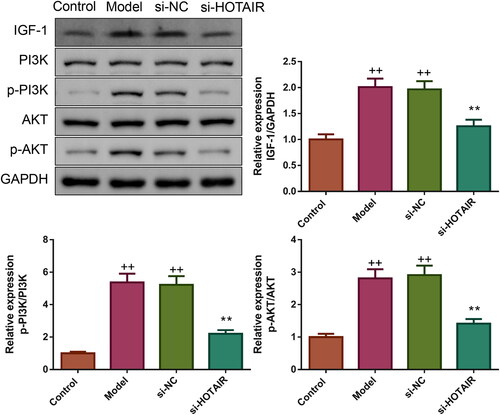
HOTAIR downregulation suppressed IGF-1 expression and PI3K/Akt pathway in ovarian GCs of PCOS
Furthermore, the influence of si-HOTAIR on IGF-1 expression as well as PI3K and Akt phosphorylation was also evaluated by western blotting. The expression of IGF-1 and phosphorylation of PI3K and Akt were elevated significantly in the Model and si-NC groups (p < .01, ). However, upon transfection with si-HOTAIR, IGF-1 expression, as well as PI3K and Akt phosphorylation showed a trend to the Control group (p < .01).
HOTAIR downregulation attenuated PCOS in vitro via IGF-1 mediated PI3K/Akt pathway
For exploring the role of IGF-1-mediated PI3K/Akt pathway in the attenuation of PCOS by si-HOTAIR, the PCOS ovarian GCs transfected with si-HOTAIR were treated with 20 μM LY294002 (Akt inhibitor) or 20 μM IGF-1. The results of the CCK-8 assay were exhibited in , relative to the si-HOTAIR group, the si-HOTAIR + LY294002 group showed increased cell viability while the si-HOTAIR + IGF-1 group displayed decreased cell viability (p < .05). In addition, flow cytometry results also found that the apoptosis rate of ovarian GCs was lower in the si-HOTAIR + LY294002 and higher in the si-HOTAIR + IGF-1 group than the si-HOTAIR group (p < .01, ). The relative expressions of Bcl-2, Bax, and Bad proteins depicted in further demonstrated that LY294002 inhibited while IGF-1 facilitated the apoptosis of ovarian GCs from PCOS rats ().
Figure 8. HOTAIR downregulation improved PCOS in vitro via IGF-1-mediated PI3K/Akt pathway. A: The viability of the ovarian GCs was tested by CCK-8. B: The apoptosis of the ovarian GCs was detected by flow cytometry. C: The Bcl-2, Bax, and Bad protein expressions of the ovarian GCs were detected by western blotting.
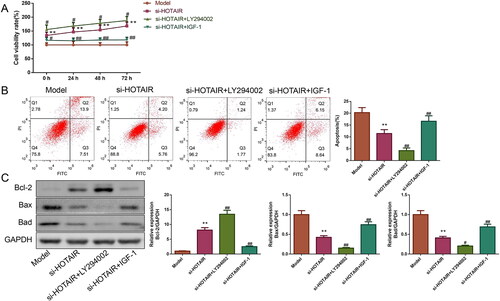
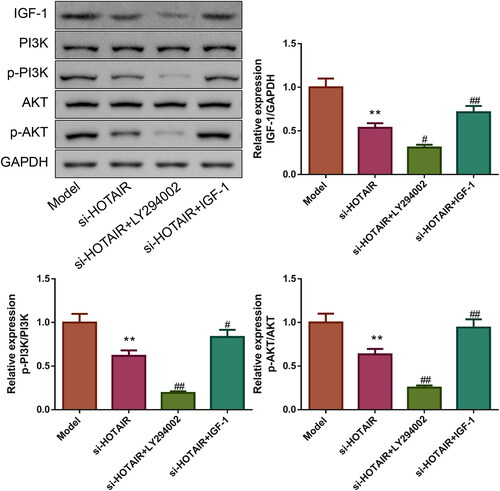
Finally, we attempted to elucidate the mechanism by which si-HOTAIR alleviated PCOS via detecting the expressions of PI3K/Akt pathway-related proteins (). Compared to the si-HOTAIR group, the expression of IGF-1 and the ratios of p-PI3K/PI3K and p-Akt/Akt were downregulated in the si-HOTAIR + LY294002 group but upregulated in the si-HOTAIR + IGF-1 group (p < .05).
