Abstract
Objective: Polycystic ovarian syndrome (PCOS) is a prevalent gynecologic disorder, often associated with abnormal follicular development. Cangfu Daotan decoction (CFD) is a traditional Chinese medicine formula that is effective in alleviating PCOS clinically, but the specific mechanism remains unclear. Forkhead box K1 (FOXK1) is associated with cellular function. This study aimed to explore the effects of CFD and FOXK1 on PCOS.Methods: High-fat diet and letrozole were combined to establish PCOS rat models. Next, primary GCs were extracted from those PCOS rats. Then, GC cells were transfected with si-FOXK1 or oe-FOXK1. CFD-contain serum was prepared, and experiments were conducted to investigate the regulation of FOXK1 by CFD.Results: FOXK1 was highly expressed in GCs of PCOS rats. Further investigation revealed that FOXK1 overexpression resulted in inhibition of proliferation and DNA synthesis, along with promotion of apoptosis and autophagy in GCs. Additionally, it was found that FOXK1 promoted the expressions of the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway-related proteins. Interestingly, treatment with CFD reversed all the effects of FOXK1 overexpression in GCs. Conclusion: This study demonstrated that CFD exerted a protective role in PCOS by inhibiting FOXK1, which provided a research basis for the application of CFD in PCOS, and suggested that FOXK1 is a novel therapeutic target in PCOS treatment.
Introduction
Polycystic ovarian syndrome (PCOS) is a prevalent gynecological endocrine disorder that affects approximately 6–15% of women of reproductive age worldwide [Citation1]. Clinically, it is characterized by sporadic ovulation or anovulation and hyperandrogenemia, which can lead to menstrual irregularities even infertility [Citation2]. At present, western medicine is based on symptomatic treatment, which has certain efficacy but relatively many side effects and is easy to relapse after discontinuation of medication [Citation3,Citation4]. Therefore, we are eager to carry out in-depth basic research on empirical formulas with definite clinical efficacy in order to provide stronger evidence for the promotion and application of Chinese medicine in the treatment of PCOS.
Aberrant follicular development is widely believed to be a significant factor in the development of PCOS [Citation5]. Abnormal follicular development is the main pathological basis for anovulation and clinical endocrine alterations in PCOS patients, while apoptosis and autophagy of ovarian granulosa cells (GCs) are crucial in the process of follicular development and atresia [Citation6]. GCs supply nutrients and growth regulators for oocyte development, DNA synthesis is a core process for cell formation and proliferation, thus, the DNA synthetic ability of GCs is important for PCOS development [Citation7]. In addition, both excessive apoptosis and autophagy will hinder follicle growth and ultimately affect the developmental potential of oocytes [Citation8]. It was found that promoting follicle development can achieve the improvement of metabolic syndrome and enhancement of reproductive function in PCOS patients [Citation9]. Cangfu Daotan decoction (CFD), a well-known Chinese medicine prescription, is effective in facilitating follicular development in PCOS patients [Citation10]. CFD also can regulate lipid and sex hormone levels in PCOS rats to improve pregnancy outcomes [Citation11]. However, the underlying molecular mechanisms of how CFD improves follicular development in patients with PCOS still need to be explored in depth.
The forkhead box (FOX) is a superfamily of transcription factors with a highly conserved DNA binding domain [Citation12]. FOXK1 phosphorylation inhibits autophagy-related gene expression by increasing its nuclear localization and binding to the transcriptional repressor complex [Citation13]. In addition, FOXK1 has been reported to modulate cell apoptosis in numerous cancers, such as glioma [Citation14], breast cancer [Citation15], and so on. Thus, we hypothesized that FOXK1 may be involved in PCOS follicular development by negatively regulating GCs autophagy. The AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) signaling pathway is closely associated with GCs autophagy [Citation16]. Several published studies have reported that some drugs alleviate disease by regulating autophagy [Citation17] and apoptosis [Citation18] through the AMPK/mTOR signaling pathway. In addition, p53 in the nucleus can promote cellular autophagy by regulating AMPK/mTOR levels [Citation19]. Thus, the AMPK/mTOR pathway appears to be a very promising target for the action of follicular developmental abnormalities in the pathogenesis of PCOS. However, whether the regulation of FOXK1 in PCOS is related to the AMPK/mTOR pathway and the effect of CFD on it is unclear.
Therefore, we proposed to isolate primary GCs and then intervene with cell transfection technology. The effect of FOXK1 on GCs autophagy and apoptosis in PCOS and the role of the AMPK/mTOR pathway in this process were investigated, thereby further validating the interventional effect of CFD and providing an experimental basis for its use in the clinical treatment of PCOS.
Materials and methods
Animal ethology
Eighteen 6-week-old female SD rats were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd, (SCXK (Shanghai) 2017-0005). Rats were maintained in an environment with a temperature of (22 ± 2)°C and a humidity of (55 ± 5)%.
