Abstract
Background
Polycystic ovary syndrome (PCOS) is a complicated gynecological endocrine disease that occurs in women of childbearing age. Protocatechuic acid is a phenol-rich compound derived from herbs and owns vital functions in numerous diseases. Howbeit, protocatechuic acid’s impact on PCOS is unknown.
Methods
A combination of in vivo and in vitro models was examined in this study. C57BL/6 mice were injected subcutaneously daily with dehydroepiandrosterone to establish a PCOS mouse model, and protocatechuic acid was intraperitoneally injected into PCOS mice. Granulosa cells of PCOS ovaries were also isolated. The function of protocatechuic acid was appraised using enzyme-linked immunosorbent assay, hematoxylin–eosin staining, reactive oxygen species (ROS) and LC3 levels analysis, flow cytometry, quantitative real-time PCR, and western blot. Meanwhile, the mechanism of protocatechuic acid was assessed with a series of molecular experiments.
Results
Protocatechuic acid owned no apparent toxic effect on mice. Functionally, protocatechuic acid owned a function of mitigating PCOS in vivo. Meanwhile, protocatechuic acid repressed ROS, autophagy, and apoptosis of PCOS ovarian granulosa cells in vitro. Mechanistically, rescue assays elucidated that the protective function of protocatechuic acid against PCOS was interrelated to the activation of the PI3K/AKT/mTOR axis.
Conclusion
Protocatechuic acid alleviated PCOS symptoms in mice through PI3K signaling in granulosa cells to reduce ROS levels and apoptosis.
Introduction
Polycystic ovary syndrome (PCOS) is a prominent cause of ovulation dysfunction and impacts approximately 20% of women of reproductive age globally [Citation1,Citation2]. The principal clinical symptoms of PCOS are irregular menstruation, infertility, oligoovulation, and accompanied metabolic abnormalities [Citation3]. Despite the huge incidence of PCOS and its adverse effects on women’s health, little is known about its exact pathogenesis. Thus, elucidating PCOS pathogenesis is conducive to investigating effective drugs for PCOS.
Cumulative studies elucidate that granulosa cells are essential in follicular formation. For example, the normal proliferation of granulosa cells offers an appropriate microenvironment for oocyte maturation, initiation of growth, and follicular atresia [Citation4]. Dysfunction associated with granulosa cells might lead to abnormal folliculin and eventually to anovulatory infertility [Citation5]. Reactive oxygen species (ROS) are produced in numerous pathological processes [Citation6]. The increase of ROS modifies cell signaling proteins, which in turn mediates pathological processes such as atherosclerosis and inflammation [Citation7,Citation8]. The antioxidant mechanism in vivo maintains ROS at low contents, which is conducive to cell normal function [Citation9]. Critically, previous studies expound that reducing ovarian granulosa cell apoptosis and ROS levels in cells is conducive to alleviating PCOS. For instance, kisspeptin reduces ROS production and accelerates ovarian granulosa cell proliferation via activating PI3K/AKT and ERK axes, alleviating apoptosis and oxidative stress [Citation10]. The GTPase immunity-associated protein (GIMAP) 7 accelerates the PCOS process by inducing oxidative stress and apoptosis of PCOS ovarian granulosa cells [Citation11]. Hence, restraint of granulosa cell apoptosis and ROS levels in cells supplies novel therapeutic strategies for PCOS.
Autophagy affects cell survival by maintaining cellular bioenergy and removing proteins and damaged organelles [Citation12]. In ovaries, oocytes do not develop normally without autophagy, and defects in follicular autophagy lead to female infertility [Citation13,Citation14]. As has been reported, some pathophysiological changes of PCOS are interrelated to the autophagy and apoptosis of ovarian granulosa cells. For instance, chemerin is overexpressed in PCOS rats, and chemerin enhances autophagy through PI3K/Akt/mTOR, thus speeding up the PCOS process [Citation15]. Guizhi Fuling Wan is a kind of traditional Chinese medicine used to remove blood stasis and dissipate phlegm in the treatment of gynecological diseases. Guizhi Fuling Wan alleviates ovulation disorder in PCOS rats by restraining granulocellular autophagy and accelerating follicular development [Citation16]. Thus, the discovery of drugs that repress the autophagy of granulosa cells owns the potential to alleviate PCOS.
