Abstract
Background
Polycystic ovary syndrome (PCOS) is one of the most prevalent endocrine disorders in gynecology with severe metabolic abnormalities. Therefore, identifying effective treatments and drugs for PCOS is important. We aimed to investigate effect of the traditional Chinese medicine (TCM) Rubus chingii Hu (R. chingii) on ovarian function and insulin resistance (IR) of PCOS rat models, and to explore the underlying mechanisms
Methods
A PCOS rat model was established by subcutaneous injection of dehydroepiandrosterone (DHEA) solution for 20 days. PCOS rats were randomly divided into a control group (CON), model group (MOD), metformin group (MET), TCM R. chingii group (RCG), and RCG + Ad-TXNIP groups. After 28 days of treatment, the samples were collected for subsequent experiments.
Results
R. chingii treatment alleviated hormone imbalance and IR while improving ovarian pathology in the PCOS model. R. chingi inhibited the activation of the thioredoxin-interacting protein (TXNIP)/NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome in the ovarian tissue of PCOS rats. Furthermore, TXNIP overexpression hindered the protective effect of R. chingii intervention in PCOS rats, as evidenced by the increase of homeostasis model assessment of insulin resistance (HOMA-IR), luteinizing hormone (LH), testosterone (T), C-reactive protein (CRP) levels, and atretic follicles.
Conclusion
R. chingii intervention improved ovarian polycystic development by suppressing the TXNIP/NLRP3 inflammasome, which may be an effective treatment for PCOS.
Introduction
Polycystic ovary syndrome (PCOS), a common female reproductive endocrine disease associated with anovulation, hyperandrogenism, and insulin resistance (IR), is one of the main causes of female anovulatory infertility [Citation1]. The incidence is 5-10% among reproductive women, which leads to 70% anovulatory infertility [Citation2]. PCOS also increases the risk of type 2 diabetes and cardiovascular diseases [Citation1]. Thus, finding an effective treatment for PCOS blocking the course, and preventing the occurrence of complications will be of important significance.
To date, there are no truly effective approved therapies available for the treatment of PCOS owing to the complexity of its abnormal metabolism and diverse clinical manifestations [Citation3]. Studies have shown that follicular dysplasia and endocrine disorders are important etiologies of PCOS [Citation4,Citation5]. There are 60-80% of women have varying degrees of insulin resistance (IR) in clinical practice [Citation6]. The insulin sensitizer metformin is currently considered an effective drug for the treatment of IR, and has a certain efficacy in regulating reproductive endocrine disorders and metabolic abnormalities in patients [Citation7]. Metformin restores ovulation in approximately 30-50% of patients [Citation8]. However, it is a class B drug for safety classification. Several studies have indicated that traditional Chinese medicines (TCM) have excellent effects on endocrinopathy and infertility. TCM has long been used to restore the reproductive function of PCOS patients and rectify their endocrine disorders without adverse effects [Citation9]. Importantly, tonifying the kidneys and invigorating the blood are key approaches for treating PCOS. Rubus chingii Hu (R. chingii), also known as raspberry, is an important TCM that can improve kidney function and treat excessive polyuria [Citation10]. A study substantiated its antioxidant activity in the kidney and serum of aging mouse models [Citation11]. However, the mechanism underlying the effects of R. chingii on PCOS has not yet been elucidated.
It is well known the link between the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome and various diseases, such as cognitive decay, cardiovascular risk, and reproductive endocrine disorder. Activation of the NLRP3 inflammasome has a crucial role in obesity-induced inflammation, IR, and type 2 diabetes mellitus (T2DM) [Citation12]. A recent study confirmed that activation of the NLRP3 inflammasome plays a key role in aging-associated chronic inflammation and IR [Citation13]. The NLRP3 inhibitor tranilast ameliorated mouse gestational diabetes mellitus (GDM) symptoms (including hyperglycemia, insulin insufficiency, and insulin resistance) and decreased the expression of proinflammatory cytokines [Citation14]. The NLRP3 inflammasome is formed in ovarian granulosa cells (GCs) of PCOS patients, which induces oxidative stress, affects cellular metabolism, and damages cell proliferation [Citation15]. Moreover, diacerein ameliorated letrozole-induced PCOS in rats by suppressing NLRP3 immunoreactivity [Citation16]. The thioredoxin-interacting protein (TXNIP)-related pathway is important for the activation of NLRP3 inflammatory vesicles. Under a variety of precipitating stimuli, TXNIP disassociates from the protein complex and binds to NLRP3, triggering its activation and finally regulating the activation process of the NLRP3 inflammasome [Citation17]. However, the role of TXNIP/NLRP3 pathway in PCOS has not been fully studied.
