Abstract
Objectives
Endometriosis is a common benign gynaecological disease that significantly compromises the quality of life of patients. To date, invasive surgery is the method of choice to visually and histologically confirm endometriosis. Thus, there is a major interest to develop noninvasive diagnostic tools. Oxidative stress is one of the proposed mechanisms of pathogenesis and may be involved in pelvic pain, dysmenorrhea, dyspareunia, and infertility in endometriosis patients. Thus, markers of oxidative stress may serve as diagnostic biomarkers for endometriosis.
Design
This prospective case–control study assessed erythrocyte superoxide dismutase (SOD), erythrocyte glutathione peroxidase (GPX), serum hexanoyl lysine (HEL) and peritoneal fluid HEL.
Participants/Materials, Setting, and Methods
We enrolled 86 women with primary infertility; the case group included 57 women with endometriosis, and the control group included 29 women with unexplained primary infertility. All the patients underwent laparoscopy, and the diagnosis was confirmed histologically. RANDOX and RANSEL reagents were used to determine the levels of SOD and GPX, respectively, and ELISA was used to determine the levels of HEL.
Results
We found no statistically significant differences in the erythrocyte levels of GPX (p value 0.623) or SOD (p value 0.122) or the serum or peritoneal fluid levels of HEL (p value 0.562 and 0.329 accordingly).
Conclusions
SOD, GPX, and HEL levels most likely do not differ between patients with unexplained infertility and patients with endometriosis.
Introduction
Endometriosis is a common benign gynaecological disease with an estimated prevalence of 10% worldwide, which increases to 50% in women with infertility or chronic pain [Citation1]. Endometriosis significantly compromises the quality of life of women and is a major cause of infertility. It is a heterogeneous disease that is categorized into endopelvic (ovarian, peritoneal, or deep infiltrating endometriosis) and extrapelvic endometriosis, and the most frequently used staging system for endometriosis is the revised American Fertility Society score [Citation2]. There are multiple theories about the etiology of the disease; however, it is generally accepted that the disease develops due to the implantation of endometrial cells through retrograde menstruation [Citation3]. The gold standard for diagnosis is visual surgical (laparoscopic) inspection of the pelvic organs, coupled with histological confirmation [Citation2]. Nonspecific symptoms and the need for invasive diagnostic procedures are the reasons why it can take a long time to obtain a definitive diagnosis of endometriosis [Citation4].
There is a major interest for the development of noninvasive diagnostic tools and techniques for treating endometriosis. Potential biomarkers for endometriosis are oxidative stress markers. Oxidative stress is involved in the pathophysiology of endometriosis and is thus also an interesting potential target for the management of endometriosis [Citation5].
It is widely accepted that oxidative stress, defined as an imbalance between reactive oxygen species (ROS) and antioxidants, is involved in the pathophysiology of endometriosis, causing a general inflammatory response in the peritoneal cavity [Citation6–9]. ROS modulate the proliferation of endometriotic cells [Citation9]. Ferrero et al. showed that functions related to oxidative stress response were significantly enriched in human oocytes from women with endometriosis compared to oocytes from healthy donors, suggesting that the increased oxidative stress described in serum and follicular fluid of women with endometriosis could affect their oocyte quality [Citation10]. To date, myeloperoxidase (MPO), superoxide dismutase (SOD), and glutathione peroxidase (GPX) have been investigated as potential biomarkers of oxidative stress and inflammation in endometriosis patients.
Dorien et al. studied the involvement of MPO, a proinflammatory enzyme and marker of neutrophil activation and oxidative stress, in endometriosis [Citation11]. Total and active MPO levels did not differ significantly between endometriosis patients and controls.
SOD is an antioxidant enzyme that converts superoxide anion radicals into hydrogen peroxide and thus represents the first line of defence against oxygen free radicals [Citation12], protecting cells and extracellular components against cellular damage. GPX is another antioxidant enzyme that prevents the formation of an extremely toxic hydroxyl radical and reduces lipid or nonlipid hydroperoxides [Citation13]. Two studies have demonstrated that GPX is not altered in endometriosis, whereas SOD is significantly decreased [Citation13,Citation14]. Ekarattanawong et al. suggested SOD and GPX as biomarkers of endometriosis [Citation13]. Using receiver operating characteristic curve analysis, they revealed that combined test with both SOD and GPX could be useful as diagnostic test [Citation13].