Until now, PCOS has mainly been treated with drugs. For instance, Chen et al. validated that resveratrol mitigates PCOS through transzonal projections within oocyte-granulosa cell communication [Citation17]. Ma et al. expounded that Soy isoflavones ameliorate ovarian morphology and hormone disorders in PCOS rats by enhancing anti-inflammatory and antioxidant capacity [Citation18]. Protocatechulic acid is a phenol-rich compound derived from herbs, grains, and fruits, including plums, grapes, almonds, onions, grain brown rice, and citrus fruits [Citation19]. Protocatechuic acid owns a wide range of biological functions, containing anti-inflammatory, antioxidant, and antiviral [Citation20,Citation21]. However, the impact of protocatechuic acid on PCOS has not been reported.
In the current study, protocatechuic acid alleviated PCOS symptoms in mice in vivo. Meanwhile, protocatechuic acid reduced ROS, autophagy, and apoptosis of PCOS ovarian granulosa cells in vitro. Based on these findings, this research aimed to further reveal the mechanism by which protocatechuic acid alleviated PCOS, supplying promising drugs for PCOS remedies.
Materials and methods
Animals
C57BL/6 mice (4-week-old, female) were offered by Cyagen (Suzhou, China). All mice were kept in 12-h light/dark cycle with feed and water. This research was approved by the animal ethics committee in the Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (2022DW-64-02).
Construction of PCOS mice
The mice are divided into seven groups, including control, PCOS, protocatechuic acid low, protocatechuic acid medium, protocatechuic acid high, metformin, and compound groups. Each group was randomly assigned to six mice. C57BL/6 mice were injected subcutaneously daily with dehydroepiandrosterone (DHEA, 6 mg/100 g body weight, dissolved in 0.1 mL of sesame oil, Sigma, Shanghai, China) for 21 days to establish a PCOS mouse model [Citation22]. The control group consisted of normally reared mice. For mice in protocatechuic acid low, protocatechuic acid medium, and protocatechuic acid high, 10, 20, and 40 mg/kg protocatechuic acid (HY-N0294, 99.99%, C7H6O4, MedChemExpress, New Jersey, USA) were intraperitoneally injected into PCOS mice for another 8 days [Citation23,Citation24]. For mice in metformin, metformin (HY-B0627, 99.64%, C4H11N5, MedChemExpress) was administered to PCOS mice by oral gavage through drinking water at a dose of approximately 200 mg/kg/day for 8 days [Citation25]. For mice the compound group, PCOS mice were daily injected with 2 mg of LY294002 (Selleck) for 8 days. The body weight of mice was examined weekly. Eight days later, all mice were anesthetized and sacrificed, and their blood, urine, ovarian tissues, heart, liver, spleen, lung, and kidney were separated for subsequent research.
Enzyme-linked immunosorbent assay
Mouse blood samples were centrifuged (4 °C, 2500 rpm, 15 min) to acquire serum samples. Serum levels of luteinizing hormone (LH), testosterone (T), follicle stimulation hormone (FSH), anti-Müllerian hormone (AMH), and estradiol (E2) were examined using commercial kits offered by Abcam (Cambridge, UK).
Aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), white blood cell count (WBC), red blood cell count (RBC), and platelets (PLT) contents in blood samples were determined using Automated Hematology Analyzer (Mindray, Shenzhen, China).
Hematoxylin–eosin staining
Mouse ovarian, heart, liver, spleen, lung, and kidney tissues were embedded in paraffin and made into 5 μm sections. Thereafter, sections were fixed with 4% paraformaldehyde (Beyotime, Shanghai, China) for 3 h. Sections were dyed with Hematoxylin and Eosin Kits (Abcam). All histological sections were visualized using an optical microscope (WUMO, Shanghai, China). Pathological analysis of mouse ovarian tissues was evaluated by two independent observers who were not aware of group information.
Isolation of PCOS ovarian granulosa cells
Granulosa cells were collected from ovarian tissues using PBS (containing 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, Gibco™) follicular puncture following the reported methods [Citation26]. The isolated ovarian granulosa cells were maintained in DMEM medium/F12 (Procell, Wuhan, China) with 1% gentamicin (Procell) and 10% FBS (Procell), and cells were grown in 5% CO2 at 37 °C.