Preparation of CFD-containing serum
Eight SD rats were randomly divided into control group and CFD group (n = 4) after 1 week of adaptation. Then, the rats in the CFD group were gavaged with 15 g/kg/day of CFD (Jingyuetang Pharmaceutical) for 5 days, while the rats in the control group received an equal amount of saline [Citation20]. One hour after the last administration, blood samples were collected from the abdominal aorta and the drug-containing serum was obtained by centrifugation. The serum was inactivated in a water bath at 56 °C for 30 min, and stored at −80 °C.
Establishment of PCOS rat model
Ten SD rats were randomly allocated into control and PCOS groups (n = 5). As in previous studies, a high-fat diet combined with letrozole (Shanghai yuanye) was used to establish a PCOS model [Citation21,Citation22]. The rats in the control group were given normal feed and gavaged with 0.5% carboxymethyl cellulose solution (CMC) (Shanghai yuanye), while the rats in the PCOS group were given high-fat feed and gavaged with letrozole (1 mg/kg/day) dissolved in 0.5% CMC for 21 days [Citation22,Citation23]. During this period, vaginal smears were performed daily to assess whether the PCOS had been successfully induced [Citation24].
Extraction and culture of primary GCs in rats
The rats from the control and PCOS groups were administered 25 IU of pregnant mare’s serum gonadotropin (PMSG) (Solarbio) via intraperitoneal injection [Citation25]. After 48 h, bilateral ovaries of the rats were extracted and GCs were separated mechanically in accordance with a previous study [Citation26]. Following filtration using a cell sieve, GCs were cultured in a DMEM/F12 medium (Gibco) containing 10% fetal bovine serum (Gibco) in a 37 °C, 5% CO2 incubator. Cell morphology was observed under a light microscope.
Cell grouping and intervention
Following the isolation of primary GCs from both the control and PCOS groups, FOXK1 mRNA and protein expressions were examined. To investigate the impact of FOXK1 on GCs, 4 subgroups were set up: si-NC group, si-FOXK1 group, vector group, and oe-FOXK1 group. Cells were transfected with corresponding RNA or vector, respectively. Moreover, to further identify the regulatory function of CFD, we set up the following groups: vector + 10%NRS group, oe-FOXK1 + 10%NRS group, and oe-FOXK1 + 10%CFD group. Cells were transfected with vector or oe-FOXK1 and cultured in a medium with CFD-containing or CFD-free serum.
Cell transfection
FOXK1 in GCs was silenced through transfection with small interfering RNA (siRNA). si-FOXK1 and negative control were synthesized by GenePharma (Shanghai). The si-FOXK1 was transfected into the cells using lipofectamine 2000 (ThermoFisher) according to the operating instructions. The pcDNA3.1-FOXK1 and pcDNA3.1 vector were purchased from invitrogen (ThermoFisher). pcDNA3.1-FOXK1 and pcDNA3.1 vectors were transfected with lentivirus (ThermoFisher) and the transfection efficiency was detected after 48 h.
Quantitative real-time PCR (qRT-PCR)
The GCs were harvested and cellular mRNA was extracted using trizol (Yeasen Biotechnology) and organic solvents (Yeasen Biotechnology). Reverse transcription and qRT-PCR reactions were performed according to the kit instructions (Beijing ComWin). qRT-PCR primer sequences used in the study were listed in .
Table 1. Primer sequence for qRT-PCR.
CCK-8 assay
CCK-8 assay was employed to evaluate cell proliferation. After intervening cells as described above, they were inoculated in 96-well plates and incubated for a certain time (12 h, 24 h, 48 h, 72 h, 96 h). Following removal of the medium and washing with PBS solution, 10 μL of CCK-8 solution (Beyotime) was added to each well. The optical density (OD) value at 450 nm was measured after 2 h of incubation.
EdU assay
The DNA synthesis capacity of GCs was detected by EdU kit. The Edu working solution was prepared according to kit requirements. The cells were inoculated in 12-well plates, and 500 µL of Edu solution (Beyotime) was added to each well and then incubated for 4 h. Following this, cells were fixed, transparent, stained for nuclei, and observed under an inverted microscope.
Flow cytometry
Flow cytometry was utilized to detect apoptosis. In short, cells were inoculated in 6-well plates and treated as above mentioned protocol for 24 h. Then, cells were collected and washed with PBS. Afterward, 5 μL Annexin V-FITC (BD Pharmingen) and 10 μL PI (BD Pharmingen) were added and the reaction was performed at room temperature and protected from light for 15 min. Finally, a binding buffer was added, and the apoptosis rate was detected via flow cytometry.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
The TUNEL assay was adopted to analyze the apoptosis of cells based on the manufacturer’s instructions. In short, after treatment, the cells were fixed by 4% paraformaldehyde (PFA) and rinsed by PBS. Next, the cells were incubated with 0.3% Triton X-100 in PBS for 5 min. Following this, the cells were stained with the staining mixture from the TUNEL kit (Beyotime) at room temperature. The nuclei in the apoptotic cells were stained with DAPI. The number of positive cells was calculated using ImageJ software.