Discussion
PCOS, the leading cause of infertility in women, is a complex and multifactorial disease. Given the continued increase in the incidence of PCOS in recent years and its negative impact on women of reproductive age, it is important to explore the development mechanism of PCOS and develop effective therapies for PCOS patients [Citation19]. In this study, from PCOS animal model and cellular model, we found that downregulated lncRNA HOTAIR could improve PCOS through IGF-1-mediated PI3K/Akt pathway.
DHEA induction is a classic and common method for establishing PCOS models in rodents. High androgen level in the serum is one of the most important clinical features of PCOS [Citation20]. Injection of DHEA leads to an increase in androstenedione level in mice, leading to a constant state of hyperandrogenism, which impedes normal development of the follicle and ultimately results in ovulation disorders and ovarian polycystic changes [Citation21]. A published report has found that DHEA-induced PCOS rats exhibit symptoms similar to the clinical characteristics observed in patients with PCOS, such as obesity, hyperandrogenism, as well as morphological alterations in the polycystic ovary [Citation22]. In addition, Xing et al. have demonstrated that the rats exposed to DHEA exhibited typical PCOS-like features, such as increased bodyweight, upregulated ovarian index, disturbed estrous cycle, abnormal sex hormone contents in the serum, as well as aberrant ovarian morphology [Citation23]. Therefore, in the present study, rat PCOS models were set up by subcutaneous injection of DHEA. Consistently, we observed similar symptoms to those described previously in DHEA-exposed rats, such as increased body weight and ovarian weight, aberrant serum hormone levels, as well as abnormal estrous cycle and ovarian morphology, indicating the successful establishment of PCOS rat models.
HOTAIR, a well-studied lncRNA, is highly expressed in some gynecological diseases and accumulating evidence indicates that HOTAIR overexpression is associated with a poor clinical outcome [Citation24]. It has been reported that HOTAIR overexpression leads to paclitaxel resistance in ovarian cancer [Citation25], whereas HOTAIR knockdown suppresses cell proliferation and arrests cell cycle at the G1 stage [Citation26]. In addition, Lee et al. have revealed that HOTAIR expression is significantly higher in cervical cancer patients, and this increase is strongly related to lymph node metastasis, lymphovascular space invasion, tumor size as well as patient’s prognosis. They also performed cellular experiments and proved that the downregulating HOTAIR expression can alleviate cervical cancer [Citation27]. In line with previous research, this study found that all abnormalities in PCOS rats were improved after downregulating HOTAIR expression, suggesting that inhibiting HOTAIR expression could attenuate PCOS in vivo.
Follicular dysplasia is another cardinal characteristic of patients with PCOS, and numerous studies have revealed that abnormal follicular development in PCOS is strongly related to the apoptosis of GCs [Citation28]. As a guarder of ovum, GCs generate growth factors and steroid hormones, provide nutrients for oocytes, and maintain a specialized microenvironment, thus playing a vital role in follicle development [Citation28]. Ovarian GCs apoptosis will accelerate the recruitment of follicles into the growth phase, thereby reducing the follicular reserve capacity of the ovary and decreasing the fecundity in females [Citation29]. Published studies have revealed that follicular development will be atretic if 10% of GCs undergo apoptosis [Citation30], and nearly all follicles are disappeared through programmed cell death due to apoptosis of granulosa cells [Citation31]. Bcl-2, Bax, and Bad are the members of the Bcl-2 family, which plays a crucial role in the mitochondrial apoptosis pathway. Aberrant expressions of Bcl-2, Bax, and Bad proteins can promote the apoptosis of GCs in PCOS rats [Citation19]. In this research, we observed the apoptosis rates were elevated, and the protein expressions of the Bcl-2 family were abnormal in PCOS rats’ ovarian tissues or GCs. However, upon downregulation of HOTAIR expression, the apoptosis was suppressed and the expressions of apoptosis-associated proteins were reversed, suggesting that downregulation of HOTAIR expression might alleviate PCOS by regulating GCs apoptosis.