Cell treatment
To verify whether PI3K/AKT/mTOR axis mediated PCOS ovarian granulosa cell growth, PCOS ovarian granulosa cells were exposed to 25 µmol/L LY294002 (HY-10108, 99.92%, C19H17NO3, MedChemExpress) for 1.5 h [Citation27]. LY294002 (Selleck) is a PI3K inhibitor.
ROS and LC3 levels analysis
PCOS ovarian granulosa cells (3 × 104) were seeded into 12-well plates overnight. Then cells were dyed with 1 μM DCFH2DA probe (MedChemExpress) at 37 °C for 25 min. Then ROS fluorescence was tested using a fluorescence microscope (WUMO).
PCOS ovarian granulosa cells were immobilized with 4% paraformaldehyde (Beyotime) and blocked with 2% BSA (Absin, Shanghai, China) for 25 min. Cells were exposed to anti-LC3 (ab232940, 1/1000) overnight at 4 °C. Cells were washed with PBS and incubated with fluorescently coupled secondary antibody (Abcam, 1/2000) for 1.5 h. The nucleus was dyed by DAPI (Abcam) and stained cells were observed under an inverted fluorescence microscope (WUMO).
Flow cytometry
PCOS ovarian granulosa cell apoptosis was examined using a flow cytometer. After ovarian granulosa cells (1 × 105) were gathered and were resuspended in binding buffer (500 μL), cells were dyed with Annexin V-FITC (5 μL) and propidium iodide (PI, 5 μL) for 20 min in the dark. Apoptotic cells were estimated by flow cytometry (Millipore, Shanghai, China) and assessed with FlowJo 10.0 software (Tree Star, Ashland, USA).
Quantitative real-time PCR
Total RNAs were isolated from PCOS ovarian granulosa cells with TRIzol (Beyotime) as per the manufacturer’s protocol. Then, cDNA was synthesized with QuantiTect® Reverse Transcription Kits (Takara, Dalian, China). Real-time PCR was implemented on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, USA) with SYBR Green® fast qPCR Mix (Takara). Relative levels were calculated with 2–ΔΔCT methods. All data were normalized to β-actin. Primer sequences are listed in .
Table 1. Primer sequences.
Western blot
Followed by PCOS ovarian granulosa cells were lysed in RIPA (Beyotime), and protein contents were assessed with BCA Protein Assay Kits (Sangon, Shanghai, China). Proteins were separated using 10% SDS-PAGE (Sangon) and were further transferred onto PVDF membranes (Millipore). Membranes were blocked with 5% skim milk, and membranes were then exposed to primary antibodies against PI3K p85 (ab191606, 1/1000), p-PI3K p85 (ab182651, 1/1000), AKT (ab179463, 1/10,000), p-AKT (ab38449, 1/1000), mTOR (ab134903, 1/10,000), p-mTOR (ab109268, 1/2000), and β-actin (ab8227, 1/2000) at 4 °C overnight. After membranes were washed, membranes were further exposed to secondary antibodies (1/2000) for 1.5 h. All antibodies were offered by Abcam. All bands were visualized with an enhanced chemiluminescent detection system (Yeasen, Shanghai, China) and Image J software (National Institutes of Health, Bethesda, USA).
Statistical analysis
Data were exhibited as mean ± SD. Differences among two or multiple groups were assessed with unpaired Student t test or one-way ANOVA followed by Tukey’s post-test. p < .05 was considered statistically significant.
Results
Construction and verification of the PCOS mouse model
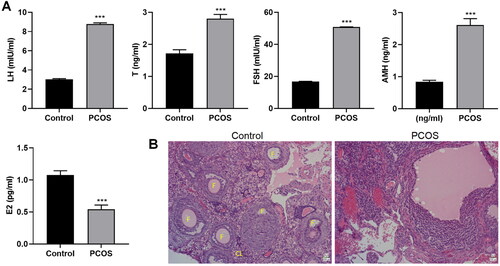
To estimate the effect of protocatechuic acid on PCOS, a mouse PCOS model was constructed. As shown in , serum LH, T, FSH, and AMH levels were increased, and E2 was lessened in PCOS. Meanwhile, the corpus luteum of PCOS mice was prominently reduced, and the arrangement was looser compared with the control (). To summarize, PCOS mice were successfully established.