Immunofluorescence staining
Immunofluorescence staining was used to detect LC3 expressions in GCs of PCOS rats. Cells were fixed with 4% PFA (Beyotime) for 10 min. Then, 0.5% Triton X-100 (Beyotime) was applied to permeabilize the cell membrane. After rinsing by PBS, 3% BSA was added to the cells. Subsequently, LC3 antibody (Abcam) and secondary antibody were used to incubate the cells. Following washing, nuclear staining reagent DAPI (Beyotime) was added. After blocking, the fluorescence was observed under an inverted fluorescence microscope.
Monodansylcadaverine (MDC) staining
Autophagy was assessed by MDC staining. Following treatment, cells were washed two times and then stained with MDC staining(Beyotime) at room temperature for 40 min. Subsequently, the cells were washed by a wash buffer, fixed with 4% PFA, and rinsed again. Finally, the cells were viewed under a microscope and the autophagy was assessed by counting the green dot number.
Western blot
Cellular proteins were extracted and the concentration was determined by a BCA kit (Beyotime). The target protein bands were obtained by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Then, PVDF membranes (Millipore) were placed into incubation cartridges containing bovine serum albumin (BSA) (BioFRoxx) for 2 h. After antigen-antibody reaction, ECL luminescent reagents (Solarbio) were added and exposed. Information regarding the antibodies used in the study could be found in .
Table 2. Antibody information of western blot.
Statistical analysis
The data was analyzed by SPSS statistical software, and expressed as mean ± standard deviation. When the measures between multiple groups conformed to a normal distribution, data were analyzed by One-way-ANOAY if the variances were homogeneous, and further comparisons between groups were made using the Tukey test. If the variances were not homogeneous, independent samples t-test was used. Otherwise, Kruskal-Wallis H-test was used. Statistically significant differences were indicated as p < .05.
Results
FOXK1 was highly expressed in PCOS-derived GCs
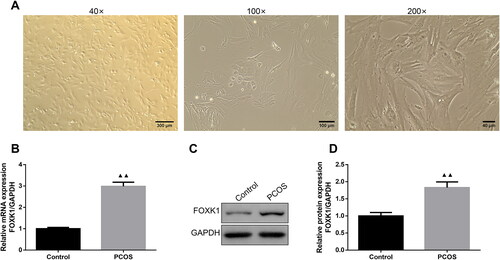
The primary rat ovarian GCs were shown in . Specifically, the cells were long spindle-shaped, and arranged in a swirl-like pattern, the nuclei were large and round, and the cytoplasm was rich in vacuoles and granules (). qRT-PCR and western blot were used to detect the expression level of FOXK1 in the ovarian GCs of PCOS rats, and showed the expression levels of FOXK1 mRNA and protein were elevated in the GCs of PCOS rats compared with the control group.
Figure 1. FOXK1 is highly expressed in PCOS-derived GCs. (A) The morphology of rat ovarian GCs was observed. (Magnification, 40×, 100×, 200×). (B) qRT-PCR was used to detect FOXK1 mRNA level in GCs of PCOS rats. (C,D) Western blot was used to detect FOXK1 protein expression level in GCs of PCOS rats. n = 3, compared to the control group, ▲p < .05, ▲▲p < .01. Note. FOXK1: forkhead box K1; PCOS: polycystic ovarian syndrome; GCs: granulosa cells; qRT-PCR: quantitative real-time PCR.
FOXK1 was successfully silenced or overexpressed and FOXK1 inhibited GCs proliferation in PCOS rats
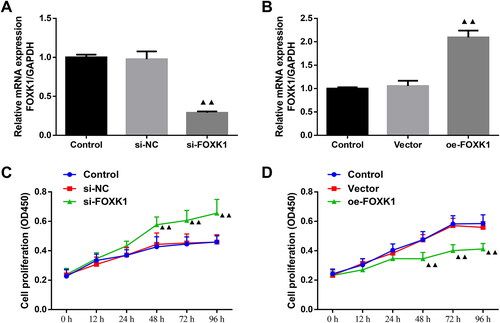
The transfection efficiency of FOXK1 silencing and overexpression in PCOS rat GCs was detected by qRT-PCR (). The expression level of FOXK1 mRNA was reduced in GCs from PCOS rats in the si-FOXK1 group compared with the si-NC group (). Conversely, compared with the vector group, the expression level of FOXK1 mRNA was elevated in GCs from PCOS rats in the oe-FOXK1 group (). Then, CCK-8 assay indicated no significant differences in cell proliferation at 0–96 h between the control and si-NC group as well as the control and vector group (). Notably, cell proliferation of the si-FOXK1 group was increased at 48, 72 and 96 h compared with the si-NC group, whereas the cell proliferation of the oe-FOXK1 group was decreased after 48, 72 and 96 h compared with the vector group ().