Hexanoyl lysine (HEL) was identified as a lipid hydroperoxide-modified lysine residue and is considered to be a useful marker of early lipid peroxidation derived from protein modification [Citation15]. Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals and is used as an indicator of oxidative stress in cells and tissues. Studies have assessed HEL in certain diseases, such as metabolic syndrome, systemic sclerosis, and Sjögren syndrome [Citation16,Citation17]; however, to the best of our knowledge, no studies have examined the involvement of HEL in endometriosis.
In this study, we compared the levels of oxidative stress biomarkers in infertile endometriosis patients and infertile controls. We aimed to evaluate oxidative stress markers as potential diagnostic biomarkers of endometriosis in patients with infertility and to contribute to a better understanding of the pathophysiology of endometriosis. According to the published literature, our study is the first to study HEL in endometriosis patients.
Materials and methods
This study was designed as a prospective case–control study. We examined the levels of erythrocytes SOD, and GPX, serum HEL and peritoneal fluid HEL.
Patient selection
We enrolled 86 women with primary infertility (); the case group included 57 women with endometriosis, and the control group included 29 women with unexplained primary infertility. All patients underwent laparoscopy due to infertility (or endometriosis), and the diagnosis was confirmed histologically. All patients had a body mass index (BMI) within the normal range, a regular menstrual cycle (21–35 days), and normal partner semen analyses. The exclusion criteria included patients who had undergone hormonal therapy in the last year, had irregular menstrual cycles, previous pelvic surgery or had the following diseases: autoimmune, malignant or suspected malignant, or previous pelvic inflammatory disease, leiomyoma uteri, or polycystic ovaries. As presented in 26% of patients in endometriosis group had ovarian endometriosis, 28% peritoneal and 38.6% ovarian and peritoneal at same time; the minority of patients (7%) had deep infiltrative endometriosis. 35% of patients with endometriosis was classified as stage I, 5.4% as stage II, 52.6% as stage III, and 7% as stage IV according to Revised American Fertility score system.
Table 1. Clinical characteristics of the patients enrolled in this study.
Informed consent was obtained from all the participants before their inclusion in this study. This study was conducted with the approval of the Medical Ethics Committee of the Republic of Slovenia (No. 0120-049/2016-4) and according to The Code of Ethics of the World Medical Association (Declaration of Helsinki). Trial registration number: NCT04591548.
Sample and data collection
All patients who met the inclusion criteria were additionally evaluated. They filled out a questionnaire on their health history, stress levels, use of medications, and types of pain (dysmenorrhea, dyspareunia, or chronic pain), using a validated visual analogue scale. Based on the laparoscopy and histology results, patients were classified into either the case group (only women with endometriosis and no other pathology) or control group (only women without any pathology).
Blood samples were collected 1–3 days before surgery according to a strict standard operating procedure [Citation18,Citation19] and were tested for SOD, GPX, and HEL. Two tubes of blood were taken from the subjects. One tube was with EDTA anticoagulant (BD Vacuainer #368861) (3 ml) for erythrocyte collection and SOD and GPX determination and the other tube was without anticoagulant for serum preparation (BD Vacuainer #369032) (4 ml) for HEL determination. The tube with anticoagulant was centrifuged for 10 min at 2500× g, the plasma was separated and the remaining cells were washed three times with saline and then haemolysed with ice-cold water. The resulting haemolysate was stored at minus 80 degrees Celsius until analysis. The tube without anticoagulant was centrifuged for 15 min at 1400× g and the serum was then frozen at −80°Cuntil analysis.