Thus, this study investigated whether R. chingii therapy has beneficial effects on hormone balance and the restoration of ovarian function in a PCOS Sprague-Dawley (SD) rat model. Moreover, the effects of R. chingii on the modulation of the TXNIP/NLRP3 pathway in ovarian tissue were measured.
Methods and materials
Experimental animals
Female SD rats (4 weeks old, 60 ± 5 g) were purchased from the Laboratory Animal Center of the Medical Department of Xi’an Jiaotong University (animal facilities use certificate no: SCXK (Shaan) 2018-001). The feeding environment was 25 ± 1 °C, relative humidity 50%-60%, and a light/darkness for 12 h. The rats were allowed free access to food and water. After one week of acclimation, the rats were used to establish the PCOS model. This study was approved by the Ethics Committee of the Qinghai University Medical College (No. 20180310). Animal studies followed the ARRIVE 2.0 guidelines.
PCOS rat model establishment and drug delivery
Eighty rats were randomly divided into two groups: the normal group (n = 10), SD rats fed regularly without any intervention, and the model group (n = 70). The model rats were injected subcutaneously with 0.2 ml dehydroepiandrosterone (DHEA) solution (6 mg/100 g), which was dissolved in soybean oil (Shanghai yuanye Bio-Technology Co., Ltd, China) for 20 consecutive days. The morphology of vaginal epithelial cells was observed by conventional Pap staining to select model rats from the 11th day. During the two estrus cycles (10 days), continuous keratinization suggested that the PCOS model was successfully constructed (, 69 SD rats) according to previous reports [Citation18,Citation19]. Among them, 64 rats qualified as PCOS-IR models in this experiment (HOMA-IR >2.8) [Citation20]. Insulin resistance (HOMA-IR) indices were calculated as HOMA-IR = fasting plasma glucose (FPG, mmol/L) ×serum fasting insulin (FINS, mU/L)/22.5.
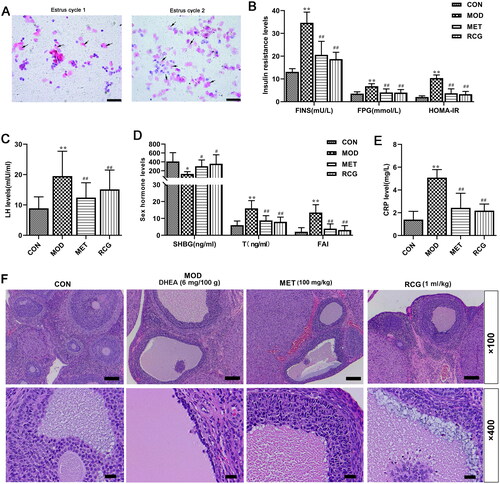
Figure 1. R. chingii treatment alleviated the disruption of serum hormone levels in the PCOS model. (A) Vaginal exfoliated smear in rats. Scale, 200 μm in the PCOS model group, the exudated vaginal cells showed continuous keratinization in two estrous cycles (10 d). (B-E) Fasting plasma glucose (FGP) was measured by Biochemical Analyzer. Serum fasting insulin (FINS), luteinizing hormone (LH), testosterone (T), C-reactive protein (CRP), and sex hormone binding globulin (SHGB) levels were detected by ELISA. Homeostasis model assessment of insulin resistance (HOMA-IR) = FPG × FINS/22.5, free androgen index (FAI) = T/SHBG × 100. (F) Morphological changes of ovary tissue (H&E, × 100 and × 400). *p < 0.05 vs. control, **p < 0.01 vs. control, ##p < 0.01 vs. PCOS.
Rats with successful modeling were randomly divided into the model group (MOD); metformin group (MET), the rats were orally gavaged with 2 ml (100 mg/kg/d); TCM R. chingii group (RCG), the rats were given orally with 10 ml/kg/d R. chingii decoction (20 g R. chingii slices were dissolved in 200 ml water for 30 min and then boiled for 20 min); R. chingii + Ad-TXNIP group, PCOS rats were injected with 100 µL Ad-TXNIP adenovirus (1 × 1011 PFU) through the tail vein and then treated with R. chingii. The rats were administered the medicine for four weeks. The rats in the control group (CON) were administered the same amount of solvent. The rats in each group were continuously injected with DHEA to prevent ovulation.