Figure 2. FOXK1 is successfully silenced or overexpressed and FOXK1 inhibits GCs proliferation in PCOS rats. (A) Transfection efficiency of FOXK1 silencing in GCs of PCOS rats was examined by qRT-PCR. (B) qRT-PCR was used to detect the transfection efficiency of FOXK1 overexpression in GCs of PCOS rats. (C) CCK-8 assay detected the effect of silencing FOXK1 on proliferation in GCs of PCOS rats. (D) CCK-8 assay was used to detect the effect of overexpression of FOXK1 on GCs proliferation in PCOS rats. n = 3, compared to the vector group, ▲p < .05, ▲▲p < .01.
FOXK1 inhibited the DNA synthesis capacity of GCs in PCOS rats
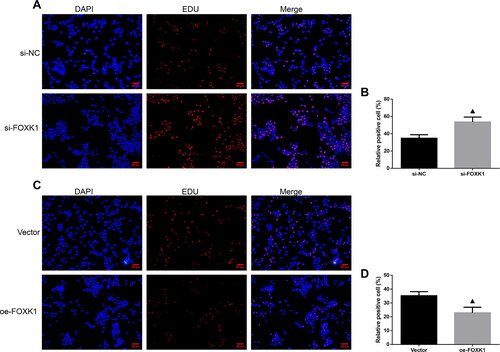
EDU staining was performed to examine the effect of silencing or overexpression of FOXK1 on the DNA synthesis capacity of GCs in PCOS rats (). Compared with the si-NC group, the si-FOXK1 group increased the number of orange-red fluorescent cells and enhanced red fluorescence intensity (). Conversely, compared with the vector group, the oe-FOXK1 group exhibited a decreased number of orange-red fluorescent cells and a reduction in the intensity of red fluorescence ().
Figure 3. FOXK1 inhibits the DNA synthesis capacity of GCs in PCOS rats. (A,B) EDU staining was used to detect the effect of silencing FOXK1 on the ability of GCs DNA synthesis in PCOS rats. (C,D) EDU staining was used to detect the effect of overexpression of FOXK1 on the ability of GCs DNA synthesis in PCOS rats. Magnification, 200×. n = 3, compared to the vector group, ▲p < .05, ▲▲p < .01.
FOXK1 promoted apoptosis in GCs of PCOS rats
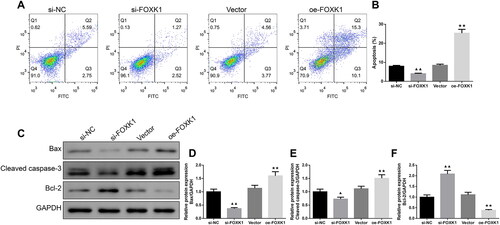
Flow cytometry suggested that the apoptosis rate of GCs in the si-FOXK1 group was lower than that of the si-NC group, while the apoptosis rate in the oe-FOXK1 group was higher than in the vector group (). Further analysis by Western blot also showed decreased Bax and cleaved caspase-3 levels in the si-FOXK1 group, and elevated levels of Bcl-2 protein, relative to the si-NC group (). Conversely, Bax and cleaved caspase-3 protein expressions were increased and Bcl-2 protein expression was decreased in GCs of PCOS rats in the oe-FOXK1 group compared with the vector group ().
Figure 4. FOXK1 promotes apoptosis in GCs of PCOS rats. (A,B) Flow cytometry was used to detect the effect of silencing or overexpression of FOXK1 on GCs apoptosis in PCOS rats. (C–F) Effects of silencing or overexpression of FOXK1 on the apoptosis-related proteins Bax, cleaved caspase-3, and Bcl-2 expression levels in GCs were examined by western blot. n = 3, compared to the si-NC group, ▲p < .05; ▲▲p < .01; compared to the vector group, *p < .05, **p < .01. Note. Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-Associated X.
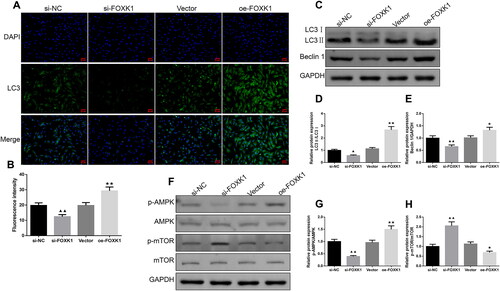
FOXK1 promoted GCs autophagy and AMPK/mTOR pathway protein levels in PCOS rats
Immunofluorescence staining revealed that the intensity of LC3 fluorescence expression was diminished in the cells of the si-FOXK1 group compared with the si-NC group, and enhanced in the cells of the oe-FOXK1 group compared with the vector group (). In addition, as shown in , the levels of LC3 II/I, Beclin 1, p-AMPK/AMPK protein were decreased and p-mTOR/mTOR protein was increased in the cells of the si-FOXK1 group compared with the si-NC group. However, oe-FOXK1 promoted LC3 II/I, Beclin 1, p-AMPK/AMPK protein expressions and decreased p-mTOR/mTOR protein expression in cells ().