Figure 1. Establishment of polycystic ovary syndrome mouse model. A mouse model of PCOS was constructed with DHEA. (A) Serum levels of LH, T, FSH, AMH, and E2 were examined with ELISA. (B) Pathological changes of mice ovarian tissues were estimated using hematoxylin–eosin (HE) staining (×200). The follicles (F) and corpus luteum (CL) are shown on the control group ovarian micrographs. ***p < .001 vs. control.
Modulation of protocatechuic acid in PCOS mice
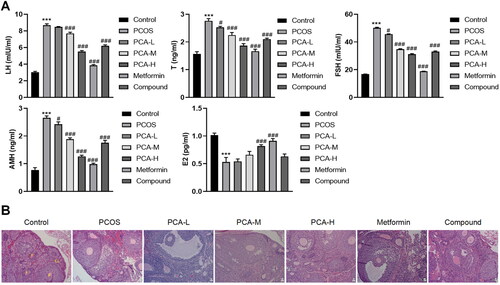
To further delve into whether protocatechuic acid modulated hormone levels of PCOS mice, different doses of protocatechuic acid were applied to treat PCOS mice. ELISA data corroborated that serum LH, T, FSH, and AMH levels were elevated, and E2 was decreased in PCOS, yet these impacts were abolished after protocatechuic acid treatment (). Also, protocatechuic acid reduced corpus luteum in PCOS mice in comparison with PCOS (). To sum up, protocatechuic acid owned the function of relieving PCOS mice.
Figure 2. Protocatechuic acid influences PCOS mice. Following PCOS mouse model was constructed, 10, 20, and 40 mg/kg protocatechuic acid were intraperitoneally injected into PCOS mice. (A) Analysis of LH, T, FSH, AMH, and E2 levels by ELISA. (B) Comparison of pathological changes of mice ovarian tissues with HE staining (×200). The follicles (F) and corpus luteum (CL) are shown on the control group ovarian micrographs. ***p < .001 vs. control; #p < .05; ###p < .001 vs. PCOS.
Biosafety evaluation of protocatechuic acid on mice
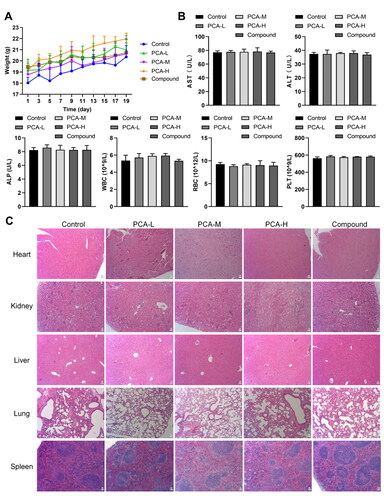
Subsequently, the safety of protocatechuic acid was evaluated in mice. As displayed in , low, medium, and high doses of protocatechuic acid did not conspicuously change the body weight of mice. AST, ALT, ALP, WBC, RBC, and PLT are commonly applied to indicate liver or kidney function [Citation28]. We further enunciated that there were no remarkable changes in AST, ALT, ALP, WBC, RBC, and PLT levels after protocatechuic acid treatment (). Moreover, HE staining further expounded that protocatechuic acid possessed no obvious impact on the heart, liver, spleen, lung, and kidney of mice (). In general, protocatechuic acid owned no conspicuous toxic effect on mice.
Figure 3. Analysis of the toxic effect of protocatechuic acid on mice. (A) Detection of body weight of mice. (B) Aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), white blood cell count (WBC), red blood cell count (RBC), and platelets (PLT) contents were checked with Automated Hematology Analyzer. (C) Pathological changes in the heart, liver, spleen, lung, and kidney in mice were appraised using HE staining (×200).
Protocatechuic acid mediates ROS, autophagy, and apoptosis of ovarian granulosa cells isolated from PCOS mice
Modulation of protocatechuic acid on PCOS ovarian granulosa cells was further investigated. LC3 is an autophagy-related molecule [Citation29]. As shown in , LC3 levels was increased in PCOS ovarian granulosa cells, yet this impact was reversed after protocatechuic acid treatment. ROS levels were increased in PCOS, while protocatechuic acid abolished this increase (). Meanwhile, apoptosis of PCOS ovarian granulosa cells was enhanced in PCOS, and protocatechuic acid abolished this impact ().