Specimen collection
The rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (100 mg/kg) and were sacrificed. Blood was drawn from the abdominal aorta and the serum was separated. Subsequently, the left ovary was fixed in 4% paraformaldehyde (PFA) for hematoxylin and eosin (H&E) staining. In addition, the remaining ovary tissues at −86 °C.
H&E stain
The left ovary tissue was fixed with 4% PFA for 24 h and embedded in paraffin for histopathological analysis using H&E staining. Briefly, each rat ovary paraffin section was denatured and dehydrated using xylene and graded ethanol. After staining with hematoxylin and eosin, paraffin sections were washed with distilled water and dehydrated with graded ethanol and xylene. The pathological features of the ovarian tissue were evaluated using a light microscope in five random fields.
RNA extraction and quantitative real-time PCR (RT-qPCR)
Total RNA was isolated from ovary tissue using TRIzol reagent (HeFei BoMei Biotechnology Co. Ltd, China) following the manufacturer’s protocol. cDNA was prepared using an SYBR® Premix Ex TaqTM II kit (Takara Clontech, Kyoto, Japan). RT-qPCR was performed in triplicate using a StepOnePlus real-time PCR system (Thermo Fisher Scientific, Waltham, MA, UK). The results were normalized using β-actin as an internal control. The primer sequences used are listed in . Relative expression of each gene was calculated using the 2-ΔΔCt method.
Table 1. Primer sequences for RT-qPCR.
Western blot analysis
Ovary tissues were homogenized using a homogenizer with magnetic beads. The total protein was extracted using RIPA buffer (Beyotime, Shanghai, China). The protein concentration was determined using a BCA kit (Beyotime, Shanghai, China). Total protein (40 µg/sample) was separated by 10% SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dried milk overnight at 4 °C and incubated with the following corresponding protein antibodies: TXNIP (1:300, Abcam, #188865), NLRP3 (1:1000, Abcam, #263899), ASC (1:1000, ABclonal, #A1170), P-caspase-1 (1:1000, Abcam, #1872), C-caspase-1 (Caspase1 p10 + P12, 1:500, Abcam, #238972), Insulin receptor substrate-1 (IRS-1; 1:1000, Abcam, #40777), and β-actin (1/100000, ABclonal, #AC038). The membranes were then washed with Tris-buffered saline/0.1% Tween (TBST) and incubated for 3 h with HRP Goat anti-rabbit IgG (1:5000, Abcam, #6721). The bands were visualized using an ECL system (Affinity Biosciences, Cincinnati, Ohio, USA), and β-actin was used as an internal control. The net optical density was measured using the Quantity One software (Tanon, Shanghai, China).
Enzyme-linked immunosorbent assay (ELISA)
The levels of FINS, luteinizing hormone (LH), SHBG, T, and C-reactive protein (CRP) in the serum of each group of rats were measured using ELISA kits (Elabscience, Wuhan, China), following the manufacturer’s instructions. Absorbance was measured at a wavelength of 450 nm and estimated using an enzyme-linked immune monitor (Tecan, Switzerland).
Statistical analysis
The data are presented as mean ± standard deviation. Statistical analysis was performed using SPSS 20.0 (IBM Corp.). One-way analysis of variance (ANOVA) with Tukey’s post-hoc test of means was used for comparisons between groups. Differences with a p < 0.05 were considered statistically significant.
Results
R. chingii treatment restored the disruption of serum hormone levels in the PCOS model
We first determined whether the PCOS model was successfully established. Our results showed that the serum fasting insulin (FINS), fasting plasma glucose (FPG), homeostasis model assessment of insulin resistance (HOMA-IR), luteinizing hormone (LH), testosterone (T), free androgen index (FAI), and C-reactive protein (CRP) levels were increased, while sex hormone-binding globulin (SHGB) levels were decreased after DHEA injection when compared with control rats (), suggesting that the established model reflected disordered hormone levels. Moreover, this disruption of serum hormone levels in the PCOS model group was significantly improved by R. chingii treatment, which was similar to the results obtained in the metformin treatment group ().
Furthermore, we investigated whether R. chingii could restore ovarian characteristics and morphological changes in a PCOS model. H&E staining results indicated that the structure of ovarian tissue in the PCOS group was in a state of disorder, with apparent cystic dilatation in the ovarian follicles (). Additionally, the atretic follicle increased, and the granulosa cell layer was disordered (). In contrast, R. chingii treatment recovered the ovarian structure, manifested as a well-arranged granular layer of cells and normally developing follicles ().