Figure 5. FOXK1 promotes GCs autophagy and AMPK/mTOR pathway protein levels in PCOS rats. (A,B) Immunofluorescence was used to examine the effect of silencing or overexpressing FOXK1 on the LC3 expression level in the GCs of PCOS rats. (Magnification, 200×). (C–H) Western blot was used to test the effect of silencing or overexpression of FOXK1 on autophagy and p-AMPK/AMPK, p-mTOR/mTOR protein expression levels in the GCs of PCOS rats. n = 3, compared to the si-NC group, ▲p < .05, ▲▲p < .01; compared to the vector group, *p < .05, **p < .01. Note. AMPK: AMP-activated protein kinase; mTOR: mammalian target of rapamycin.
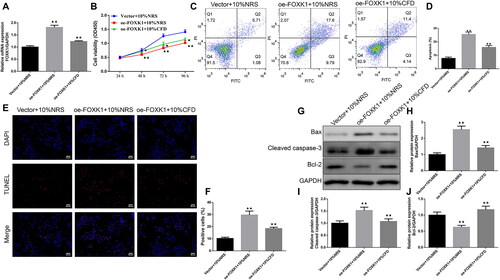
CFD reversed the effects of FOXK1 on GCs proliferation and apoptosis in PCOS rats
The qRT-PCR results revealed that the mRNA expression level of FOXK1 was higher in the oe-FOXK1 + 10%NRS group than the vector + 10%NRS group, while CFD caused a reduction in the FOXK1 mRNA level (). We observed that cell viability was reduced at 48, 72 and 96 h in the oe-FOXK1 + 10%NRS group compared to the vector + 10%NRS group, while cell viability was elevated after CFD intervention (). Furthermore, apoptosis was also assessed, and the results showed that overexpression of FOXK1 led to an increase in apoptosis rate, TUNEL-positive cell percentage, Bax, cleaved caspase-3 protein levels and a reduction in Bcl-2 levels, while CFD reversed these changes ().
Figure 6. CFD reverses the effect of FOXK1 on GCs proliferation and apoptosis in PCOS rats. (A) qRT-PCR was used to examine the effect of CFD on the FOXK1 mRNA expression level in the GCs of PCOS rats. (B) CCK-8 was used to detect the effect of CFD on GCs proliferation in PCOS rats. (C,D) The effect of CFD on GCs apoptosis rate was detected by flow cytometry. (E,F) The effect of CFD on GCs apoptosis rate was measured by TUNEL staining (Magnification, 200×). (G–J) Western blot was used to detect the effect of CFD on the expression levels of apoptosis-related proteins Bax, cleaved caspase-3, and Bcl-2 in GCs of PCOS rats. n = 3, compared to the vector + 10%NRS group, ▲p < .05, ▲▲p < .01; compared to the Oe-FOXK1 + 10%NRS group, *p<.05, **p < .01. Note. NRS: normal rat serum.
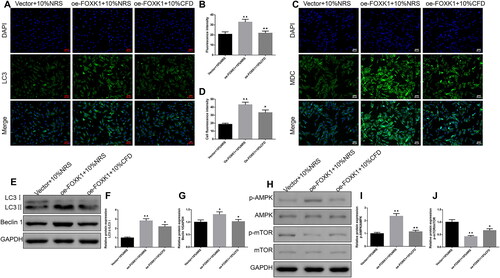
CFD reversed the effects of FOXK1 on the regulation of GCs autophagy and AMPK/mTOR pathway in PCOS rats
Immunofluorescence results suggested that LC3 fluorescence expression intensity was enhanced in cells of the oe-FOXK1 + 10%NRS group compared with the vector + 10%NRS group (). MDC is a marker for autolysosomes; the results of demonstrated that the oe-FOXK1 + 10%NRS group exhibited stronger fluorescence than the vector + 10%NRS group. Moreover, the LC3 II/I, Beclin 1, p-AMPK/AMPK protein expression levels were elevated and p-mTOR/mTOR protein levels were decreased (). Similarly, the situations were reversed by CFD treatment
Figure 7. CFD reverses the effect of FOXK1 on the regulation of GCs autophagy and AMPK/mTOR pathway in PCOS rats. (A,B) The effect of CFD on the expression level of LC3 in GCs of PCOS rats was detected by immunofluorescence. Magnification, 200×. (C,D) The effect of CFD on autophagy in GCs of PCOS rats was detected by MDC staining. Magnification, 200×. (E–G) Western blot was used to detect the effects of CFD on the expression levels of LC3 II/I, Beclin 1, p-AMPK/AMPK and p-mTOR/mTOR proteins in GCs of PCOS rats. n = 3, compared to the vector + 10%NRS group, ▲p < .05, ▲▲p < .01; compared to the Oe-FOXK1 + 10%NRS group, *p < .05, **p < .01.