Figure 4. Protocatechuic acid impacts ROS, autophagy, and apoptosis of PCOS ovarian granulosa cells. Ovarian granulosa cells were isolated from PCOS and control mice. (A) ROS levels were tested by immunofluorescence assay. (B) Detection of LC3 levels using immunofluorescence analysis. (C) Apoptosis of PCOS ovarian granulosa cells was examined with flow cytometry. (D) MRNA levels of PI3K p85, AKT, and mTOR were checked with quantitative real-time PCR (qRT-PCR). E: Comparison of protein levels of PI3K p85, AKT, and mTOR using Western blot. ***p < .001 vs. control; ##p < .01; ###p < .001 vs. PCOS.
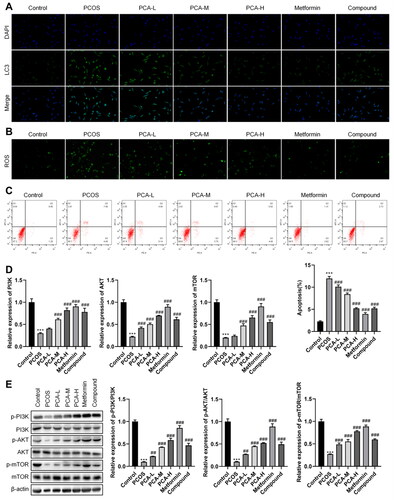
Previous studies confirm that PI3K/AKT/mTOR axis partakes in mediating the PCOS process [Citation30,Citation31]. As displayed in , mRNA and protein levels of PI3K p85, AKT, and mTOR were lessened in PCOS, yet protocatechuic acid abolished this effect. In a word, protocatechuic acid repressed ROS, autophagy, and apoptosis of PCOS ovarian granulosa cells. Also, protocatechuic acid promoted PI3K/AKT/mTOR axis activation in PCOS.
Verification of PI3K/AKT/mTOR axis on PCOS ovarian granulosa cells
Followed by PCOS ovarian granulosa cells were treated with LY294002, we further affirmed that protocatechuic acid induced low LC3 levels, while this impact was abolished after LY294002 treatment (). Meanwhile, analysis of ROS levels exhibited alike trends (). Also, PCOS ovarian granulosa cell apoptosis was weakened, yet LY294002 reversed this impact ().
Figure 5. PI3K/AKT/mTOR axis mediates PCOS ovarian granulosa cells. PCOS ovarian granulosa cells were exposed to 25 µmol/L LY294002 for 1.5 h. (A and B) Comparison of ROS levels with immunofluorescence analysis. (C) LC3 levels were examined using an immunofluorescence assay. (D) Analysis of PCOS ovarian granulosa cell apoptosis with flow cytometry. (E and F) MRNA and protein levels of PI3K p85, AKT, and mTOR were tested by qRT-PCR and Western blot. ***p < .001 vs. control; ###p < .001 vs. PCOS; $p < .05; $$$p < .001 vs. PCA-H.
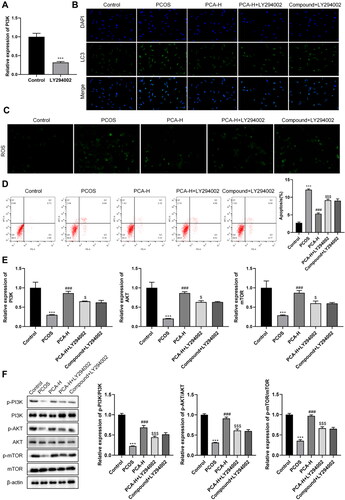
Subsequently, PI3K, AKT, and mTOR expressions were higher in protocatechuic acid than that in PCOS, while LY294002 further abolished this trend (). Summarizing, PI3K/AKT/mTOR axis was responsible for PCOS ovarian granulosa cells.
Discussion
PCOS is the most common endocrine disease in women of childbearing age, and environmental and genetic mechanisms exert prominent functions in the etiology of PCOS [Citation32]. The purpose of this study was to investigate the function and potential mechanism of protocatechuic acid in PCOS. The findings of this study were presented below: (1) Protocatechuic acid exerted a function of mitigating PCOS mice in vivo. (2) Protocatechuic acid restrained ROS, autophagy, and apoptosis of PCOS ovarian granulosa cells in vitro. (3) Protocatechuic acid has a protective effect on PCOS and promotes the activation of PI3K/AKT/mTOR.