R. chingii treatment attenuated cell apoptosis and IR in the ovary of PCOS rats
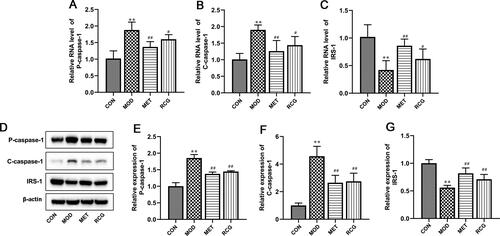
We then investigated the effects of R. chingii treatment on apoptosis and IR in PCOS rats. As shown in , R. chingii treatment significantly inhibited the enhanced apoptosis in PCOS rats, characterized by a decrease in P-caspase-1 and C-caspase-1 expression in the ovary tissue. Decreased activity of insulin receptor substrate-1 (IRS-1) is associated with IR. Compared to normal rats, the RNA and protein expression of IRS-1 was significantly reduced in PCOS-IR rats, which was blocked by R. chingii treatment ().
Figure 2. R. chingii treatment attenuated cell apoptosis and IR in the ovary of PCOS rats. (A-C) P-caspase-1, C-caspase-1 and IRS-1 RNA levels were examined using RT-qPCR. (B-E) P-caspase-1, C-caspase-1 and IRS-1 protein levels were analyzed using western blot. β-actin is a loading control. **p < 0.01 vs. control, #p < 0.05 vs. PCOS, ##p < 0.01 vs. PCOS.
R. chingii treatment inhibited gene expression of TXNIP/NLRP3 pathway in the ovary of PCOS rats
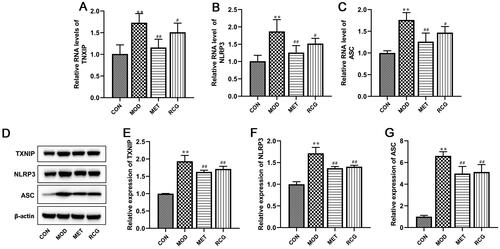
Next, we explored whether the TXNIP/NLRP3 pathway is involved in R. chingii-induced ovarian recovery in the PCOS model. RT-qPCR and Western blot analysis showed that the RNA and protein levels of key genes in the TXNIP/NLRP3 pathway, including TXNIP, NLRP3, and ASC, were enhanced in the PCOS model, whereas the application of R. chingii drastically decreased their expression levels ().
Figure 3. R. chingii treatment inhibited gene expression of TXNIP/NLRP3 pathway in the ovary of PCOS rats. (A-C) TXNIP, NLRP3, and ASC RNA levels were examined using RT-qPCR. (B-E) TXNIP, NLRP3, and ASC protein levels were examined by western blot. β-actin is a loading control. **p < 0.01 vs. control, #p < 0.05 vs. PCOS, ##p < 0.01 vs. PCOS.
R. chingii treatment suppressed cell apoptosis and IR via deactivating the TXNIP/NLRP3 pathway
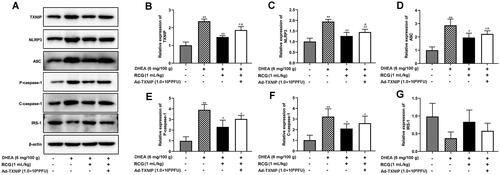
Next, we explored whether R. chingii treatment reduced apoptosis and IR by regulating the TXNIP/NLRP3 pathway. PCOS rats were co-treated with R. chingii, followed by injection with AD-TXNIP adenovirus, which stably overexpressed TXNIP. As shown in , the protein levels of TXNIP, NLRP3, and ASC were significantly upregulated in the AD-TXNIP-treated PCOS rats, suggesting that TXNIP overexpression significantly activated the TXNIP/NLRP3 pathway. Furthermore, in PCOS rats, inhibition of P-caspase-1, C-caspase-1, and IRS-1 expression induced by R. chingii treatment was reversed by TXNIP overexpression. These results imply that R. chingii treatment suppressed cell apoptosis and IR by deactivating the TXNIP/NLRP3 pathway.
Figure 4. R. chingii treatment suppressed cell apoptosis and IR via deactivating TXNIP/NLRP3 pathway. PCOS rats were co-treated with R. chingii followed by injecting with AD-TXNIP adenovirus which was stably overexpressed TXNIP through the tail vein. TXNIP, NLRP3, ASC, P-caspase-1, C-caspase-1, and IRS-1 protein levels were examined by western blot. β-actin is a loading control. **p < 0.01 vs. control, #p < 0.05 vs. PCOS, ##p < 0.01 vs. PCOS, &p < 0.05 vs. R. chingii treatment.