Discussion
In the present study, we observed abnormal high expression of FOXK1 in GCs of PCOS rats. By extracting primary GCs and silencing or overexpressing FOXK1, we confirmed that FOXK1 promoted apoptosis and autophagy in GCs of PCOS-IR rats, and the underlying mechanism was related to the AMPK/mTOR signaling pathway. Importantly, CFD treatment reversed the effect of FOXK1 and thus protected GCs from apoptosis and autophagy.
FOX has been found to be involved in the regulation of biological processes such as glucose metabolism [Citation27], apoptosis [Citation28] and autophagy [Citation29]. FOX is negatively regulated as a downstream gene of insulin signaling [Citation30], which is involved in the regulation of oocyte mass and is increased in PCOS patients [Citation31]. Another study has revealed that as an evolutionarily conserved transcription factor, FOXK1 dysregulation is thought to affect cell fate and promote disease progression [Citation32]. Studies have shown that FOXK1 is aberrantly expressed in a variety of pathological processes [Citation33,Citation34]. In addition, FOXK1 can also induce aerobic glycolysis to reprogram cellular metabolism and affect cellular function [Citation35]. In this experiment, we also found abnormally high FOXK1 expression in GCs of PCOS rats. Next, we interfered with FOXK1 expression in GCs by silencing or overexpression. Through CCK-8 and EDU experiments, we found that FOXK1 effectively inhibited the proliferation and DNA synthesis abilities of GCs in PCOS.
Research has indicated that FOXK1 serves as a crucial factor in DNA damage-mediated autophagy by functioning as an autophagy-associated (ATG) transcriptional repressor [Citation36]. Bowman et al. also have discovered that FOXK1 protein inhibited starvation-induced atrophy and the initiation of the autophagic program, and the possible mechanism was related to the specific recruitment of the Sin3 complex to limit the expression of autophagic genes [Citation37]. In addition, FOXK1 overexpression was found to inhibit apoptosis in esophageal cancer cells [Citation38], glioma cells [Citation14], and hepatoma cells [Citation39]. However, there were some different findings in our study. After overexpression of FOXK1, the expression levels of LC3 II/I, Beclin 1, Bax, Cleaved caspase-3, which are proteins related to autophagy and apoptosis, were upregulated in GCs of PCOS rats while Bcl-2 expression was decreased, suggesting that FOXK1 triggered autophagy and apoptosis in GCs of PCOS rats. Therefore, to further investigate the underlying molecular mechanisms, we examined the effects of FOXK1 on proteins involved in the AMPK/mTOR pathway. The results suggested that FOXK1 promoted the phosphorylation of AMPK and inhibits the phosphorylation of mTOR. Indeed, the AMPK/mTOR pathway is known to promote autophagy and autophagy-dependent apoptosis [Citation40,Citation41]. Activation of the AMPK/mTOR pathway has been shown to promote GCs autophagy [Citation42]. Liu et al. also have found that rat ovarian GCs apoptosis and autophagy activation were associated with activation of the AMPK/mTOR pathway [Citation43]. Therefore, we hypothesized that the promotion of GCs autophagy and apoptosis in PCOS rats by FOXK1 overexpression was dependent on AMPK/mTOR pathway activation.
Next, we explored the regulatory effects of CFD on GCs in PCOS rats. Our results showed that CFD effectively reversed the effects of FOXK1 on GCs, as evidenced by promoting cell proliferation and inhibiting autophagy and apoptosis. Furthermore, the AMPK/mTOR pathway was also inhibited in GCs after CFD treatment. In view of this, our next experiment intends to investigate the role of the AMPK/mTOR pathway in the regulation of GCs by FOXK1 and CFD. Of course, there were some limitations in the study. The main limitations of the study were the lack of in vivo validation and only applied blank cells in qRT-PCR and CCK-8 assays. In the future, we are going to further verify the protective effects and mechanisms of CFD using PCOS animal models. Moreover, blank cells will be applied across the study to make the key findings of the study more convincing.
In conclusion, this study explored the role and mechanism of CFD. Specifically, CFD reversed FOXK1 expression in PCOS-associated GCs, thereby activating the AMPK/mTOR pathway, inhibiting apoptosis and autophagy to alleviate PCOS in vitro. These findings provided a research basis for the application of CFD in PCOS, and suggested that FOXK1 is a novel therapeutic target in PCOS treatment.
Ethical approval
Ethical review was conducted by the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center: SYXK (Zhejiang) 2021-0033.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xu B, Dai W, Liu L, et al. Metformin ameliorates polycystic ovary syndrome in a rat model by decreasing excessive autophagy in ovarian granulosa cells via the PI3K/AKT/mTOR pathway. Endocr J. 2022;69(7):1–9. doi:10.1507/endocrj.EJ21-0480.