Protocatechuic acid is the principal benzoic acid derivative in vegetables and fruits [Citation21]. Protocatechuic acid possesses biological activity against diabetes, aging, and inflammation [Citation33]. Traditionally, protocatechuic acid is nontoxic and a relatively safe oral compound [Citation34]. Homoplastically, our experimental data revealed that protocatechuic acid had no obvious toxic effect on mice, which was mainly reflected in weight, liver and kidney function, and heart, liver, spleen, lung, and kidney tissues of mice. Emerging evidence expounds that protocatechuic acid owns protective functions in various human diseases. For example, protocatechuic acid effectively protects liver cells from H2O2-induced oxidative stress and cell death [Citation35]. High doses of protocatechuic acid prevent cognitive impairment, hinting that protocatechuic acid might be a protective agent for Alzheimer’s disease [Citation36]. Crucially, we further confirmed that protocatechuic acid exerted a function of alleviating PCOS mice, which was alike to the aforementioned conclusions.
Granulosa cells are the main somatic cell type of ovarian follicles and partake in follicular formation through proliferation, acquisition of gonadotropin reactivity, and production of autocrine/paracrine factors [Citation37]. Previous studies demonstrate that enhancement of granulosa cell apoptosis is commonly observed in PCOS pathological process [Citation38,Citation39]. As has been reported, ovulating PCOS women enhance granulosa cell proliferation in their ovaries compared with normally ovulating PCOS women [Citation40]. Similarly, our results also authenticated that protocatechuic acid repressed PCOS ovarian granulosa cell apoptosis in vitro.
Previous studies expound that ROS and autophagy mediate the progression of various human diseases, including PCOS. For instance, the transferrin receptor raises iron content, mediates ROS release, induces lipid peroxidation, and further stimulates ferroptosis of human granulosa-like tumor cells in PCOS [Citation41]. Guizhi Fuling Wan can alleviate PCOS in rats by inhibiting autophagy in granulosa cells [Citation16]. Downregulation of sestrin 1 represses human granulosa-like tumor cell proliferation through repressing autophagy, implying that downregulation of sestrin 1 might be a potential novel treatment strategy for PCOS [Citation42]. Similar to these findings, our data proved that protocatechuic acid reduced ROS and autophagy of PCOS ovarian granulosa cells, hinting that protocatechuic acid owned a function of mitigating PCOS in vitro.
Accumulated research shows that PI3K/AKT/mTOR axis partakes in the occurrence and development of human diseases [Citation43,Citation44]. Crucially, recent research clarifies that miR-18b-5p produced by exosomes from follicular fluid mitigates PCOS by promoting the activation of PI3K/Akt/mTOR [Citation45]. Guizhi Fuling Wan and Liuwei Dihuang pill can alleviate polycystic ovary syndrome rats by promoting PI3K/AKT/mTOR signaling pathway [Citation16,Citation46]. Analogously, we also proved that protocatechuic acid activated PI3K/AKT/mTOR axis in PCOS ovarian granulosa cells. Meanwhile, we further validated that the protective function of protocatechuic acid against PCOS was interrelated to the activation of PI3K/AKT/mTOR.
Taken together, this research illustrated that protocatechuic acid exerted a function of mitigating PCOS in vivo and in vitro. Also, the protective function of protocatechuic acid against PCOS was interrelated to the activation of PI3K/AKT/mTOR, implying that protocatechuic acid might be a promising drug for PCOS.
Ethical approval
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the animal ethics committee in the Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (2022DW-64-02).
Author contributions
Conceptualization: Feihong Wang, Yong Tan. Methodology: Yanyun Yin, Xiaowei Nie. Validation: Yijie Zou, Xingli Tong, Yun Tong. Investigation: Yijie Zou, Xingli Tong, Yun Tong. Data curation: Feihong Wang, Yong Tan. Writing-original draft preparation: Feihong Wang, Yanyun Yin, Xiaowei Nie. Writing-review and editing: Feihong Wang, Yong Tan. Supervision: Yong Tan. Project administration: Yong Tan. Funding acquisition: Yong Tan. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Dumesic DA, Oberfield SE, Stener-Victorin E, et al. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):1–9. doi: 10.1210/er.2015-1018.