Peritoneal fluid samples were collected during laparoscopy, before any intra-abdominal procedures were performed. The pneumoperitoneum was accessed using a Veress needle at the umbilicus, and peritoneal fluid was aspirated from the Douglas space using a 2 mm needle. In the case of blood contamination of peritoneal fluid, the patient was excluded from study. Peritoneal fluid samples (3 ml) were collected into 12 ml plastic tubes (Greiner, Monroe, North Carolina, USA) and stored at 4 °C. The samples were centrifuged within 1 h of collection (900× g for 5 min at 4 °C), aliquoted, and stored at −80 °C.
Biochemical evaluation of oxidative stress
Superoxide dismutase (SOD)
We used RANSOD diagnostic kit produced by RANDOX (Randox Laboratories Ltd., Crumlin, County Antrim, UK) reagent for quantitative determination of SOD in erythrocytes [Citation20]. The method employs xanthine and xanthine oxidase to generate superoxide radicals, which react with chromogen to form a red formazan dye. SOD activity is then measured by the decrease in product formation due to inactivation of superoxide radicals. Absorbance was measured at 505 nm. The performance of this method is as follows: 3.6%–4.6% within-run precision and 5.9%–7.0% between-run precision.
Glutathione peroxidase (GPX)
We used RANSEL diagnostic kit produced by RANDOX (Randox Laboratories Ltd., Crumlin, County Antrim, UK) reagent for quantitative determination of GPX in erythrocytes [Citation21]. GPX catalyzes the oxidation of glutathione by hydroperoxide. In the presence of glutathione reductase and NADPH, oxidized glutathione is converted to the reduced form. The decrease in absorbance was measured at 340 nm. The performance of this method was as follows: 3.2%–4.9% within-run precision and 4.4%–7.3% between-run precision. Both enzymes were measured on a Roche Modular P analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Hexanoyl lysine (HEL)
The ELISA kit uses the competitive enzyme immunoassay technique together with a polyclonal anti-HEL antibody and an HEL- horseradish peroxidase (HRP) conjugate [Citation17,Citation22]. The assay sample and buffer were incubated together with HEL-HRP conjugate in pre-coated wells for 1 h. Afterwards, the wells were decanted, washed five times, and incubated with a substrate for HRP enzyme. The product of the enzyme-substrate reaction forms a blue complex. Finally, a stop solution was added to stop the reaction, turning the solution yellow. The color intensity was measured spectrophotometrically at 450 nm in a microplate reader. The performance of this method, i.e. the within-run and between-run precision, is <10%.
Statistical analysis
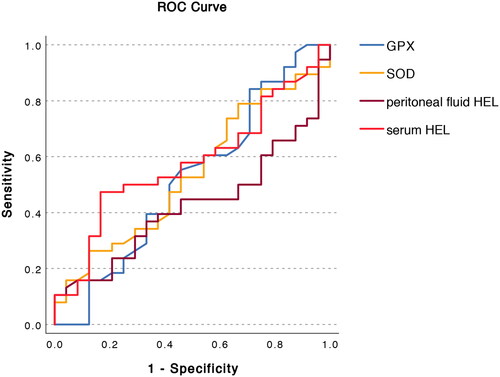
SPSS for Windows version 22 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The values of oxidative stress markers were analyzed with the Mann-Whitney U test. p < 0.05 was considered significant. A receiver operating characteristic curve (ROC) curve was generated for SOD, GPX and HEL.
Results
The clinical characteristics of endometriosis patients and control infertile patients are presented in . There were no significant differences between the case and control groups for either menstrual phase or for any of the characteristics examined ().
We found no statistically significant differences in GPX or SOD activity or HEL serum or peritoneal fluid levels ( and ). We observed a trend of higher SOD levels in endometriosis patients, which, however, was not statistically significant. We did not find statistically significant correlations between GPX, SOD, and HEL levels and dysmenorrhea, dyspareunia, and chronic pelvic pain or between the severity of endometriosis and the levels of oxidative stress biomarkers. ROC analysis revealed no prediction value for selected biomarkers candidates ( and ).
Figure 1. Levels of superoxide dismutase (SOD), glutathione peroxidase (GPX), serum and peritoneal hexanoyl lysine (HEL) in endometriosis patients (n = 57) and controls (n = 29).