- Zhang Y, Hu M, Jia W, et al. Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J Endocrinol. 2020;246(3):247–263. doi:10.1530/JOE-20-0155.
- Ren M, Liu J, Zhang Y, et al. Effects of different ovulation induction regimens on sex hormone levels and serum CTRP3 and CTRP15 levels in patients with Polycystic Ovary Syndrome (PCOS). Biomed Res Int. 2022;2022:6027878. doi:10.1155/2022/6027878.
- Wu Y, Tu M, Huang Y, et al. Association of metformin with pregnancy outcomes in women with polycystic ovarian syndrome undergoing in vitro fertilization: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8):e2011995. doi:10.1001/jamanetworkopen.2020.11995.
- Calcaterra V, Verduci E, Cena H, et al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: the role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients. 2021;13(6):1848. doi:10.3390/nu13061848.
- Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–2733. doi:10.1080/15548627.2021.1938914.
- Knapp KM, Sullivan R, Murray J, et al. DONSONLinked-read genome sequencing identifies biallelic pathogenic variants in as a novel cause of Meier-Gorlin syndrome. J Med Genet. 2020;57(3):195–202. doi:10.1136/jmedgenet-2019-106396.
- Xie F, Zhang J, Zhai M, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162(1):73–82. doi:10.1530/REP-20-0643.
- Rashid R, Mir SA, Kareem O, et al. Polycystic ovarian syndrome-current pharmacotherapy and clinical implications. Taiwan J Obstet Gynecol. 2022;61(1):40–50. doi:10.1016/j.tjog.2021.11.009.
- Liu S, Zhang Y, Yang F, et al. Modified Cangfu Daotan decoction ameliorates polycystic ovary syndrome with insulin resistance via NF-κB/LCN-2 signaling pathway in inflammatory microenvironment. Front Endocrinol (Lausanne). 2022;13:975724. doi:10.3389/fendo.2022.975724.
- Yi W, Li X, Chen K, et al. Effects of Cangfu Daotan Decoction on obese polycystic ovary syndrome and its mechanism. Steroids. 2021;165:108740. doi:10.1016/j.steroids.2020.108740.
- Zhang X, Jiang L, Liu H. Forkhead box protein O1: functional diversity and post-translational modification, a new therapeutic target? Drug Des Devel Ther. 2021;15:1851–1860. doi:10.2147/DDDT.S305016.
- Wang Y, Qiu W, Liu N, et al. Forkhead box K1 regulates the malignant behavior of gastric cancer by inhibiting autophagy. Ann Transl Med. 2020;8(4):107. doi:10.21037/atm.2019.12.123.
- Ji Z, Jiang H, Zhang P. FOXK1 promotes cell growth through activating wnt/β-catenin pathway and emerges as a novel target of miR-137 in glioma. Am J Transl Res. 2018;10(6):1784–1792.
- Li Z, Qu M, Wan HX, et al. FOXK1 promotes malignant progression of breast cancer by activating PI3K/AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(22):9978–9987.
- Zhang J, Huang L, Shi X, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020;12(23):24270–24287. doi:10.18632/aging.202143.
- Li H, Pang B, Nie B, et al. Dioscin promotes autophagy by regulating the AMPK-mTOR pathway in ulcerative colitis. Immunopharmacol Immunotoxicol. 2022;44(2):238–246. doi:10.1080/08923973.2022.2037632.
- Ni X, Shang F-S, Wang T-F, et al. Ellagic acid induces apoptosis and autophagy in Colon cancer through the AMPK/mTOR pathway. Tissue Cell. 2023;81:102032. doi:10.1016/j.tice.2023.102032.
- Zhu J, Ao H, Liu M, et al. UBE2T promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung adenocarcinoma. J Transl Med. 2021;19(1):374. doi:10.1186/s12967-021-03056-1.
- Jiang X-L, Tai H, Xiao X-S, et al. Cangfudaotan decoction inhibits mitochondria-dependent apoptosis of granulosa cells in rats with polycystic ovarian syndrome. Front Endocrinol (Lausanne). 2022;13:962154. doi:10.3389/fendo.2022.962154.
- Zhang Y, Zhou H, Ding C. The ameliorative effect of CangFu Daotan Decoction on polycystic ovary syndrome of rodent model is associated with m6A methylation and Wnt/β-catenin pathway. Gynecol Endocrinol. 2023;39:2181637.
- Wang MX, Yin Q, Xu X. A rat model of polycystic ovary syndrome with insulin resistance induced by letrozole combined with high fat diet. Med Sci Monit. 2020;26:e922136. doi:10.12659/MSM.922136.
- Zhou Y, Lan H, Dong Z, et al. Rhamnocitrin attenuates ovarian fibrosis in rats with letrozole-induced experimental polycystic ovary syndrome. Oxid Med Cell Longev. 2022;2022:5558599. doi:10.1155/2022/5558599.