R. chingii treatment restored the ovary disorder through TXNIP/NLRP3 pathway
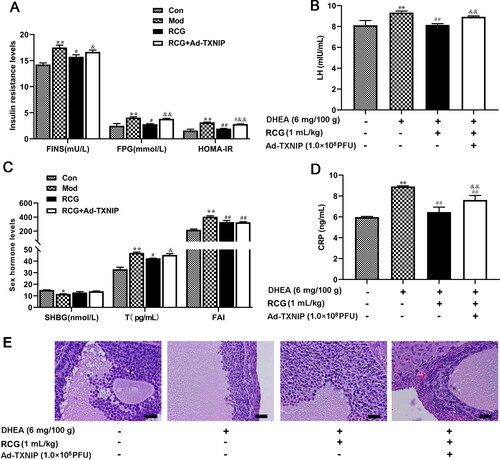
Finally, we assessed whether TXNIP overexpression hindered the balancing effect of R. chingii treatment on hormone levels in our PCOS rat model. We observed significant upregulation of FINS, FPG, HOMA-IR, LT, T, and CRP levels in Ad-TXNIP co-induced animals relative to the R. chingii-treated group (–D). Compared with R. chingii treatment, TXNIP overexpression partially disrupted normal tissue morphology, with oocytes again being absent in follicles and a decrease in the number of granule cell layers (). This suggests that inhibition of the TXNIP/NLRP3 pathway is a key mediator of the observed ability of R. chingii treatment to protect against PCOS pathology.
Figure 5. R. chingii treatment restored the ovary disorder through TXNIP/NLRP3 pathway. PCOS rats were co-treated with R. chingii followed by injecting with AD-TXNIP adenovirus which was stably overexpressed TXNIP through the tail vein. (A-D) FPG was measured by Biochemical Analyzer. FINS, LH, T, CRP, and SHGB levels were detected by ELISA. HOMA-IR = FPG × FINS/22.5, FAI = T/SHBG × 100. (F) Morphological changes of ovary tissue (H&E, × 400). **p < 0.05 vs. control, **p < 0.01 vs. control, #p < 0.05 vs. PCOS, ##p < 0.01 vs. PCOS, &p < 0.05 vs. R. chingii treatment, &&p < 0.01 vs. R. chingii treatment.
Discussion
The clinical symptoms of patients diagnosed with PCOS are characterized by metabolic and hormonal disorders [Citation21]. IR and hyperinsulinemia (HI) play important roles in PCOS [Citation22]. Elevated serum insulin levels usually stimulate the interaction between insulin and insulin-like growth factor-1 (IGF-1) receptors, which increases LH and androgen levels. HI can inhibit the production and secretion of sex hormone-binding globulin (SHGB) from the liver, manifesting as hyperandrogenism [Citation23]. High androgen concentration in the ovaries further leads to follicular atresia and eventually to persistent anovulation and infertility [Citation23]. Furthermore, CRP is an acute reactive protein that appears after inflammation injury, which is associated with IR [Citation24] and is upregulated in a model of PCOS [Citation25]. Our results showed that, in PCOS model rats, R. chingii treatment decreased FAI and IR while inhibiting LH and CRP levels, suggesting that disordered hormonal imbalance and low-grade inflammation in the PCOS model were improved.