Recently, an increasing number of scholars have investigated IGF-1-mediated PI3K/Akt pathway in relation to PCOS. Their research suggests that IGF-1-mediated PI3K/Akt pathway has an important role in regulating ovarian GC apoptosis in PCOS [Citation32]. HOTAIR positively regulates IGF-1 expression by inducing methylation of the miR-196b gene promoter [Citation33] or controlling miR-130a expression [Citation15]. Then, IGF-1 can stimulate the PI3K/Akt pathway by affecting TSC1/2 phosphorylation [Citation34], thereby further regulating the downstream proteins of the PI3K/Akt pathway, such as Bcl-2 and Bax [Citation35]. Some studies have found that activation of the PI3K/Akt pathway can ameliorate ovarian dysfunction [Citation36]. However, it is worth noting that the effect of PI3K/Akt on PCOS seems to be dual. The overactive PI3K/Akt pathway will produce many immature follicles, which further exacerbate PCOS [Citation14]. Some available data have proved that the PI3K/Akt pathway predominantly affects ovarian GCs [Citation37]. For example, Abramovich et al. have demonstrated that inhibiting the PI3K/Akt pathway can facilitate the proliferation of GCs and prevent the apoptosis of follicles [Citation38]. In addition, Fukuda et al. have proved that the PI3K/Akt pathway is associated with androgen generation, and believe that activating the PI3K/Akt pathway is the primary cause of PCOS [Citation39]. There are very few studies have mentioned the use of the PI3K/Akt pathway inhibitor in the treatment of PCOS. In this study, we observed the conditions of PCOS models, both in vivo and in vitro, were alleviated after treatment with si-HOTAIR, furthermore, the expression of IGF-1 as well as phosphorylation of PI3K and Akt were also downregulated, which were consistent with previous research. Interestingly, after culture with Akt inhibitor, ovarian GCs exhibited higher cell viability and lower apoptosis, while treatment with IGF-1 produced an opposite effect. All of these implied that downregulating HOTAIR expression might improve PCOS through IGF-1 mediated PI3K/Akt pathway.
Although the presented data illustrated the role and mechanism of HOTAIR in PCOS based on animal and cell experiments, there is no useful clinical information to support these findings, which is the limitation of the study. To address this, in the future, we will perform clinical experiments to further validate the key funding of the paper.
In conclusion, the present study demonstrated that downregulation of HOTAIR expression can alleviate PCOS both in vivo and in vitro and the specific mechanism is associated with the IGF-1 mediated PI3K/Akt pathway. These important findings of the study may provide a solid experimental basis for HOTAIR as a promising therapeutic target for PCOS.
Acknowledgment
Not applicable.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
All the data are contained in the manuscript.
Additional information
Funding
References
- Yu Y, Tan P, Zhuang Z, et al. DIA proteomics analysis through serum profiles reveals the significant proteins as candidate biomarkers in women with PCOS. BMC Med Genomics. 2021;14(1):1. doi: 10.1186/s12920-021-00962-7.
- Fan H, Hong X, Zeng J, et al. Differences in the individual curative effect of acupuncture for obese women with polycystic ovary syndrome based on metagenomic analysis: study protocol for a randomized controlled trial. Trials. 2021;22(1):454. doi: 10.1186/s13063-021-05426-y.
- Gongadashetti K, Gupta P, Dada R, et al. Follicular fluid oxidative stress biomarkers and ART outcomes in PCOS women undergoing in vitro fertilization: a cross-sectional study. Int J Reprod Biomed. 2021;19(5):449–11. doi: 10.18502/ijrm.v19i5.9254.