- Prajapati DP, Patel M, Dharamsi A. Beneficial effect of polyherbal formulation in letrozole induced Polycystic ovarian syndrome (PCOS). J Tradit Complement Med. 2022;12(6):575–583. doi:10.1016/j.jtcme.2022.08.003.
- Roshanfekr Rad M, Nejati V, Razi M, et al. Nano-micelle curcumin; a hazardous and/or boosting agent? Relation with oocyte in-vitro maturation and pre-implantation embryo development in rats. Iran J Pharm Res. 2020;19(2):242–250. doi:10.22037/ijpr.2019.14799.12671.
- Kafshgiri SK, Parivar K, Baharara J, et al. Movento influences development of granulosa cells and ovarian follicles and FoxO1 and Vnn1 gene expression in BALB/c mice. Iran J Basic Med Sci. 2016;19(11):1209–1215.
- Wang L, Guo Y ,Pan M, et al. Functions of forkhead box O on glucose metabolism in abalone Haliotis discus hannai and its responses to high levels of dietary lipid. Genes (Basel). 2021;12(2):297. doi:10.3390/genes12020297.
- Wang Z, Liu Y, Liu X, et al. Activation of forkhead box O3a by mono(2-ethylhexyl)phthalate and its role in protection against mono(2-ethylhexyl)phthalate-induced oxidative stress and apoptosis in human cardiomyocytes. J Appl Toxicol. 2021;41(4):618–631. doi:10.1002/jat.4070.
- Zhang M, Sui W, Xing Y, et al. Angiotensin IV attenuates diabetic cardiomyopathy via suppressing FoxO1-induced excessive autophagy, apoptosis and fibrosis. Theranostics. 2021;11(18):8624–8639. doi:10.7150/thno.48561.
- Li Y, Sair AT, Zhao W, et al. Ferulic acid mediates metabolic syndrome via the regulation of hepatic glucose and lipid metabolisms and the insulin/IGF-1 receptor/PI3K/AKT pathway in palmitate-treated HepG2 cells. J Agric Food Chem. 2022;70(46):14706–14717. doi:10.1021/acs.jafc.2c05676.
- Wang H, Cai H, Wang X, et al. HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat Commun. 2019;10(1):5719.
- Liu Y, Ding W, Ge H, et al. FOXK transcription factors: regulation and critical role in cancer. Cancer Lett. 2019;458:1–12. doi:10.1016/j.canlet.2019.05.030.
- Wencong M, Jinghan W, Yong Y, et al. FOXK1 promotes proliferation and metastasis of gallbladder cancer by activating AKT/mTOR signaling pathway. Front Oncol. 2020;10:545. doi:10.3389/fonc.2020.00545.
- Zheng S, Yang L, Zou Y, et al. Long non-coding RNA HUMT hypomethylation promotes lymphangiogenesis and metastasis via activating FOXK1 transcription in triple-negative breast cancer. J Hematol Oncol. 2020;13(1):17. doi:10.1186/s13045-020-00852-y.
- Sukonina V, Ma H, Zhang W, et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature. 2019;566(7743):279–283. doi:10.1038/s41586-019-0900-5.
- Chen Y, Wu J, Liang G, et al. CHK2-FOXK axis promotes transcriptional control of autophagy programs. Sci Adv. 2020;6(1):eaax5819. doi:10.1126/sciadv.aax5819.
- Bowman CJ, Ayer DE, Dynlacht BD. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat Cell Biol. 2014;16(12):1202–1214. doi:10.1038/ncb3062.
- Chen D, Wang K, Li X, et al. FOXK1 plays an oncogenic role in the development of esophageal cancer. Biochem Biophys Res Commun. 2017;494(1–2):88–94. doi:10.1016/j.bbrc.2017.10.080.
- Xing B, Shen C, Yang Q, et al. miR-144-3p represses hepatocellular carcinoma progression by affecting cell aerobic glycolysis via FOXK1. Int J Exp Pathol. 2023;104(3):117–127. doi:10.1111/iep.12468.
- Sun D, Tao W, Zhang F, et al. Trifolirhizin induces autophagy-dependent apoptosis in Colon cancer via AMPK/mTOR signaling. Signal Transduct Target Ther. 2020;5(1):174.
- Zhang Y, He N, Zhou X, et al. Betulinic acid induces autophagy-dependent apoptosis via Bmi-1/ROS/AMPK-mTOR-ULK1 axis in human bladder cancer cells. Aging (Albany NY). 2021;13(17):21251–21267. doi:10.18632/aging.203441.
- Wang L, Liu Q, Kitamoto T, et al. Identification of insulin-responsive transcription factors that regulate glucose production by hepatocytes. Diabetes. 2019;68(6):1156–1167. doi:10.2337/db18-1236.
- Liu T, Di Q-N, Sun J-H, et al. Effects of nonylphenol induced oxidative stress on apoptosis and autophagy in rat ovarian granulosa cells. Chemosphere. 2020;261:127693. doi:10.1016/j.chemosphere.2020.127693.