Insulin resistance (IR) refers to a decrease in the sensitivity of peripheral tissues to insulin, resulting in a decrease in the biological efficacy of insulin. To maintain normal blood glucose levels, the body compensates by secreting more insulin, resulting in HI [Citation26]. The study showed roughly 60%-80% of PCOS patients have IR [Citation6]. HI due to IR leads to increased responsiveness of granulosa cells to luteinizing hormone (LH) in the ovarian microenvironment, inhibiting the proliferation of granulosa cells in the follicle and preventing the development of a dominant follicle and ovulation [Citation27]. The function of ovarian granulosa cells in PCOS patients is affected by hyperinsulinemia, and the uptake and utilization of glucose are decreased, resulting in insufficient energy for follicle production and developmental disorders [Citation28]. IR is closely associated with impaired insulin signaling. Insulin receptors bind to IRS-l and activate downstream regulators of this pathway [Citation29]. IRS-1 has tyrosine and serine kinase activity. When the insulin receptor is abnormally expressed, IRS-1 overactivates serine kinase activity and inhibits tyrosine kinase phosphorylation [Citation29]. IRS-1 is a possible mechanism for insulin resistance in patients with PCOS-IR. Previous work showed that electro-acupuncture treatment ameliorated ovarian IR in PCOS patients through upregulation of the IRS-1/PI3K/GLUT4 signaling pathway [Citation30]. In a rat model of PCOS-IR, total flavonoids decreased p-IRS-1Ser307, increased IRS-1 and p-IRS-1Tyr895, and ameliorated histopathological changes in the ovary and pancreas [Citation31]. Furthermore, modified Cangfu Daotan decoction (MCDD) plays a role in improving ovarian function in PCOS-IR rats by upregulating the expression of INSR/IRS-1/GLUT4 in the insulin signaling pathway in the inflammatory environment [Citation32]. Similar to previous reports, in the present study, IRS-1 expression was significantly decreased in the ovarian tissue of PCOS-IR rats, which was increased by R. chingii treatment. These data suggest that R. chingii exerts a therapeutic effect against PCOS-IR by upregulating IRS-1expression.
TXNIP, a member of the α-suppressor superfamily, participates in various biological metabolic processes such as the regulation of mitochondrial oxidative phosphorylation, programmed cell death, and glucose metabolism. Activated TXNIP binds to NLRP3 to activate the downstream inflammasome pathway, resulting in the release of inflammatory factors and cell apoptosis. Apoptosis-associated speck-like proteins, including CARD (ACS) and caspase-1, are important components of the NLRP3 inflammasome. TXNIP inhibition significantly reduces NLRP3, ACS, and caspase-1 activity [Citation33]. A previous study confirmed that patients with PCOS have higher serum TXNIP [Citation34]. TXNIP/NLRP3 inflammasome pathway plays an important role in IR and insulin secretion. Various TXNIPs participate in regulating pancreatic β cell biology and the expression of insulin transcripts, including zinc finger Ebox-binding homeobox 1 (Zeb1) [Citation35] and islet amyloid polypeptide protein (IAPP) [Citation36]. More importantly, TXIP-mediated palmitate (PA)-induced IR was observed in skeletal muscle cells [Citation37]. Further in vitro studies implied that AMPK-mediated TXNIP degradation enhanced glucose uptake and the expression of insulin signaling molecules, including p-IRS-1, IRS-1, and GLUT-1 in differentiated 3T3-L1 adipocytes [Citation38]. Notably, TXNIP significantly limits insulin receptor sensitivity in the brain by amplifying inflammation and oxidative stress [Citation35]. Similarly, this study confirmed that R. chingii treatment contributed to increased IRS-1 expression in the PCOS model, which may be mechanistically associated with diminished activity of the TXNIP/NLRP3 inflammasome. Moreover, TXNIP overexpression blocked the improvement in IR and hormonal disorders induced by R. chingii treatment.
In contrast, the TXNIP/NLRP3 inflammasome also plays a key role in the maintenance of ovarian function. In a mouse model of DHEA-induced PCOS, TXNIP was upregulated in the ovaries, and knockdown of TXNIP ameliorated ovarian injury and inflammation [Citation39]. In granulosa cells, knockdown of TXNIP attenuates inflammation and activation of the NLRP3 inflammasome [Citation39]. The upregulated expression of NLRP3, caspase-1, and IL-1β was tested in granulosa cells from patients with ovary insufficiency [Citation40]. NLRP3 knockdown improved the survival and pregnancy rates of patients with ovarian cancer [Citation40]. In NLRP3-deficient or -silencing female mice, serum FSH and estradiol levels decreased, whereas fertilization ability increased [Citation40]. A recent study showed that increased NLRP3 inflammasome expression suppressed folliculogenesis and fibrosis in a PCOS mouse model [Citation41]. NLRP3/Caspase-1 signaling pathway participates in oxidative stress-triggered ovarian granulosa cell apoptosis [Citation40]. The present study further demonstrated that NLRP3 overexpression reversed the restoration of the pathological state of ovarian tissue induced by R. chingii treatment. Accordingly, NLRP3 inflammasome usually results in ovarian dysfunction or infertility.
Conclusion
This study provides novel information that R. chingii treatment restores normal sexual hormone levels and attenuates IR and follicular atresia through inhibition of TXNIP/NLRP3 inflammasome signaling. Importantly, dysfunction of the TXNIP/NLRP3 inflammasome is related to sexual hormone secretion disorder and the pathogenesis of PCOS. In general, these findings may provide a new therapeutic strategy for PCOS.