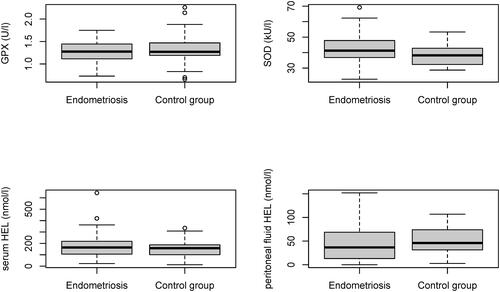
Table 2. Values of oxidative stress markers in endometriosis patients (n = 57) and disease-free controls (n = 29).
Table 3. Area under the curve the selected biomarker candidates.
Discussion
The pathogenesis of endometriosis remains obscure, and, despite research efforts, there is still no reliable noninvasive alternative to surgical visualization (for obtaining a definitive diagnosis of endometriosis) [Citation23]. Oxidative stress markers have been proposed to facilitate early diagnosis of endometriosis, and several of them have been studied in relation to endometriosis; however, the conclusions are inconsistent [Citation11–13]. In the future, an imbalance in the oxidation–reduction reaction status may represent a therapeutic target.
The first objective of this study was to evaluate the levels of SOD and GPX activity in erythrocytes, and HEL in serum samples and peritoneal fluid in infertile patients with endometriosis compared to infertile patients without endometriosis. According to literature review we hypothesized that SOD and GPX levels are decreased, whereas MPO and HEL are increased in the blood and peritoneal fluid of endometriosis patients. However, we found no statistically significant differences in erythrocytes, serum or peritoneal fluid levels of these oxidative stress markers between the two groups. Several published studies have linked unexplained infertility to oxidative stress [Citation24]. The pathophysiology of unexplained infertility remains unclear; however, some studies suggest that ROS play a role. It has been shown that abnormal increases in ROS levels in follicular fluid negatively correlate with oocyte development, embryonic development, and pregnancy outcome, and physiological ROS levels are required for normal oocyte development [Citation25–28]. Studies have reported that concentrations of malondialdehyde, a marker of lipid peroxidation, are increased in the peritoneal cavity in cases of unexplained infertility, whereas the concentrations of antioxidants are not increased [Citation29–31]. A randomized study investigated N-acetyl cysteine, a powerful antioxidant, as a cofactor to clomiphene citrate for inducing ovulation in women with unexplained infertility but demonstrated that N-acetyl cysteine was ineffective [Citation32]. Paszkowski et al. reported the importance of selenium (an essential part of the selenoenzyme GPX) in the follicular fluid of infertile patients who presented for in vitro fertilization treatment [Citation33]. Comparing patients with tubal factor, male factor, and unexplained infertility, they found selenium and GPX levels to be the lowest in patients with unexplained infertility. They concluded that reduced antioxidant levels in ovarian follicles may cause unexplained infertility [Citation33].
Based on our results we hypothesize that lower antioxidant capacity and consequently higher ROS are determinant factors in the pathophysiology of unexplained infertility and infertility in endometriosis patients. The mechanism of infertility can be explained in some endometriosis patients as the result of distorted pelvic anatomy; however, some patients present no anatomical reason for infertility. There are multiple theories regarding the etiology of endometriosis (e.g. the celomic metaplasia, Mullerian rest, induction, stem-cell, and retrograde menstruation theories). However, none of these theories can, as yet, explain all types of endometriosis [Citation3]. The retrograde menstruation theory is one of the most commonly accepted theories [Citation3]. According to this theory, the accumulation of iron from erythrocytes evokes oxidative stress [Citation13]. Studies of markers of oxidative stress and inflammation have suggested MPO, SOD, and GPX as potential biomarkers of endometriosis [Citation11,Citation13,Citation14]. Prieto et al. suggested that (lower) SOD and GPX blood levels could represent biomarkers of endometriosis. However, they compared endometriosis patients with patients with tubal or male factor of infertility or healthy oocyte donors [Citation14]. In our study, we observed the opposite: a nonsignificant trend of higher SOD levels in endometriosis patients. We hypothesize that oxidative stress levels in cases of unexplained infertility differ from those in cases with other causes of infertility. However, no study to date has evaluated the levels of these biomarker candidates in patients with different etiologies of infertility. Our unexpected results could be due to our different study cohort.