- Sharpe A, Morley L, Tang T, et al. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;12(12):CD013505. doi: 10.1002/14651858.Cd013505.
- Kim C, Chon S, Lee S. Effects of lifestyle modification in polycystic ovary syndrome compared to metformin only or metformin addition: a systematic review and meta-analysis. Sci Rep. 2020;10(1):7802. doi: 10.1038/s41598-020-64776-w.
- Sun Y, Wang W, Shen Q, et al. Waist circumference coupled with either HDL-C or TG can be used as a diagnostic marker for metabolic syndrome in Chinese women with polycystic ovary syndrome. Int J Endocrinol. 2018;2018:6102085. doi: 10.1155/2018/6102085.
- Hancerliogullari N, Tokmak A, Kuru Pekcan M, et al. Evaluation of serum human epididymis protein 4 levels in women with polycystic ovary syndrome. Cureus. 2019;11(9):e5736. doi: 10.7759/cureus.5736.
- Legro R, Barnhart H, Schlaff W, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. doi: 10.1056/NEJMoa063971.
- Tan S, Pastori C, Penas C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 2018;17(1):74. doi: 10.1186/s12943-018-0822-0.
- Zhen J, Li J, Li X, et al. Downregulating lncRNA NEAT1 induces proliferation and represses apoptosis of ovarian granulosa cells in polycystic ovary syndrome via microRNA-381/IGF1 axis. J Biomed Sci. 2021;28(1):53. doi: 10.1186/s12929-021-00749-z.
- Zhou Y, Chen D, Chu Y, et al. HOTAIR[effects of targeting lncRNA on the invasion and nude mouse tumorigenicity of epithelial ovarian cancer cells]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49(3):352–357.
- Qiu H, Wang X, Guo R, et al. HOTAIR rs920778 polymorphism is associated with ovarian cancer susceptibility and poor prognosis in a Chinese population. Future Oncol. 2017;13(4):347–355. doi: 10.2217/fon-2016-0290.
- Zou J, Li Y, Liao N, et al. Identification of key genes associated with polycystic ovary syndrome (PCOS) and ovarian cancer using an integrated bioinformatics analysis. J Ovarian Res. 2022;15(1):30. doi: 10.1186/s13048-022-00962-w.
- Li T, Mo H, Chen W, et al. Role of the PI3K-Akt signaling pathway in the pathogenesis of polycystic ovary syndrome. Reprod Sci. 2017;24(5):646–655. doi: 10.1177/1933719116667606.
- Jiang B, Xue M, Xu D, et al. Down-regulated lncRNA HOTAIR alleviates polycystic ovaries syndrome in rats by reducing expression of insulin-like growth factor 1 via microRNA-130a. J Cell Mol Med. 2020;24(1):451–464. doi: 10.1111/jcmm.14753.
- Zhang X, Hu P, Xie Y, et al. Long noncoding RNA HOTAIR promotes endometrial carcinoma cell proliferation by binding to PTEN via the activating phosphatidylinositol 3-Kinase/akt signaling pathway. Mol Cell Biol. 2019;39(23):e00251-19. doi: 10.1128/mcb.00251-19.
- Li Z, Qian J, Li J, et al. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2019;18(1):435–442. doi: 10.3892/etm.2019.7629.
- Jiajie T, Yanzhou Y, Hoi-Hung A, et al. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci Rep. 2017;7:41304. doi: 10.1038/srep41304.
- Wang C, Ding C, Hua Z, et al. Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome via IGF-1-PI3K/Akt-Bax/bcl-2 pathway. Mediators Inflamm. 2020;2020:8865647. doi: 10.1155/2020/8865647.
- Cao J, Maowulieti G, Yu T. Effect of testosterone on the expression of PPARγ mRNA in PCOS patients. Exp Ther Med. 2019;17(3):1761–1765. doi: 10.3892/etm.2018.7101.
- Erlandsson M, Bian L, Jonsson I, et al. Metastasin S100A4 is a mediator of sex hormone-dependent formation of the cortical bone. Mol Endocrinol. 2013;27(8):1311–1321. doi: 10.1210/me.2012-1398.