Authors’ contributions
Huizhen and Yongping Li conceived and designed the experiments. Li, Li, Zhang, Tong, and Sa performed the experiments and acquired data. Huizhen Li and Ying Zhang analyzed the data. Yuping Sa and Wenping Sun contributed the reagents and materials. Yongping Li wrote the manuscript. Yongping Li obtained the funding. Yongping Li and Huizhen Li supervised the experiments and wrote the manuscript.
Disclosure statement
There are no declarations of interest.
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):1–8. doi: 10.1097/AOG.0000000000002698.
- Gu Y, Zhou G, Zhou F, et al. Life modifications and PCOS: old story but new tales. Front Endocrinol (Lausanne). 2022;13:808898. doi: 10.3389/fendo.2022.808898.
- Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(4):272–277. doi: 10.1080/09513590.2017.1395841.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24.
- Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008.
- Shang Y, Zhou H, Hu M, et al. Effect of diet on insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105(10):dgaa425. doi: 10.1210/clinem/dgaa425.
- Zhao H, Xing C, Zhang J, et al. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18(1):171. doi: 10.1186/s12978-021-01207-7.
- Morgante G, Massaro MG, Di Sabatino A, et al. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol Endocrinol. 2018;34(1):4–9. doi: 10.1080/09513590.2017.1370644.
- Moini Jazani A, Nasimi Doost Azgomi H, Nasimi Doost Azgomi A, et al. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru. 2019;27(2):863–877. doi: 10.1007/s40199-019-00312-0.
- Su XH, Duan R, Sun YY, et al. Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: an in vivo and in vitro approach. J Physiol Pharmacol. 2014;65:417–424.
- Zeng HJ, Liu Z, Wang YP, et al. Studies on the anti-aging activity of a glycoprotein isolated from Fupenzi (Rubus chingii Hu.) and its regulation on klotho gene expression in mice kidney. Int J Biol Macromol. 2018;119:470–476. doi: 10.1016/j.ijbiomac.2018.07.157.
- Litwiniuk A, Bik W, Kalisz M, et al. Inflammasome NLRP3 potentially links obesity-associated low-grade systemic inflammation and insulin resistance with Alzheimer’s disease. Int J Mol Sci. 2021;22(11):5603. doi: 10.3390/ijms22115603.
- He M, Chiang HH, Luo H, et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 2020;31(3):580–591.e585. doi: 10.1016/j.cmet.2020.01.009.
- Cao J, Peng Q. NLRP3 inhibitor tranilast attenuates gestational diabetes mellitus in a genetic mouse model. Drugs R D. 2022;22(1):105–112. doi: 10.1007/s40268-022-00382-7.
- Liu Y, Liu H, Li Z, et al. The release of peripheral immune inflammatory cytokines promote an inflammatory cascade in PCOS patients via altering the follicular microenvironment. Front Immunol. 2021;12:685724. doi: 10.3389/fimmu.2021.685724.
- Ibrahim YF, Alorabi M, Abdelzaher WY, et al. Diacerein ameliorates letrozole-induced polycystic ovarian syndrome in rats. Biomed Pharmacother. 2022;149:112870. doi: 10.1016/j.biopha.2022.112870.
- Zhang M, Hu G, Shao N, et al. Thioredoxin-interacting protein (TXNIP) as a target for Alzheimer’s disease: flavonoids and phenols. Inflammopharmacology. 2021;29(5):1317–1329. doi: 10.1007/s10787-021-00861-4.
- Kim EJ, Jang M, Choi JH, et al. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): post-pubertal improve PCOS’s features. Front Endocrinol (Lausanne). 2018;9:735. doi: 10.3389/fendo.2018.00735.
- Morsi AA, E AM, Razik HFA, et al. Histomorphological changes in a rat model of polycystic ovary syndrome and the contribution of stevia leaf extract in modulating the ovarian fibrosis, VEGF, and TGF-β immunoexpressions: comparison with metformin. Acta Histochem Cytochem. 2022;55(1):9–23. doi: 10.1267/ahc.21-00081.
- Coniglio RI, Meroño T, Montiel H, et al. HOMA-IR and non-HDL-C as predictors of high cholesteryl ester transfer protein activity in patients at risk for type 2 diabetes. Clin Biochem. 2012;45(7-8):566–570. doi: 10.1016/j.clinbiochem.2012.02.005.
- Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303(3):631–643. doi: 10.1007/s00404-020-05951-2.
- Hernández-Jiménez JL, Barrera D, Espinoza-Simón E, et al. Polycystic ovarian syndrome: signs and feedback effects of hyperandrogenism and insulin resistance. Gynecol Endocrinol. 2022;38(1):2–9. doi: 10.1080/09513590.2021.2003326.
- Wu LM, Wang YX, Zhan Y, et al. Dulaglutide, a long-acting GLP-1 receptor agonist, can improve hyperandrogenemia and ovarian function in DHEA-induced PCOS rats. Peptides. 2021;145:170624. doi: 10.1016/j.peptides.2021.170624.
- Kim KE, Heo JS, Han S, et al. Blood concentrations of lipopolysaccharide-binding protein, high-sensitivity C-reactive protein, tumor necrosis factor-α, and Interleukin-6 in relation to insulin resistance in young adolescents. Clin Chim Acta. 2018;486:115–121. doi: 10.1016/j.cca.2018.07.042.
- Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789. doi: 10.3390/ijms22073789.
- Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. doi: 10.4093/dmj.2021.0280.
- Zeng X, Xie YJ, Liu YT, et al. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–221. doi: 10.1016/j.cca.2019.11.003.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940.
- Ren N, Kim E, Li B, et al. Flavonoids alleviating insulin resistance through inhibition of inflammatory signaling. J Agric Food Chem. 2019;67(19):5361–5373. doi: 10.1021/acs.jafc.8b05348.
- Xiang S, Xia MF, Song JY, et al. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-insulin resistance of phlegm-dampness syndrome. Chin J Integr Med. 2021;27(5):330–335. doi: 10.1007/s11655-020-3219-z.
- Peng MF, Tian S, Song YG, et al. Effects of total flavonoids from Eucommia ulmoides Oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J Ethnopharmacol. 2021;273:113947. doi: 10.1016/j.jep.2021.113947.
- Liu S, Zhang Y, Yang F, et al. Modified Cangfu Daotan decoction ameliorates polycystic ovary syndrome with insulin resistance via NF-κB/LCN-2 signaling pathway in inflammatory microenvironment. Front Endocrinol (Lausanne). 2022;13:975724. doi: 10.3389/fendo.2022.975724.
- Abais JM, Xia M, Li G, et al. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J Biol Chem. 2014;289(39):27159–27168. doi: 10.1074/jbc.M114.567537.
- Wu J, Wu Y, Zhang X, et al. Elevated serum thioredoxin-interacting protein in women with polycystic ovary syndrome is associated with insulin resistance. Clin Endocrinol (Oxf). 2014;80(4):538–544. doi: 10.1111/cen.12192.
- Nasoohi S, Parveen K, Ishrat T. Metabolic syndrome, brain insulin resistance, and Alzheimer’s disease: thioredoxin interacting protein (TXNIP) and inflammasome as core amplifiers. J Alzheimers Dis. 2018;66(3):857–885. doi: 10.3233/JAD-180735.
- Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. doi: 10.1038/ni.1935.
- Li M, Zhang Y, Cao Y, et al. Icariin ameliorates palmitate-induced insulin resistance through reducing thioredoxin-interacting protein (TXNIP) and suppressing ER stress in C2C12 myotubes. Front Pharmacol. 2018;9:1180. doi: 10.3389/fphar.2018.01180.
- Zhao W, Pu M, Shen S, et al. Geniposide improves insulin resistance through AMPK-mediated txnip protein degradation in 3T3-L1 adipocytes. Acta Biochim Biophys Sin (Shanghai). 2021;53(2):160–169. doi: 10.1093/abbs/gmaa157.
- Wang Y, Yang J, Wang Y, et al. Upregulation of TXNIP contributes to granulosa cell dysfunction in polycystic ovary syndrome via activation of the NLRP3 inflammasome. Mol Cell Endocrinol. 2023;561:111824. doi: 10.1016/j.mce.2022.111824.
- Hou J, Lei Z, Cui L, et al. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol Environ Saf. 2021;212:112012. doi: 10.1016/j.ecoenv.2021.112012.
- Wang D, Weng Y, Zhang Y, et al. Exposure to hyperandrogen drives ovarian dysfunction and fibrosis by activating the NLRP3 inflammasome in mice. Sci Total Environ. 2020;745:141049. doi: 10.1016/j.scitotenv.2020.141049.