Our data analysis revealed that SOD, GPX, and HEL levels most likely do not differ between patients with unexplained infertility and patients with endometriosis. The p values observed in this study suggest that larger sample numbers would not change this result.
Nevertheless, the present study has some limitations. First, the sample numbers are relatively small; however, as explained above, it is questionable whether a larger sample population would produce a different result. Furthermore, the relatively small sample number is mainly due to strict standard operating procedures, which simultaneously represent the strength of our study. The second limitation is a lack of validation from a different study population without endometriosis or unexplained infertility. Our results suggest that oxidative stress markers are not useful for detecting endometriosis in infertile populations. Future studies are needed, especially studies comparing endometriosis patients, healthy fertile controls, and patients with different etiologies of infertility. Even though oxidative stress biomarkers may not be valuable diagnostic biomarkers for specific diseases, in future they might help in identifying patients that benefit from specific treatments. To conclude, more studies are needed that would investigate the role of oxidative stress in the pathophysiology of infertility and evaluate new therapeutic targets for treating endometriosis and unexplained infertility.
Authors’ contributions
V.J., T.L.R., H.B.F., and J.O.: conception and design of the study, data acquisition, data analysis. I.V.: data analysis. V.J.: drafting the manuscript. J.O.: biochemical analyses of oxidative stress markers. T.L.R., H.B.F., and J.O.: critical revision of the manuscript for intellectual content. V.J., H.B.F., T.L.R., and J.O. initiated the project and were responsible for the study. All authors have approved the final version of the manuscript.
Statement of ethics
Informed consent was obtained from all the participants before their inclusion in the study. The study was conducted with the approval of the Medical Ethics Committee of the Republic of Slovenia (No. 0120-049/2016-4) and according to The Code of Ethics of the World Medical Association (Declaration of Helsinki). Trial registration number: NCT04591548.
Supplemental Material
Download MS Word (54.3 KB)Acknowledgements
The authors thank their study participants who kindly donated their samples and time. The authors thank the personnel of the Department of Obstetrics and Gynaecology, University Medical Centre Ljubljana, Slovenia, especially Mrs. Tanja Lončar. The authors also thank Mrs. Vera Troha Poljančič at the Clinical Institute of Clinical Chemistry and Biochemistry, University Medical Centre Ljubljana, and dr. Eva Lasič for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Rogers PA, D’Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 world congress of endometriosis in montpellier, France. Reprod Sci. 2013;20(5):1–6. doi: 10.1177/1933719113477495.
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi: 10.1093/humrep/det457.
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstetrics Gynec. 1927;14(4):422–469. doi: 10.1016/S0002-9378(15)30003-X.
- Rogers PA, Adamson GD, Al-Jefout M, et al. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202–226. doi: 10.1177/1933719116654991.
- Amreen S, Kumar P, Gupta P, et al. Evaluation of oxidative stress and severity of endometriosis. J Hum Reprod Sci. 2019;12(1):40–46. doi: 10.4103/jhrs.JHRS_27_17.
- Christodoulakos G, Augoulea A, Lambrinoudaki I, et al. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance. Eur J Contracept Reprod Health Care. 2007;12(3):194–202. doi: 10.1080/13625180701387266.
- Augoulea A, Mastorakos G, Lambrinoudaki I, et al. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25(2):75–81. doi: 10.1080/09513590802485012.
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28.
- Scutiero G, Iannone P, Bernardi G, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev. 2017;2017:7265238. doi: 10.1155/2017/7265238.
- Ferrero H, Corachán A, Aguilar A, et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum Reprod. 2019;34(7):1302–1312. doi: 10.1093/humrep/dez053.
- O DF, Waelkens E, Peterse DP, et al. Evaluation of total, active, and specific myeloperoxidase levels in women with and without endometriosis. Gynecol Obstet Invest. 2018;83(2):133–139. doi: 10.1159/000475664.
- Li J, Wu R, Xia B, et al. Serum levels of superoxide dismutases in patients with benign paroxysmal positional vertigo. Biosci Rep. 2020;40(5):BSR20193917.