- Bruni V, Capozzi A, Lello S. The role of genetics, epigenetics and lifestyle in polycystic ovary syndrome development: the state of the art. Reprod Sci. 2022;29(3):668–679. doi: 10.1007/s43032-021-00515-4.
- Katsigianni M, Karageorgiou V, Lambrinoudaki I, et al. Maternal polycystic ovarian syndrome in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1787–1797. doi: 10.1038/s41380-019-0398-0.
- Lai Q, Xiang W, Li Q, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12(5):518–524. doi: 10.1007/s11684-017-0575-y.
- Naji M, Aleyasin A, Nekoonam S, et al. Differential expression of miR-93 and miR-21 in granulosa cells and follicular fluid of polycystic ovary syndrome associating with different phenotypes. Sci Rep. 2017;7(1):14671. doi: 10.1038/s41598-017-13250-1.
- Zhang J, Wang X, Vikash V, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965.
- Wang Y, Li L, Zhao W, et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano. 2018;12(9):8943–8960. doi: 10.1021/acsnano.8b02037.
- Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25(7):1270–1276. doi: 10.1016/j.drudis.2020.05.001.
- Murri M, Luque-Ramírez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059.
- Sun P, Zhang Y, Sun L, et al. Kisspeptin regulates the proliferation and apoptosis of ovary granulosa cells in polycystic ovary syndrome by modulating the PI3K/AKT/ERK signalling pathway. BMC Womens Health. 2023;23(1):15.
- Xu A, Fan Y, Liu S, et al. GIMAP7 induces oxidative stress and apoptosis of ovarian granulosa cells in polycystic ovary syndrome by inhibiting sonic hedgehog signalling pathway. J Ovarian Res. 2022;15(1):141. doi: 10.1186/s13048-022-01092-z.
- Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20(10):1110–1117. doi: 10.1038/s41556-018-0201-5.
- Zhang C, Hu J, Wang W, et al. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020;34(7):9563–9574. doi: 10.1096/fj.202000605RR.
- Li X, Qi J, Zhu Q, et al. The role of androgen in autophagy of granulosa cells from PCOS. Gynecol Endocrinol. 2019;35(8):669–672. doi: 10.1080/09513590.2018.1540567.
- Luo X, Gong Y, Cai L, et al. Chemerin regulates autophagy to participate in polycystic ovary syndrome. J Int Med Res. 2021;49(11):3000605211058376. doi: 10.1177/03000605211058376.
- Liu M, Zhu H, Zhu Y, et al. Guizhi fuling wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol. 2021;270:113821. doi: 10.1016/j.jep.2021.113821.
- Chen M, He C, Zhu K, et al. Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication. Theranostics. 2022;12(2):782–795. doi: 10.7150/thno.67167.
- Ma X, Li X, Ma L, et al. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF- κB signaling pathway. Bioengineered. 2021;12(1):7215–7223. doi: 10.1080/21655979.2021.1979864.
- Mahfuz S, Mun H-S, Dilawar MA, et al. Potential role of protocatechuic acid as natural feed additives in farm animal production. Animals. 2022;12(6):741. doi: 10.3390/ani12060741.
- Lende AB, Kshirsagar AD, Deshpande AD, et al. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology. 2011;19(5):255–263. doi: 10.1007/s10787-011-0086-4.
- Cui B, Yang Z, Wang S, et al. The protective role of protocatechuic acid against chemically induced liver fibrosis in vitro and in vivo. Pharmazie. 2021;76(5):232–238.
- Xiao N, He K, Gong F, et al. Altered subsets and activities of B lymphocytes in polycystic ovary syndrome. J Allergy Clin Immunol. 2019;143(5):1943–1945.e4. doi: 10.1016/j.jaci.2019.01.007.
- Zhang X, Li C, Li J, et al. Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-κB signal pathways. Int Immunopharmacol. 2015;26(1):229–236. doi: 10.1016/j.intimp.2015.03.031.
- Wang Q, Ren X, Wu J, et al. Protocatechuic acid protects mice from influenza a virus infection. Eur J Clin Microbiol Infect Dis. 2022;41(4):589–596. doi: 10.1007/s10096-022-04401-y.
- Xiao N, Wang J, Wang T, et al. Metformin abrogates pathological TNF-α-producing B cells through mTOR-dependent metabolic reprogramming in polycystic ovary syndrome. Elife. 2022;11:e74713. doi: 10.7554/eLife.74713.