- Wu L, Wang Y, Zhan Y, et al. Dulaglutide, a long-acting GLP-1 receptor agonist, can improve hyperandrogenemia and ovarian function in DHEA-induced PCOS rats. Peptides. 2021;145:170624. doi: 10.1016/j.peptides.2021.170624.
- Xing L, Chen Y, He Z, et al. Acupuncture improves endometrial angiogenesis by activating PI3K/AKT pathway in a rat model with PCOS. Evid Based Complement Alternat Med. 2022;2022:1790041. doi: 10.1155/2022/1790041.
- Zhao W, Dong L. Long non-coding RNA HOTAIR overexpression improves premature ovarian failure by upregulating notch-1 expression. Exp Ther Med. 2018;16(6):4791–4795. doi: 10.3892/etm.2018.6750.
- Jiang J, Wang S, Wang Z, et al. HOTAIR promotes paclitaxel resistance by regulating CHEK1 in ovarian cancer. Cancer Chemother Pharmacol. 2020;86(2):295–305. doi: 10.1007/s00280-020-04120-1.
- Li J, Yang S, Su N, et al. Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the wnt/β-catenin pathway in human ovarian cancer. Tumour Biol. 2016;37(2):2057–2065. doi: 10.1007/s13277-015-3998-6.
- Lee M, Kim H, Kim S, et al. The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the notch pathway. Oncotarget. 2016;7(28):44558–44571. doi: 10.18632/oncotarget.10065.
- Wang S, Lin S, Zhu M, et al. Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/akt signaling pathway. Int J Mol Sci. 2019;20(24):6311. doi: 10.3390/ijms20246311.
- Batchvarov I, Taylor R, Bustamante-Marín X, et al. A grafted ovarian fragment rescues host fertility after chemotherapy. Mol Hum Reprod. 2016;22(12):842–851. doi: 10.1093/molehr/gaw064.
- Zhang H, Luo Q, Lu X, et al. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res Ther. 2018;9(1):20. doi: 10.1186/s13287-017-0745-5.
- Mfoundou J, Guo Y, Liu M, et al. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult Sci. 2021;100(8):101191. doi: 10.1016/j.psj.2021.101191.
- Liu M, Qiu Y, Xue Z, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):3. doi: 10.1186/s13287-019-1508-2.
- Lang Y, Xu X, Liu Y, et al. Ghrelin relieves Obesity-Induced myocardial injury by regulating the epigenetic suppression of miR-196b mediated by lncRNA HOTAIR. Obes Facts. 2022;15(4):540–549. doi: 10.1159/000523870.
- Puppa M, Gao S, Narsale A, et al. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J. 2014;28(2):998–1009. doi: 10.1096/fj.13-240580.
- Wang J, Wang S, Chen L, et al. SCARA5 suppresses the proliferation and migration, and promotes the apoptosis of human retinoblastoma cells by inhibiting the PI3K/AKT pathway. Mol Med Rep. 2021;23(3):202. doi: 10.3892/mmr.2021.11841.
- Xie F, Zhang J, Zhai M, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162(1):73–82. doi: 10.1530/rep-20-0643.
- Mani A, Fenwick M, Cheng Z, et al. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction. 2010;139(1):139–151. 19819918 doi: 10.1530/rep-09-0050.
- Abramovich D, Irusta G, Parborell F, et al. Intrabursal injection of vascular endothelial growth factor trap in eCG-treated prepubertal rats inhibits proliferation and increases apoptosis of follicular cells involving the PI3K/AKT signaling pathway. Fertil Steril. 2010;93(5):1369–1377. 19328472 doi: 10.1016/j.fertnstert.2009.01.127.
- Fukuda S, Orisaka M, Tajima K, et al. Luteinizing hormone-induced akt phosphorylation and androgen production are modulated by MAP kinase in bovine theca cells. J Ovarian Res. 2009;2(1):17. 19917087 doi: 10.1186/1757-2215-2-17.