- Ekarattanawong S, Tanprasertkul C, Somprasit C, et al. Possibility of using superoxide dismutase and glutathione peroxidase as endometriosis biomarkers. Int J Womens Health. 2017;9:711–716. doi: 10.2147/IJWH.S141021.
- Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. 2012;98(1):126–130. doi: 10.1016/j.fertnstert.2012.03.052.
- Tokuda F, Sando Y, Matsui H, et al. N epsilon-(hexanoyl) lysine, a new oxidative stress marker, is increased in metabolic syndrome, but not in obstructive sleep apnea. Am J Med Sci. 2009;338(2):127–133. doi: 10.1097/MAJ.0b013e3181a478e5.
- Shimizu K, Ogawa F, Akiyama Y, et al. Increased serum levels of N(epsilon)-(hexanoyl)lysine, a new marker of oxidative stress, in systemic sclerosis. J Rheumatol. 2008;35(11):2214–2219. doi: 10.3899/jrheum.080191.
- Sakai K, Kino S, Masuda A, et al. Determination of HEL (hexanoyl-lysine adduct): a novel biomarker for omega-6 PUFA oxidation. Subcell Biochem. 2014;77:61–72.
- Janša V, Klančič T, Pušić M, et al. Proteomic analysis of peritoneal fluid identified COMP and TGFBI as new candidate biomarkers for endometriosis. Sci Rep. 2021;11(1):20870. doi: 10.1038/s41598-021-00299-2.
- Pušić M, Klančič T, Knific T, et al. Antibody arrays identified Cycle-Dependent plasma biomarker candidates of peritoneal endometriosis. J Pers Med. 2022;12(6):852.
- Li J, Lei J, He L, et al. Evaluation and monitoring of superoxide dismutase (SOD) activity and its clinical significance in gastric cancer: a systematic review and Meta-Analysis. Med Sci Monit. 2019;25:2032–2042. doi: 10.12659/MSM.913375.
- Guillin OM, Vindry C, Ohlmann T, et al. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101.
- Kato Y, Miyake Y, Yamamoto K, et al. Preparation of a monoclonal antibody to N(epsilon)-(hexanonyl)lysine: application to the evaluation of protective effects of flavonoid supplementation against exercise-induced oxidative stress in rat skeletal muscle. Biochem Biophys Res Commun. 2000;274(2):389–393. doi: 10.1006/bbrc.2000.3150.
- Janša V, Osredkar J, Vrtačnik Bokal E, et al. Biomarkers of endometriosis: how far have we come and where are we going? ZdravVestn. 2021;90(5-6):256–265. doi: 10.6016/ZdravVestn.3056.
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–1650. doi: 10.1016/j.biocel.2010.06.001.
- Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–287. doi: 10.1111/j.1600-079X.2007.00524.x.
- Revelli A, Delle Piane L, Casano S, et al. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40.
- Pasqualotto EB, Agarwal A, Sharma RK, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81(4):973–976. doi: 10.1016/j.fertnstert.2003.11.021.
- Wiener-Megnazi Z, Vardi L, Lissak A, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013.
- Polak G, Kozioł-Montewka M, Tarkowski R, et al. [Peritoneal fluid and plasma 4-hydroxynonenal and malonyldialdehyde concentrations in infertile women]. Ginekol Pol. 2001;72(12a):1316–1320.
- Polak G, Rola R, Gogacz M[, et al. Malonyldialdehyde and total antioxidant status in the peritoneal fluid of infertile women. Ginekol Pol. 1999;70(3):135–140.
- Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49.
- Badawy A, Baker El Nashar A, El Totongy M. Clomiphene citrate plus N-acetyl cysteine versus clomiphene citrate for augmenting ovulation in the management of unexplained infertility: a randomized double-blind controlled trial. Fertil Steril. 2006;86(3):647–650. doi: 10.1016/j.fertnstert.2006.02.097.
- Paszkowski T, Traub AI, Robinson SY, et al. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236(2):173–180. doi: 10.1016/0009-8981(95)98130-9.