- Xiong Z, Li B, Wang W, et al. MiR-140 targets RAP2A to enable the proliferation of insulin-treated ovarian granulosa cells. J Ovarian Res. 2020;13(1):13. doi: 10.1186/s13048-020-0611-4.
- Diamanti-Kandarakis E, Chatzigeorgiou A, Papageorgiou E, et al. Advanced glycation end-products and insulin signaling in granulosa cells. Exp Biol Med. 2016;241(13):1438–1445. doi: 10.1177/1535370215584937.
- Nie C, Wang T, Yu H, et al. The blood parameters and liver function changed inconsistently among children between burns and traumatic injuries. PeerJ. 2019;7:e6415. doi: 10.7717/peerj.6415.
- Deng G, Li C, Chen L, et al. BECN2 (beclin 2) negatively regulates inflammasome sensors through ATG9A-dependent but ATG16L1- and LC3-independent non-canonical autophagy. Autophagy. 2022;18(2):340–356. doi: 10.1080/15548627.2021.1934270.
- Xiong H, Hu Q, Jiang Q. Protective effects of lidocaine on polycystic ovary syndrome through modulating ovarian granulosa cell physiology via PI3K/AKT/mTOR pathway. Cytotechnology. 2022;74(2):283–292. doi: 10.1007/s10616-022-00528-0.
- Cai Z, He S, Li T, et al. Plumbagin inhibits proliferation and promotes apoptosis of ovarian granulosa cells in polycystic ovary syndrome by inactivating PI3K/akt/mTOR pathway. Anim Cells Syst. 2020;24(4):197–204. doi: 10.1080/19768354.2020.1790416.
- Bjekić-Macut J, Vukašin T, Velija-Ašimi Z, et al. Polycystic ovary syndrome: a contemporary clinical approach. Curr Pharm Des. 2021;27(36):3812–3820. doi: 10.2174/18734286MTEztNDEr1.
- Khan AK, et al. Pharmacological activities of protocatechuic acid. Acta Pol Pharm. 2015;72(4):643–650.
- Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943.
- Lee WJ, Lee SH. Protocatechuic acid protects hepatocytes against hydrogen peroxide-induced oxidative stress. Curr Res Food Sci. 2022;5:222–227. doi: 10.1016/j.crfs.2022.01.006.
- Choi JR, Kim JH, Lee S, et al. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer’s disease mouse model. Food Chem Toxicol. 2020;144:111571. doi: 10.1016/j.fct.2020.111571.
- Nguyen XP, Nakamura T, Osuka S, et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction. 2019;158(1):25–34. doi: 10.1530/REP-19-0025.
- Lima PDA, Nivet A-L, Wang Q, et al. Polycystic ovary syndrome: possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages. Biol Reprod. 2018;99(4):838–852. doi: 10.1093/biolre/ioy096.
- Mikaeili S, Rashidi BH, Safa M, et al. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet. 2016;294(1):185–192. doi: 10.1007/s00404-016-4068-z.
- Stubbs SA, Stark J, Dilworth SM, et al. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab. 2007;92(11):4418–4426. doi: 10.1210/jc.2007-0729.
- Zhang L, Wang F, Li D, et al. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered. 2021;12(1):4983–4994. doi: 10.1080/21655979.2021.1956403.
- Xu X, Song X, Xu X, et al. Inhibition of sestrin 1 alleviates polycystic ovary syndrome by decreasing autophagy. Aging. 2021;13(8):11774–11785. doi: 10.18632/aging.202872.
- Shorning BY, Dass MS, Smalley MJ, et al. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci. 2020;21(12):4507.
- Xue J-F, Shi Z-M, Zou J, et al. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–1261. doi: 10.1016/j.biopha.2017.01.130.
- Zhou Z, Tu Z, Zhang J, et al. Follicular Fluid-Derived exosomal MicroRNA-18b-5p regulates PTEN-Mediated PI3K/akt/mTOR signaling pathway to inhibit polycystic ovary syndrome development. Mol Neurobiol. 2022;59(4):2520–2531. doi: 10.1007/s12035-021-02714-1.
- Qiu Z, Dong J, Xue C, et al. Liuwei dihuang pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/akt signaling pathway. J Ethnopharmacol. 2020;250:111965. doi: 10.1016/j.jep.2019.111965.
